Design of Magnetic Nanoplatforms for Cancer Theranostics
Abstract
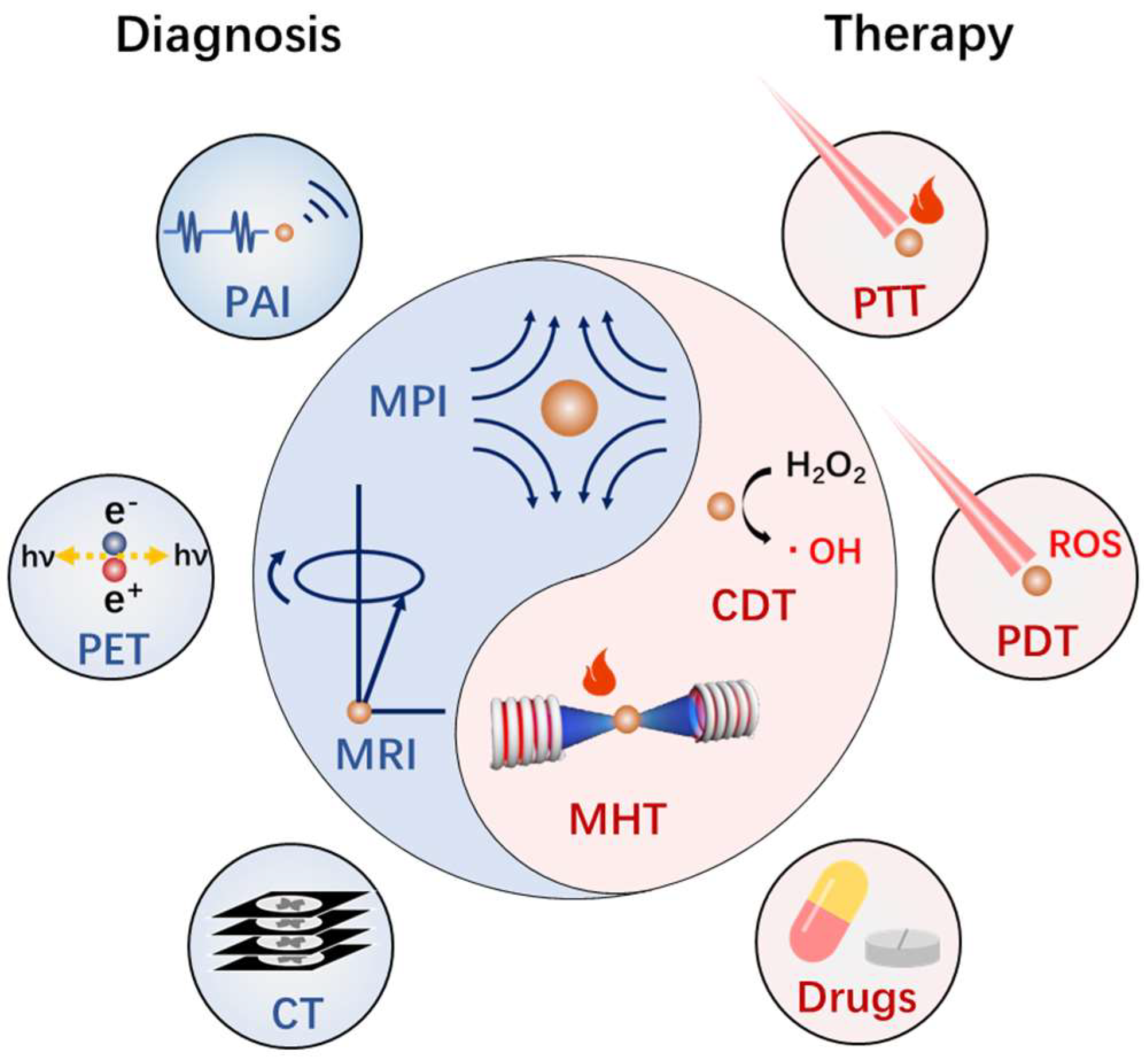
:1. Introduction
2. Controlled Synthesis of Magnetic Nanoplatforms

3. Basis of Magnetic Nanomaterials Mediated Diagnosis and Therapy of Cancer
3.1. Biosafety of Magnetic Nanoplatforms
3.2. Magnetic Resonance Imaging
3.3. Other Diagnosis Applications
3.4. Magnetic Hyperthermia
3.5. Chemodynamic Therapy
4. Implementation of Magnetotheranostic Based on Magnetic Nanoplatforms
4.1. Magnetotheranostics Based on Magnetic Nanoplatforms Only
4.2. Integration of Magnetic Nanoplatforms with Phototheransotics
4.3. Integration of Magnetic Nanoplatforms with Fluorescence Imaging
4.4. Integration of Magnetic Nanoplatforms with CT&PET/SPECT
4.5. Magnetic Nanoplatforms Carrier Based Drug Delivery
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Ferumoxytol In Iron Deficiency Anaemia in Adults with Chronic Kidney Disease. Drugs 2012, 72, 2013–2022. [Google Scholar] [CrossRef]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj–Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of Iron Oxide Nanocubes: High–Resolution In Situ Monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater. Today 2019, 31, 86–99. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Park, J.; Jin, C.; Lee, S.; Kim, J.; Choi, H. Magnetically Actuated Degradable Microrobots for Actively Controlled Drug Release and Hyperthermia Therapy. Adv. Health Mater. 2019, 8, e1900213. [Google Scholar] [CrossRef] [PubMed]
- Cazares–Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou–Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot–spot” effect for local delivery to combined magneto–photo–thermia using magneto–plasmonic hybrids. Adv. Drug Deliv. Rev. 2018, 138, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Lu, C.; Han, L.; Wang, J.; Wan, J.; Song, G.; Rao, J. Engineering of magnetic nanoparticles as magnetic particle imaging tracers. Chem. Soc. Rev. 2021, 50, 8102–8146. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Rezaeipoor, R.; Adie, S.G.; Chaney, E.J.; Oldenburg, A.L.; Marjanovic, M.; Haldar, J.P.; Sutton, B.P.; Boppart, S.A. In vivo magnetomotive optical molecular imaging using targeted magnetic nanoprobes. Proc. Natl. Acad. Sci. USA 2010, 107, 8085–8090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teijeiro–Valiño, C.; Gómez, M.A.G.; Yañez–Villar, S.; García–Acevedo, P.; Arnosa–Prieto, A.; Belderbos, S.; Gsell, W.; Himmelreich, U.; Piñeiro, Y.; Rivas, J. Biocompatible magnetic gelatin nanoparticles with enhanced MRI contrast performance prepared by single–step desolvation method. Nano Express 2021, 2, 020011. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, 2104223. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cuichen, W.; Ocsoy, I.; Zhu, G.; Yasun, E.; You, M.; Wu, C.; Zheng, J.; Song, E.; Huang, C.Z.; et al. Gold–Coated Fe3O4Nanoroses with Five Unique Functions for Cancer Cell Targeting, Imaging, and Therapy. Adv. Funct. Mater. 2013, 24, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Peng, M.L.; Li, G.; Miao, Y.Q.; Luo, H.; Jing, G.; He, Y.; Zhang, C.; Zhang, F.; Fan, H. Ultrasonication–Triggered Ubiquitous Assembly of Magnetic Janus Amphiphilic Nanoparticles in Cancer Theranostic Applications. Nano Lett. 2019, 19, 4118–4125. [Google Scholar] [CrossRef]
