On-Line Monitoring of Biofilm Accumulation on Graphite-Polypropylene Electrode Material Using a Heat Transfer Sensor
Abstract
:1. Introduction
2. Materials and Methods
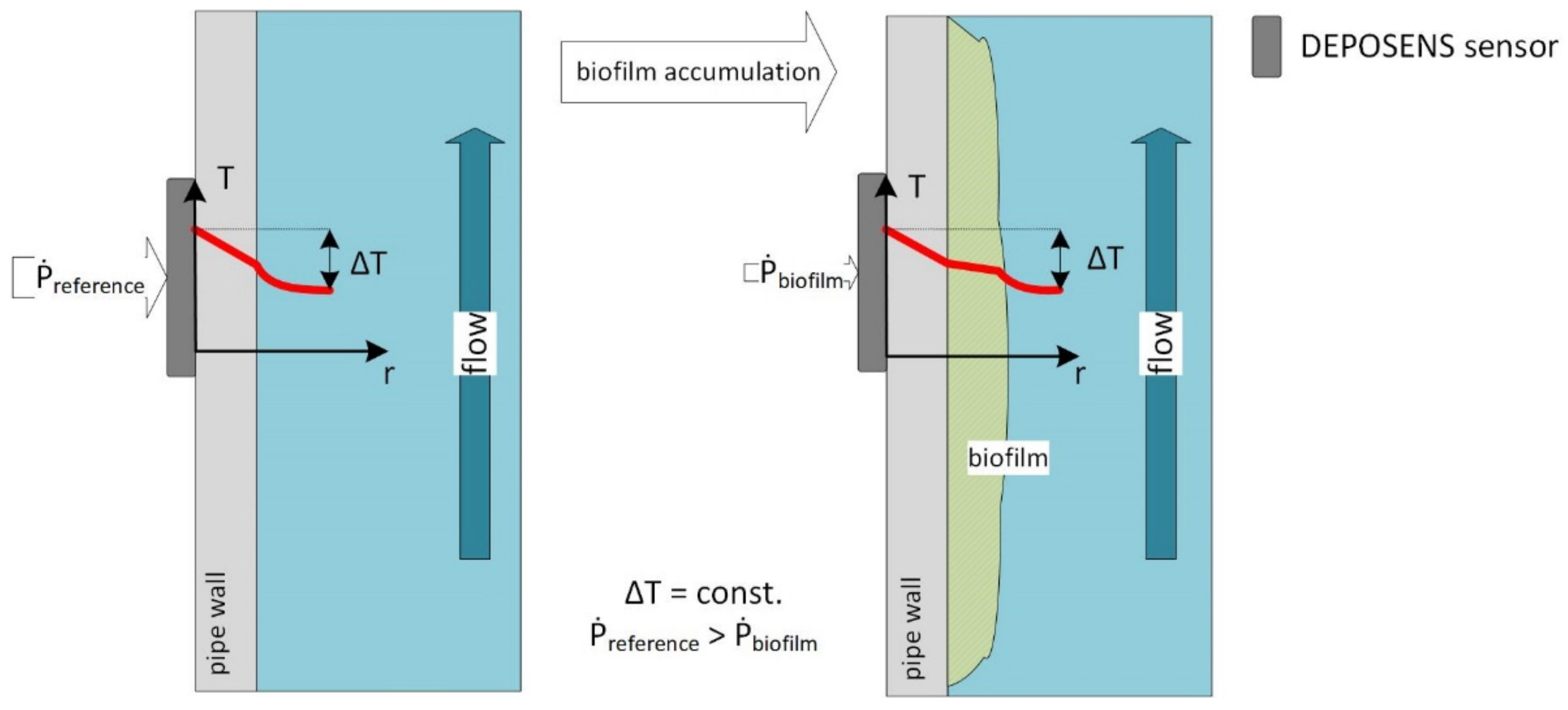
2.1. DEPOSENS Biofilm Sensor
2.2. Experimental Setup and Biofilm Cultivation
2.3. Gravimetric Biofilm Characterization
2.4. Data Analysis and Quality Control
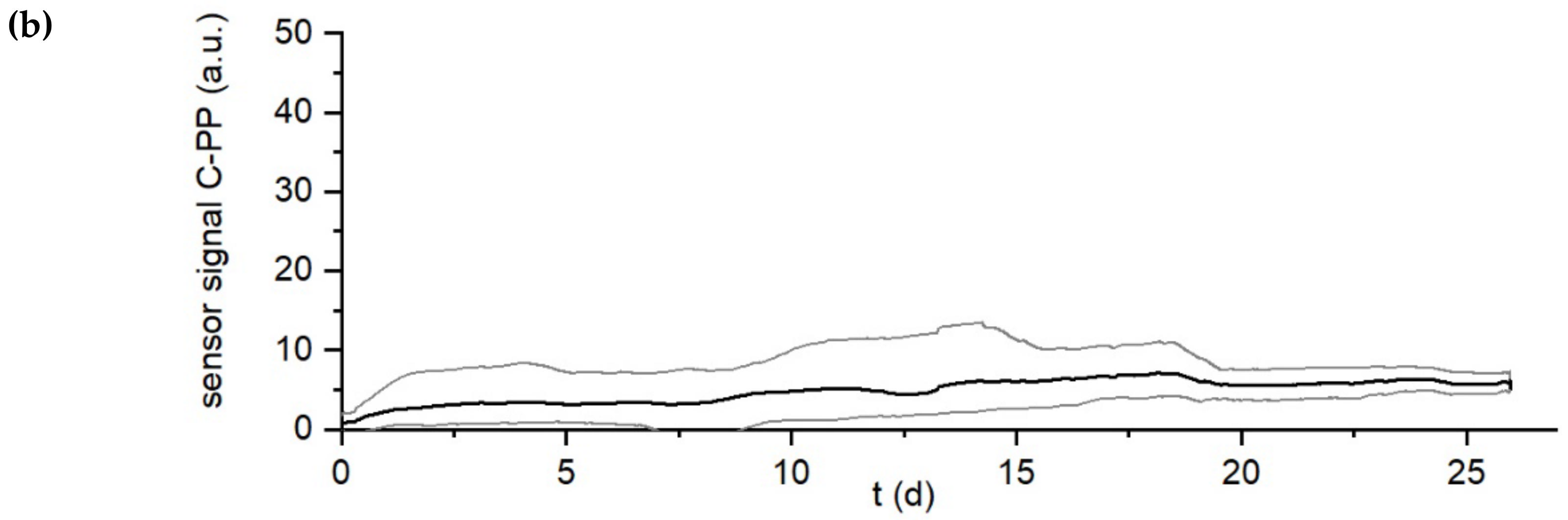
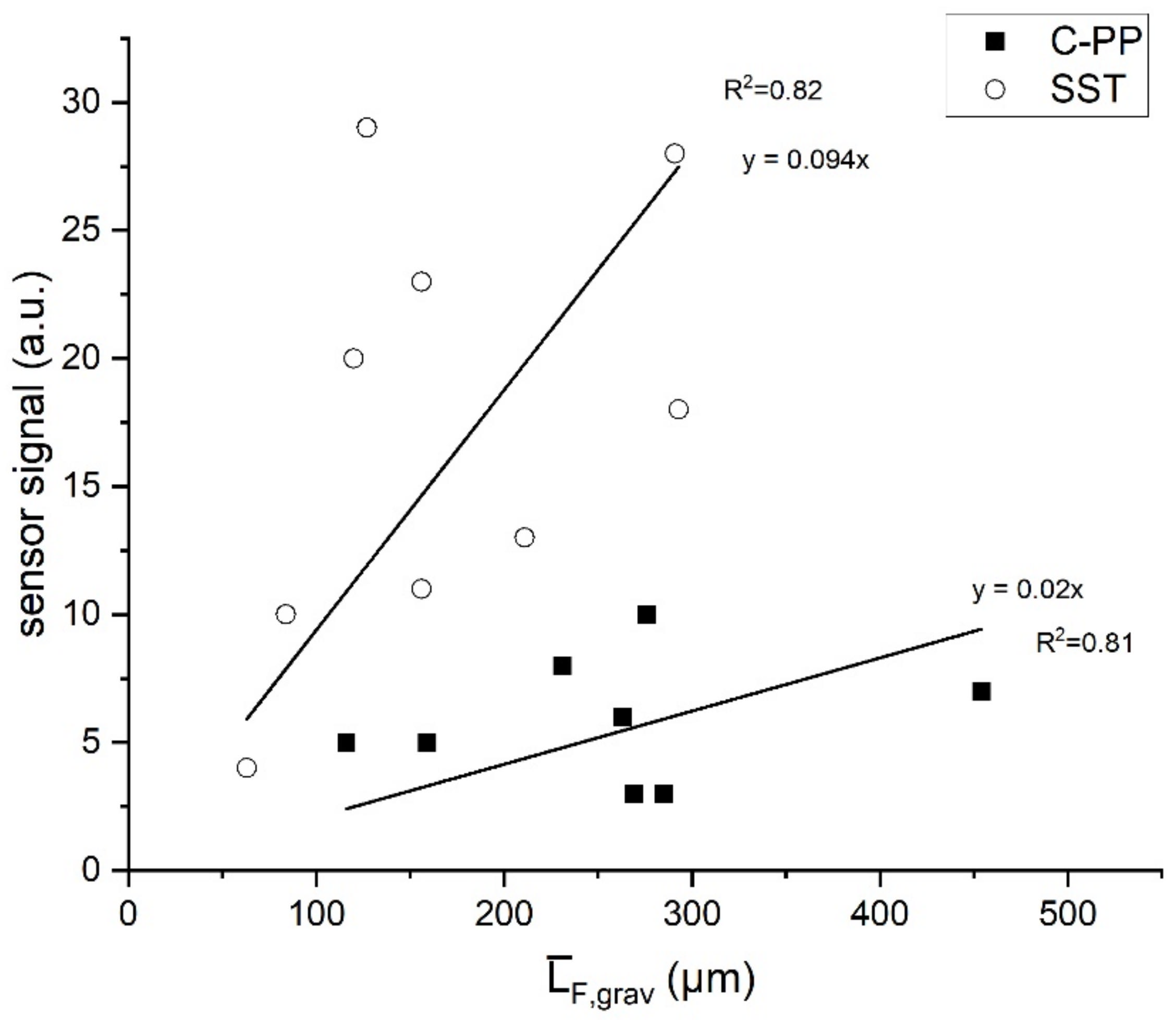
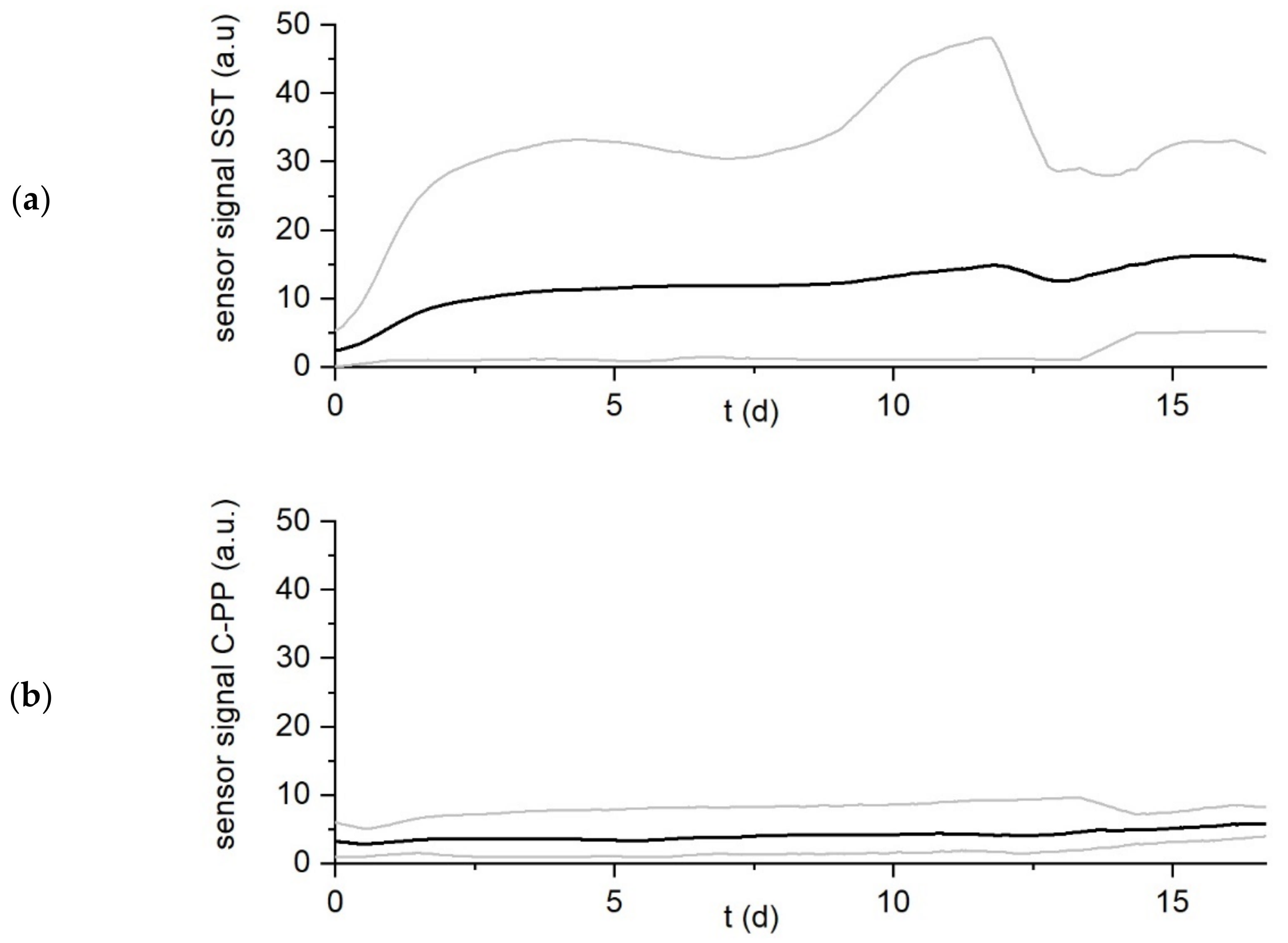
3. Results
Influence of Setting of Temperature Difference
4. Discussion
5. Conclusions
- The DEPOSENS biofilm sensor is able to identify an accumulation of biofilm on the inside of the pipe on both stainless steel and C-PP corresponding to the thickness of the accumulated biofilm. The application of the sensor on C-PP is needed for electrodes made from C-PP to have comparable biofilm growth characteristics in pipe sensors and on electrodes in BES.
- The application on the C-PP material rather than the standard stainless-steel pipe resulted in a reduction of sensitivity of the sensor, despite fairly similar thermal characteristics of the materials. The sensors on the C-PP material displayed a sensitivity (50 µm/a.u.) approximately 5-fold less than the sensor on stainless-steel (11 µm/a.u.)
- The reduced sensitivity limits the application of sensor on C-PP to technical systems with accumulating biofilm thicknesses of greater than 50 µm.
- The recommended operational settings for the application of the sensors with a temperature difference of minimum of 5 K.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sehar, S.; Naz, I. Role of the Biofilms in Wastewater Treatment. In Microbial Biofilms—Importance and Applications; Dhanasekaran, D., Thajuddin, N., Eds.; InTech: London, UK, 2016. [Google Scholar]