- Song, J.; Lin, L.; Yang, Z.; Zhu, R.; Zhou, Z.; Li, Z.-W.; Wang, F.; Chen, J.; Yang, H.–H.; Chen, X. Self–Assembled Responsive Bilayered Vesicles with Adjustable Oxidative Stress for Enhanced Cancer Imaging and Therapy. J. Am. Chem. Soc. 2019, 141, 8158–8170. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bruns, O.; Salmen, S.C.; Beisiegel, U.; Weller, H. A Highly Effective, Nontoxic T1 MR Contrast Agent Based on Ultrasmall PEGylated Iron Oxide Nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; Van De Kaa, C.H.; De La Rosette, J.; Weissleder, R. Noninvasive Detection of Clinically Occult Lymph–Node Metastases in Prostate Cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Casas, J.; Venkataramasubramani, M. Magnetic Nanoparticle Mediated Enhancement of Localized Surface Plasmon Resonance for Ultrasensitive Bioanalytical Assay in Human Blood Plasma. Anal. Chem. 2013, 85, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Gong, T.; Xu, N.; Cui, F.; Yuan, B.; Yuan, Q.; Sun, H.; Wang, L.; Liu, J. Improved Stability and Photothermal Performance of Polydopamine-Modified Fe3O4 Nanocomposites for Highly Efficient Magnetic Resonance Imaging-Guided Photothermal Therapy. Small 2020, 16, e2003969. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shi, Y.; Zhang, S.; Huang, X.; Zhang, J.; Zhang, Y.; Si, W.; Dong, X. Hydrogen Peroxide Responsive Iron–Based Nanoplatform for Multimodal Imaging–Guided Cancer Therapy. Small 2018, 15, e1803791. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, T.; Ma, X.; Ren, W.; Zhou, Z.; Zhu, G.; Zhang, A.; Liu, Y.; Song, J.; Li, Z.; et al. Multifunctional Theranostic Nanoparticles Based on Exceedingly Small Magnetic Iron Oxide Nanoparticles for T1–Weighted Magnetic Resonance Imaging and Chemotherapy. ACS Nano 2017, 11, 10992–11004. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández–García, M.P.; Guedes, A.; Tavares, P.B.; Grenèche, J.-M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One–Step Coprecipitation Route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra–large–scale syntheses of mono disperse nanocrystals. Nat Mater 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single–crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Hu, P.; Yu, L.; Zuo, A.; Guo, C.; Yuan, F. Fabrication of Monodisperse Magnetite Hollow Spheres. J. Phys. Chem. C 2008, 113, 900–906. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Accounts Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.-G.; Noh, H.-J.; Hyeon, T. Large–Scale Synthesis of Uniform and Crystalline Magnetite Nanoparticles Using Reverse Micelles as Nanoreactors under Reflux Conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.Y.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra–sensitive molecular imaging. Nat. Med. 2006, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-T.; Nah, H.; Lee, J.-H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant–Controlled Magnetic Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1234–1238. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Liu, X.L.; Jiao, J.; Ng, C.-T.; Yi, J.; E Luo, Y.; Bay, B.-H.; Zhao, L.Y.; Peng, M.L.; et al. Ultrasmall Ferrite Nanoparticles Synthesized via Dynamic Simultaneous Thermal Decomposition for High–Performance and Multifunctional T1 Magnetic Resonance Imaging Contrast Agent. ACS Nano 2017, 11, 3614–3631. [Google Scholar] [CrossRef]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.-L.; Yi, J.-B.; Yang, Y.; Fan, H.-M.; Ding, J. Stable vortex magnetite nanorings colloid: Micromagnetic simulation and experimental demonstration. J. Appl. Phys. 2012, 111, 044303. [Google Scholar] [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Accounts Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M. Innovative magnetic nanoparticle platform for magnetic resonance imaging and magnetic fluid hyperthermia applications. Curr. Opin. Chem. Eng. 2014, 4, 38–46. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, Y.; Ng, C.T.; Zhao, L.Y.; Zhang, Y.; Bay, B.H.; Fan, H.M.; Ding, J. Magnetic Vortex Nanorings: A New Class of Hyperthermia Agent for Highly Efficient In Vivo Regression of Tumors. Adv. Mater. 2015, 27, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Jia, C.-J.; Sun, L.-D.; Luo, F.; Han, X.-D.; Heyderman, L.J.; Yan, Z.-G.; Yan, C.-H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large–Scale Synthesis of Single–Crystalline Iron Oxide Magnetic Nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Yang, V.C. Magnetic brain tumor targeting and biodistribution of long–circulating PEG–modified, cross–linked starch–coated iron oxide nanoparticles. Biomaterials 2011, 32, 6291–6301. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Xie, J.; Zhang, F.; Wang, Z.-Y.; Luo, K.; Zhu, L.; Quan, Q.-M.; Niu, G.; Lee, S.; Ai, H.; et al. N–Alkyl–PEI–functionalized iron oxide nanoclusters for efficient siRNA delivery. Small 2011, 7, 2742–2749. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.-J.; Dai, Y.-L.; Ma, P.-A.; Yang, D.-M.; Li, C.-X.; Hou, Z.-Y.; Cheng, Z.-Y.; Lin, J. Poly(acrylic acid)–Modified Fe3O4Microspheres for Magnetic–Targeted and pH–Triggered Anticancer Drug Delivery. Chem. Eur. J. 2012, 18, 15676–15682. [Google Scholar] [CrossRef]
- Riedinger, A.; Leal, M.P.; Deka, S.R.; George, C.; Franchini, I.R.; Falqui, A.; Cingolani, R.; Pellegrino, T. “Nanohybrids” Based on pH–Responsive Hydrogels and Inorganic Nanoparticles for Drug Delivery and Sensor Applications. Nano Lett. 2011, 11, 3136–3141. [Google Scholar] [CrossRef]
- KC, R.B.; Lee, S.M.; Yoo, E.S.; Choi, J.H.; Ghim, H.D. Glycoconjugated chitosan stabilized iron oxide nanoparticles as a multifunctional nanoprobe. Mater. Sci. Eng. C 2009, 29, 1668–1673. [Google Scholar] [CrossRef]
- Kim, J.; Arifin, D.R.; Muja, N.; Kim, T.; Gilad, A.A.; Kim, H.; Arepally, A.; Hyeon, T.; Bulte, J.W.M. Multifunctional Capsule–in–Capsules for Immunoprotection and Trimodal Imaging. Angew. Chem. Int. Ed. 2011, 50, 2317–2321. [Google Scholar] [CrossRef]
- Nanotherm®. Available online: https://www.magforce.com/en/home/our_therapy/ (accessed on 13 December 2021).
- Wadajkar, A.S.; Menon, J.U.; Tsai, Y.-S.; Gore, C.; Dobin, T.; Gandee, L.; Kangasniemi, K.; Takahashi, M.; Manandhar, B.; Ahn, J.-M.; et al. Prostate cancer–specific thermo–responsive polymer–coated iron oxide nanoparticles. Biomaterials 2013, 34, 3618–3625. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, K.; Qi, S.; Liu, H.; Jiang, Y.; Jiang, H.; Li, J.; Zhang, H.; Cheng, Z. Affibody modified and radiolabeled gold–Iron oxide hetero–nanostructures for tumor PET, optical and MR imaging. Biomaterials 2013, 34, 2796–2806. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Di Fan, D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Zeng, J.; Jing, L.; Hou, Y.; Jiao, M.; Qiao, R.; Jia, Q.; Liu, C.; Fang, F.; Lei, H.; Gao, M. Anchoring Group Effects of Surface Ligands on Magnetic Properties of Fe3O4Nanoparticles: Towards High Performance MRI Contrast Agents. Adv. Mater. 2014, 26, 2694–2698. [Google Scholar] [CrossRef]