- Lewandowski, Z.; Boltz, J.P. Biofilms in Water and Wastewater Treatment. In Treatise on Water Science; Wilderer, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 529–570. [Google Scholar]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnisch, F.; Schröder, U. From MFC to MXC: Chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem. Soc. Rev. 2010, 39, 4433–4448. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Read, S.T.; Dutta, P.; Bond, P.L.; Keller, J.; Rabaey, K. Initial development and structure of biofilms on microbial fuel cell anodes. BMC Microbiol. 2010, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Chen, J.; Huang, H.; Liu, W.; Ye, Y.; Cheng, S. The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int. J. Hydrog. Energy 2016, 41, 16523–16528. [Google Scholar] [CrossRef]
- Marcus, A.K.; Torres, C.I.; Rittmann, B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar] [CrossRef]
- Pinck, S.; Ostormujof, L.M.; Teychené, S.; Erable, B. Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms. Microorganisms 2020, 8, 1841. [Google Scholar] [CrossRef] [PubMed]
- Semenec, L.; Franks, A.E. Delving through electrogenic biofilms: From anodes to cathodes to microbes. AIMS Bioeng. 2015, 2, 222–248. [Google Scholar] [CrossRef]
- Flemming, H.C. Role and levels of real-time monitoring for successful anti-fouling strategies—An overview. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2003, 47, 1–8. [Google Scholar] [CrossRef]