- Liu, X.; Yan, B.; Li, Y.; Ma, X.; Jiao, W.; Shi, K.; Zhang, T.; Chen, S.; He, Y.; Liang, X.-J.; et al. Graphene Oxide–Grafted Magnetic Nanorings Mediated Magnetothermodynamic Therapy Favoring Reactive Oxygen Species–Related Immune Response for Enhanced Antitumor Efficacy. ACS Nano 2020, 14, 1936–1950. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Park, J.W. Spatially nanoscale–controlled functional surfaces toward efficient bioactive platforms. J. Mater. Chem. B 2015, 3, 5135–5149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Zhou, Q.; Chen, X.; Jiao, W.; Li, G.; Peng, M.; Liu, X.; He, Y.; Fan, H. Precise Regulation of Enzyme–Nanozyme Cascade Reaction Kinetics by Magnetic Actuation toward Efficient Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 52395–52405. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, P.; Rawson, F.J.; Ho, W.K.-W.; Lee, S.-F.; Leung, K.C.-F.; Wang, X.; Beri, A.; Preece, J.A.; Ma, J.; Mendes, P.M. Surface Molecular Tailoring Using pH–Switchable Supramolecular Dendron–Ligand Assemblies. ACS Appl. Mater. Interfaces 2014, 6, 6264–6274. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Kwon, S.H.; Kwak, J.W.; Park, J.W. “Seeing and counting” individual antigens captured on a microarrayed spot with Force–Based Atomic Force Microscopy. Anal. Chem. 2010, 82, 5189–5194. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.; Lee, D.; Roy, D.; Park, J.W. Quantification of Fewer than Ten Copies of a DNA Biomarker without Amplification or Labeling. J. Am. Chem. Soc. 2016, 138, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; An, Y.-L.; Lin, M.; Gao, Z.; Zhang, J. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.; Chauhan, L.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef]
- Pisanic, T.R.; Blackwell, J.D.; Shubayev, V.I.; Fiñones, R.R.; Jin, S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 2007, 28, 2572–2581. [Google Scholar] [CrossRef]
- Wada, S.; Yue, L.; Tazawa, K.; Furuta, I.; Nagae, H.; Takemori, S.; Minamimura, T. New local hyperthermia using dextran magnetite complex (DM) for oral cavity: Experimental study in normal hamster tongue. Oral Dis. 2001, 7, 192–195. [Google Scholar] [CrossRef]
- Motoyama, S.; Ishiyama, K.; Maruyama, K.; Narita, K.; Minamiya, Y.; Ogawa, J.-I. Estimating the Need for Neck Lymphadenectomy in Submucosal Esophageal Cancer Using Superparamagnetic Iron Oxide–Enhanced Magnetic Resonance Imaging: Clinical Validation Study. World J. Surg. 2011, 36, 83–89. [Google Scholar] [CrossRef]
- Howarth, S.; Tang, T.; Trivedi, R.; Weerakkody, R.; U–King–Im, J.; Gaunt, M.; Boyle, J.; Li, Z.-Y.; Miller, S.; Graves, M.; et al. Utility of USPIO–enhanced MR imaging to identify inflammation and the fibrous cap: A comparison of symptomatic and asymptomatic individuals. Eur. J. Radiol. 2009, 70, 555–560. [Google Scholar] [CrossRef]
- Bernd, H.; De Kerviler, E.; Gaillard, S.; Bonnemain, B. Safety and Tolerability of Ultrasmall Superparamagnetic Iron Oxide Contrast Agent. Investig. Radiol. 2009, 44, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, B.; Price, T.W.; Gallo, J.; Bañobre–López, M.; Stasiuk, G.J. Smart magnetic resonance imaging–based theranostics for cancer. Theranostics 2021, 11, 8706–8737. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez–Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2018, 119, 957–1057. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Y.; Yang, L.; Wu, J.; Zhu, W.; Li, D.; Cheng, Z.; Xia, C.; Guo, Y.; Gong, Q.; et al. Negatively Charged Magnetite Nanoparticle Clusters as Efficient MRI Probes for Dendritic Cell Labeling and In Vivo Tracking. Adv. Funct. Mater. 2015, 25, 3581–3591. [Google Scholar] [CrossRef]
- Karimian–Jazi, K.; Münch, P.; Alexander, A.; Fischer, M.; Pfleiderer, K.; Piechutta, M.; Karreman, M.A.; Solecki, G.M.; Berghoff, A.S.; Friedrich, M.; et al. Monitoring innate immune cell dynamics in the glioma microenvironment by magnetic resonance imaging and multiphoton microscopy (MR–MPM). Theranostics 2020, 10, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast–enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieber, M.A.; Lengsfeld, P.; Walter, J.; Schirmer, H.; Frenzel, T.; Siegmund, F.; Weinmann, H.-J.; Pietsch, H. Gadolinium–based contrast agents and their potential role in the pathogenesis of nephrogenic systemic fibrosis: The role of excess ligand. J. Magn. Reson. Imaging 2008, 27, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Bin Na, H.; et al. Large–Scale Synthesis of Uniform and Extremely Small–Sized Iron Oxide Nanoparticles for High–Resolution T1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wisniowska, A.E.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Zhang, H.; Cai, J.; Chen, Y.; Ma, H.; Zhang, S.; Yi, J.B.; Liu, X.; Bay, B.-H.; Guo, Y.; et al. Structure–Relaxivity Mechanism of an Ultrasmall Ferrite Nanoparticle T1 MR Contrast Agent: The Impact of Dopants Controlled Crystalline Core and Surface Disordered Shell. Nano Lett. 2021, 21, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Guo, J.; Shi, Y.; Zhao, G.; Sun, S.; Sun, X. Porous yolk–shell Fe/Fe3O4 nanoparticles with controlled exposure of highly active Fe(0) for cancer therapy. Biomaterials 2020, 268, 120530. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Tay, Z.W.; Hensley, D.; Zhou, X.Y.; Fung, B.K.; Colson, C.; Lu, Y.; Fellows, B.D.; Huynh, Q.; Saayujya, C.; et al. Using magnetic particle imaging systems to localize and guide magnetic hyperthermia treatment: Tracers, hardware, and future medical applications. Theranostics 2020, 10, 2965–2981. [Google Scholar] [CrossRef] [PubMed]
- Gloag, L.; Mehdipour, M.; Ulanova, M.; Mariandry, K.; Nichol, M.A.; Hernández–Castillo, D.J.; Gaudet, J.; Qiao, R.; Zhang, J.; Nelson, M.; et al. Zero valent iron core–iron oxide shell nanoparticles as small magnetic particle imaging tracers. Chem. Commun. 2020, 56, 3504–3507. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus Iron Oxides @ Semiconducting Polymer Nanoparticle Tracer for Cell Tracking by Magnetic Particle Imaging. Nano Lett. 2017, 18, 182–189. [Google Scholar] [CrossRef]
- Oldenburg, A.L.; Crecea, V.; Rinne, S.A.; Boppart, S.A. Phase–resolved magnetomotive OCT for imaging nanomolar concentrations of magnetic nanoparticles in tissues. Opt. Express 2008, 16, 11525–11539. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Chaney, E.J.; Aksamitiene, E.; Barkalifa, R.; Spillman, D.R.; Bogan, B.J.; Boppart, S.A. Biomechanical sensing of in vivo magnetic nanoparticle hyperthermia–treated melanoma using magnetomotive optical coherence elastography. Theranostics 2021, 11, 5620–5633. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, Y.; Gao, W.; Xu, J.; Zuo, G.; Chen, Y.; Zhao, M.; Li, J.; Song, J.; Zhang, N.; et al. Magnetic Hyperthermia Ablation of Tumors Using Injectable Fe3O4/Calcium Phosphate Cement. ACS Appl. Mater. Interfaces 2015, 7, 13866–13875. [Google Scholar] [CrossRef]