- Janknecht, P.; Melo, L.F. Online Biofilm Monitoring. Rev. Environ. Sci. Biotechnol. 2003, 2, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.; Sachsenheimer, K.; Kleintschek, T.; Waldbaur, A.; Schwartz, T.; Rapp, B.E. Online monitoring of biofilm growth and activity using a combined multi-channel impedimetric and amperometric sensor. Biosens. Bioelectron. 2013, 47, 157–163. [Google Scholar] [CrossRef]
- Settu, K.; Chen, C.-J.; Liu, J.-T.; Chen, C.-L.; Tsai, J.-Z. Impedimetric method for measuring ultra-low E. coli concentrations in human urine. Biosens. Bioelectron. 2015, 66, 244–250. [Google Scholar] [CrossRef]
- Pavanello, G.; Faimali, M.; Pittore, M.; Mollica, A.; Mollica, A.; Mollica, A. Exploiting a new electrochemical sensor for biofilm monitoring and water treatment optimization. Water Res. 2011, 45, 1651–1658. [Google Scholar] [CrossRef]
- Poma, N.; Vivaldi, F.; Bonini, A.; Salvo, P.; Kirchhain, A.; Ates, Z.; Melai, B.; Bottai, D.; Tavanti, A.; Di Francesco, F. Microbial biofilm monitoring by electrochemical transduction methods. TrAC Trends Anal. Chem. 2021, 134, 116134. [Google Scholar] [CrossRef]
- Nivens, D.E.; Palmer, R.J.; White, D.C. Continuous nondestructive monitoring of microbial biofilms: A review of analytical techniques. J. Ind. Microbiol. 1995, 15, 263–276. [Google Scholar] [CrossRef]
- Schmid, T.; Helmbrecht, C.; Panne, U.; Haisch, C.; Niessner, R. Process analysis of biofilms by photoacoustic spectroscopy. Anal. Bioanal. Chem. 2003, 375, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Romero, D.F.; Behrmann, O.; Dame, G.; Urban, G.A. Dynamic thermal sensor for biofilm monitoring. Sens. Actuators A Phys. 2014, 213, 43–51. [Google Scholar] [CrossRef]
- Wieland, T.; Assmann, J.; Bethe, A.; Fidelak, C.; Gmoser, H.; Janßen, T.; Kotthaus, K.; Lübke-Becker, A.; Wieler, L.H.; Urban, G.A. A Real-Time Thermal Sensor System for Quantifying the Inhibitory Effect of Antimicrobial Peptides on Bacterial Adhesion and Biofilm Formation. Sensors 2021, 21, 2771. [Google Scholar] [CrossRef]