- Yin, P.; Shah, S.; Pasquale, N.J.; Garbuzenko, O.B.; Minko, T.; Lee, K. –B. Stem cell–based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials 2015, 81, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Moise, S.; Byrne, J.M.; El Haj, A.J.; Telling, N.D. The potential of magnetic hyperthermia for triggering the differentiation of cancer cells. Nanoscale 2018, 10, 20519–20525. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Ng, C.T.; Chandrasekharan, P.; Yang, H.T.; Zhao, L.Y.; Peng, E.; Lv, Y.B.; Xiao, W.; Fang, J.; Yi, J.; et al. Synthesis of Ferromagnetic Fe0.6Mn0.4O Nanoflowers as a New Class of Magnetic Theranostic Platform for In Vivo T1–T2Dual–Mode Magnetic Resonance Imaging and Magnetic Hyperthermia Therapy. Adv. Health Mater. 2016, 5, 2092–2104. [Google Scholar] [CrossRef]
- Yang, M.; Ho, C.; Ruta, S.; Chantrell, R.; Krycka, K.; Hovorka, O.; Chen, F.-R.; Lai, P.; Lai, C. Magnetic Interaction of Multifunctional Core–Shell Nanoparticles for Highly Effective Theranostics. Adv. Mater. 2018, 30, e1802444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X.; Chu, C.; Zhou, Z.; Chen, B.; Pang, X.; Lin, G.; Lin, H.; Guo, Y.; Ren, E.; et al. Genetically engineered magnetic nanocages for cancer magneto–catalytic theranostics. Nat. Commun. 2020, 11, 5421. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Tian, R.; Yang, Z.; Yu, G.; Lin, L.; Zhang, G.; Fan, W.; Zhang, F.; Niu, G.; et al. Activatable Singlet Oxygen Generation from Lipid Hydroperoxide Nanoparticles for Cancer Therapy. Angew. Chem. Int. Ed. 2017, 56, 6492–6496. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur–modified iron oxide as an effective Fenton–like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, L.; Chen, Y.; Shi, J. Tumor–selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, C.; Li, F.; Liao, H.; Chen, Z.; Lin, P.; Wang, N.; Zhou, Y.; Lee, J.Y.; Ding, Q.; et al. Core–Shell–Satellite Nanomaces as Remotely Controlled Self-Fueling Fenton Reagents for Imaging-Guided Triple-Negative Breast Cancer-Specific Therapy. Small 2020, 16, e2002537. [Google Scholar] [CrossRef]
- Deng, L.; Liu, M.; Sheng, D.; Luo, Y.; Wang, D.; Yu, X.; Wang, Z.; Ran, H.; Li, P. Low–intensity focused ultrasound–augmented Cascade chemodynamic therapy via boosting ROS generation. Biomaterials 2021, 271, 120710. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Zhang, Y.; Wang, Y.; Cui, M.; Li, G.; Liu, X.; Fan, H. Magnetoresponsive nanozyme: Magnetic stimulation on the nanozyme activity of iron oxide nanoparticles. Sci. China Life Sci. 2022, 65, 184–192. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, J.; Li, J.; Li, W.; Liu, Y.; Chen, C. In vivo aggregation–induced transition between T1and T2relaxations of magnetic ultra–small iron oxide nanoparticles in tumor microenvironment. Nanoscale 2017, 9, 3040–3050. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro–inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Ruiz–De–Angulo, A.; Bilbao–Asensio, M.; Cronin, J.; Evans, S.J.; Clift, M.J.; Llop, J.; Feiner, I.V.; Beadman, R.; Bascarán, K.Z.; Mareque–Rivas, J.C. Chemically Programmed Vaccines: Iron Catalysis in Nanoparticles Enhances Combination Immunotherapy and Immunotherapy–Promoted Tumor Ferroptosis. iScience 2020, 23, 101499. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near–infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2020, 50, 1111–1137. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Huang, X.; Yu, G.; Wang, S.; Zhou, Z.; Shen, Z.; Fan, W.; Liu, Y.; Davission, M.; et al. Glutathione–Responsive Self–Assembled Magnetic Gold Nanowreath for Enhanced Tumor Imaging and Imaging–Guided Photothermal Therapy. ACS Nano 2018, 12, 8129–8137. [Google Scholar] [CrossRef]
- Fu, Q.; Li, Z.; Ye, J.; Li, Z.; Fu, F.; Lin, S.-L.; Chang, C.A.; Yang, H.; Song, J. Magnetic targeted near–infrared II PA/MR imaging guided photothermal therapy to trigger cancer immunotherapy. Theranostics 2020, 10, 4997–5010. [Google Scholar] [CrossRef]
- Yang, K.; Yang, G.; Chen, L.; Cheng, L.; Wang, L.; Ge, C.; Liu, Z. FeS nanoplates as a multifunctional nano–theranostic for magnetic resonance imaging guided photothermal therapy. Biomaterials 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation–Induced Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef]
- Yan, L.; Amirshaghaghi, A.; Huang, D.; Miller, J.; Stein, J.M.; Busch, T.M.; Cheng, Z.; Tsourkas, A. Protoporphyrin IX (PpIX)–Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Nanoclusters for Magnetic Resonance Imaging and Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1707030. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, M.; Li, J.; Qin, F.; Wang, Y.; Liu, T.; Liu, J.; Sabet, Z.F.; Wang, Y.; Liu, Y.; et al. Hypoxia–Triggered Self–Assembly of Ultrasmall Iron Oxide Nanoparticles to Amplify the Imaging Signal of a Tumor. J. Am. Chem. Soc. 2021, 143, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yan, C.; Guo, Z.; Tan, G.; Niu, D.; Li, Y.; Zhu, W. Spatio-Temporally Reporting Dose-Dependent Chemotherapy via Uniting Dual-Modal MRI/NIR Imaging. Angew. Chem. Int. Ed. 2020, 59, 21143–21150. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, P.; Wang, W.; Wang, S.; Pan, X.; Zhang, F.; Li, S.; Liu, S.; Wang, H.; Gao, G.; et al. Biodegradable Poly(amino acid)–Gold–Magnetic Complex with Efficient Endocytosis for Multimodal Imaging–Guided Chemo–photothermal Therapy. ACS Nano 2018, 12, 9022–9032. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gai, S.; He, F.; Yang, P.; Zhao, Y. Multifunctional Bismuth Ferrite Nanocatalysts with Optical and Magnetic Functions for Ultrasound–Enhanced Tumor Theranostics. ACS Nano 2020, 14, 7245–7258. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, H.; Zhao, M.; Dai, C.; Zhang, S.; Peng, W.; Chen, Y. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast–Cancer Theranostics. Theranostics 2018, 8, 1648–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, Y.; Yang, W.; Xue, J.; Ding, Y.; Xie, C.; Luo, W.; Gao, F.-P.; Zhang, Z.; Zhao, Y.; et al. Comparative study of core– and surface–radiolabeling strategies for the assembly of iron oxide nanoparticle–based theranostic nanocomposites. Nanoscale 2019, 11, 5909–5913. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.L.J.; Ulmert, D.; Diop, N.-F.M.; Lupu, M.E.; Doran, M.G.; Huang, R.; Abou, D.S.; Larson, S.; Grimm, J. Non–invasive mapping of deep–tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat. Commun. 2014, 5, 3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Rosales, R.T.M.; Tavaré, R.; Glaria, A.; Varma, G.; Protti, A.; Blower, P.J. 99mTc–Bisphosphonate–Iron Oxide Nanoparticle Conjugates for Dual–Modality Biomedical Imaging. Bioconj. Chem. 2011, 22, 455–465. [Google Scholar] [CrossRef]