- Neu, T.R.; Manz, B.; Volke, F.; Dynes, J.J.; Hitchcock, A.P.; Lawrence, J.R. Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol. Ecol. 2010, 72, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol. 2015, 233–242. [Google Scholar] [CrossRef]
- Wagner, M.; Horn, H. Optical coherence tomography in biofilm research: A comprehensive review. Biotechnol. Bioeng. 2017, 114, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Triggs, G.J.; Krauss, T.F. Optical Sensing of Microbial Life on Surfaces. Appl. Environ. Microbiol. 2015, 82, 1362–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Richter, H.; Covalla, S.F.; Johnson, J.P.; Woodard, T.L.; Orloff, A.L.; Jia, H.; Zhang, M.; Lovley, D.R. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 2008, 10, 2505–2514. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial Fuel Cells: Electrode Materials. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–318. [Google Scholar]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Dumas, C.; Mollica, A.; Féron, D.; Basséguy, R.; Etcheverry, L.; Bergel, A. Marine microbial fuel cell: Use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Mustakeem, M. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Muddemann, T.; Haupt, D.R.; De Silva, L.G.S.; Jiang, B.; Kunz, U.; Bormann, H.; Niedermeiser, M.; Schlaefer, O.; Sievers, M. Integration of Upscaled Microbial Fuel Cells in Real Municipal Sewage Plants. ECS Trans. 2017, 77, 1053–1077. [Google Scholar] [CrossRef]
- Characklis, W. Biofilms; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Garcia, S.; Trueba, A.; Vega, L.M.; Madariaga, E. Quantitative Changes in Biofilms of a Seawater Tubular Heat Exchanger Subjected to Electromagnetic Fields Treatment. Int. J. Mech. Mechatron. Eng. 2018, 12, 853–856. [Google Scholar] [CrossRef]
- Bogaard, R.H. Thermal Conductivity of Selected Stainless Steels. In Thermal Conductivity 18; Ashworth, T., Smith, D.R., Eds.; Springer: Boston, MA, USA, 1985; pp. 175–185. [Google Scholar]
- Duddridge, J.E.; Kent, C.A.; Laws, J.F. Effect of surface shear stress on the attachment of Pseudomonas fluorescens to stainless steel under defined flow conditions. Biotechnol. Bioeng. 1982, 24, 153–164. [Google Scholar] [CrossRef]
- Recupido, F.; Toscano, G.; Tatè, R.; Petala, M.; Caserta, S.; Karapantsios, T.D.; Guido, S. The role of flow in bacterial biofilm morphology and wetting properties. Colloids Surfaces. B Biointerfaces 2020, 192, 111047. [Google Scholar] [CrossRef]
- Dutta, S. Mathematical Modeling of the Performance of a Rotating Biological Contactor for Process Optimisation in Wastewater Treatment. Ph.D. Dissertation, University Karlsruhe, Karlsruhe, Germany, 2007. [Google Scholar]
- Characklis, W.G.; Nevimons, M.J.; Picologlou, B.F. Influence of Fouling Biofilms on Heat Transfer. Heat Transf. Eng. 1981, 3, 23–37. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci.: Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Woon, C.W.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. Ultrasound Driven Biofilm Removal for Stable Power Generation in Microbial Fuel Cell. Energy Fuels 2017, 31, 968–976. [Google Scholar] [CrossRef]
- Islam, M.A.; Ehiraj, B.; Cheng, C.K.; Dubey, B.N.; Khan, M.M.R. Biofilm re-vitalization using hydrodynamic shear stress for stable power generation in microbial fuel cell. J. Electroanal. Chem. 2019, 844, 14–22. [Google Scholar] [CrossRef]