- Stelter, L.; Pinkernelle, J.G.; Michel, R.; Schwartländer, R.; Raschzok, N.; Morgul, M.H.; Koch, M.; Denecke, T.; Ruf, J.; Bäumler, H.; et al. Modification of Aminosilanized Superparamagnetic Nanoparticles: Feasibility of Multimodal Detection Using 3T MRI, Small Animal PET, and Fluorescence Imaging. Mol. Imaging Biol. 2009, 12, 25–34. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy–related myeloid neoplasms: When genetics and environment collide. Nat. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Reichel, D.; Sagong, B.; Teh, J.; Zhang, Y.; Wagner, S.; Wang, H.; Chung, L.W.K.; Butte, P.; Black, K.L.; Yu, J.S.; et al. Near Infrared Fluorescent Nanoplatform for Targeted Intraoperative Resection and Chemotherapeutic Treatment of Glioblastoma. ACS Nano 2020, 14, 8392–8408. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, Z.; Wang, X.; Deng, H.; Sun, L.; Lin, H.; Kang, F.; Zhang, Y.; Wang, Z.; Yang, W.; et al. Yolk–shell nanovesicles endow glutathione–responsive concurrent drug release and T1 MRI activation for cancer theranostics. Biomaterials 2020, 244, 119979. [Google Scholar] [CrossRef]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme–activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand–Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Zhang, C.; Fan, Y.; Li, D.; Cao, X.; Xia, J.; Shi, X.; Guo, R. LDH–stabilized ultrasmall iron oxide nanoparticles as a platform for hyaluronidase–promoted MR imaging and chemotherapy of tumors. Theranostics 2020, 10, 2791–2802. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic field–inducible drug–eluting nanoparticles for image–guided thermo–chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Guo, Y.; Jiao, W.; Gao, X.; Lee, W.S.V.; Wang, Y.; Deng, X.; He, Y.; Jiao, J.; et al. Electromagnetic Field-Programmed Magnetic Vortex Nanodelivery System for Efficacious Cancer Therapy. Adv. Sci. 2021, 8, 2100950. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Chen, Y.; Chen, Y.; Li, J.; Li, J.; Zhang, Z.; Zhang, Z.; Huang, C.; Huang, C.; et al. Co–delivery of microRNA–21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503. [Google Scholar] [CrossRef]
- Li, X.; Yu, K.; He, B. Magnetoacoustic tomography with magnetic induction (MAT–MI) for imaging electrical conductivity of biological tissue: A tutorial review. Phys. Med. Biol. 2016, 61, R249–R270. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38. https://doi.org/10.3390/bios12010038
Jiao W, Zhang T, Peng M, Yi J, He Y, Fan H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors. 2022; 12(1):38. https://doi.org/10.3390/bios12010038
Chicago/Turabian StyleJiao, Wangbo, Tingbin Zhang, Mingli Peng, Jiabao Yi, Yuan He, and Haiming Fan. 2022. "Design of Magnetic Nanoplatforms for Cancer Theranostics" Biosensors 12, no. 1: 38. https://doi.org/10.3390/bios12010038
APA StyleJiao, W., Zhang, T., Peng, M., Yi, J., He, Y., & Fan, H. (2022). Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors, 12(1), 38. https://doi.org/10.3390/bios12010038