| Q (L/min) | u (cm/s) | Re (–) | ∆T (K) | Number of Replicates Used |
|---|---|---|---|---|
| 3.6 | 12 | 3000 | 10 | 9 |
| 5 | 8 | |||
| 2 | 4 |
| Sensor/Pipe Material | Mean Biofilm Density(kg/m3) | Fraction of Inorganic Compounds (kg/m3) | |
|---|---|---|---|
| C-PP | 276 ± 102 (± 37%) | 24 ± 13 (± 54%) | 8 ± 5 (± 63%) |
| SST | 170 ± 84 (± 49%) | 19 ± 8 (± 42%) | 9 ± 4 (± 44%) |
| Temperature Difference (∆T) | Mean Biofilm Thickness SST (µm) | Sensitivity SST (µm/a.u.) | Mean Biofilm Thickness C-PP (µm) | Sensitivity C-PP (µm/a.u.) |
|---|---|---|---|---|
| 10 K | 170 ± 84 (± 49%) | 11 | 276 ± 102 (± 54%) | 50 |
| 5 K | 121 ± 29 (± 24%) | 9 | 193 ± 58 (± 30%) | 52 |
| 2 K | 161 ± 52 (± 32%) | 77 | 302 ± 59 (± 20%) | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netsch, A.; Horn, H.; Wagner, M. On-Line Monitoring of Biofilm Accumulation on Graphite-Polypropylene Electrode Material Using a Heat Transfer Sensor. Biosensors 2022, 12, 18. https://doi.org/10.3390/bios12010018
Netsch A, Horn H, Wagner M. On-Line Monitoring of Biofilm Accumulation on Graphite-Polypropylene Electrode Material Using a Heat Transfer Sensor. Biosensors. 2022; 12(1):18. https://doi.org/10.3390/bios12010018
Chicago/Turabian StyleNetsch, Andreas, Harald Horn, and Michael Wagner. 2022. "On-Line Monitoring of Biofilm Accumulation on Graphite-Polypropylene Electrode Material Using a Heat Transfer Sensor" Biosensors 12, no. 1: 18. https://doi.org/10.3390/bios12010018
APA StyleNetsch, A., Horn, H., & Wagner, M. (2022). On-Line Monitoring of Biofilm Accumulation on Graphite-Polypropylene Electrode Material Using a Heat Transfer Sensor. Biosensors, 12(1), 18. https://doi.org/10.3390/bios12010018




