Rapid Quantitative Detection of Live Escherichia coli Based on Chronoamperometry
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Bacterial Cells, Growth Conditions, and Intracellular Lysate Preparation
2.3. Electrochemical System
2.4. Electrochemical Measurements
2.5. Data Analysis
2.6. Analysis of Spiked Samples
2.7. Changes in E. coli Concentration in the Detection Solution
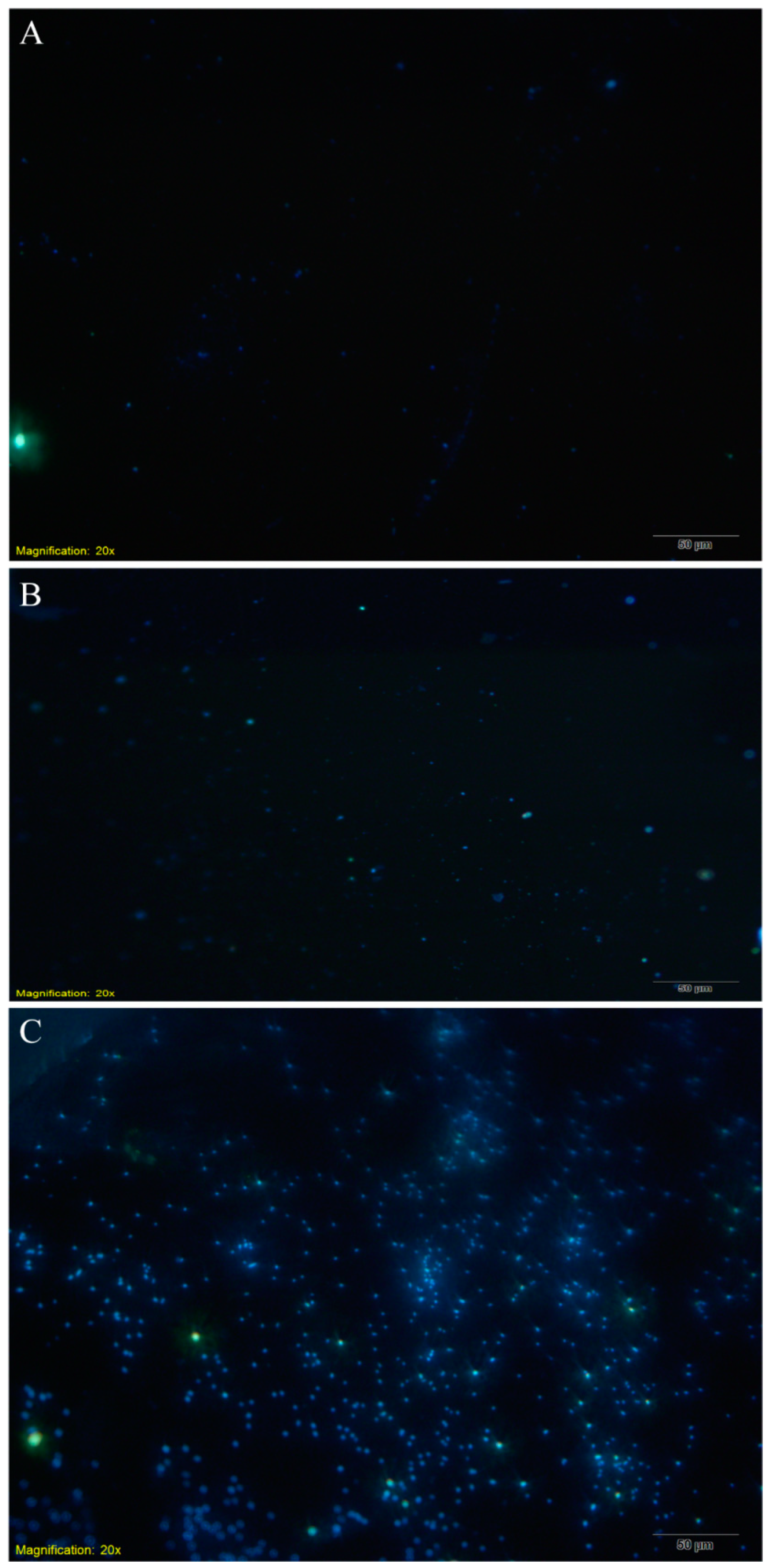
2.8. Fluorescence Microscopy
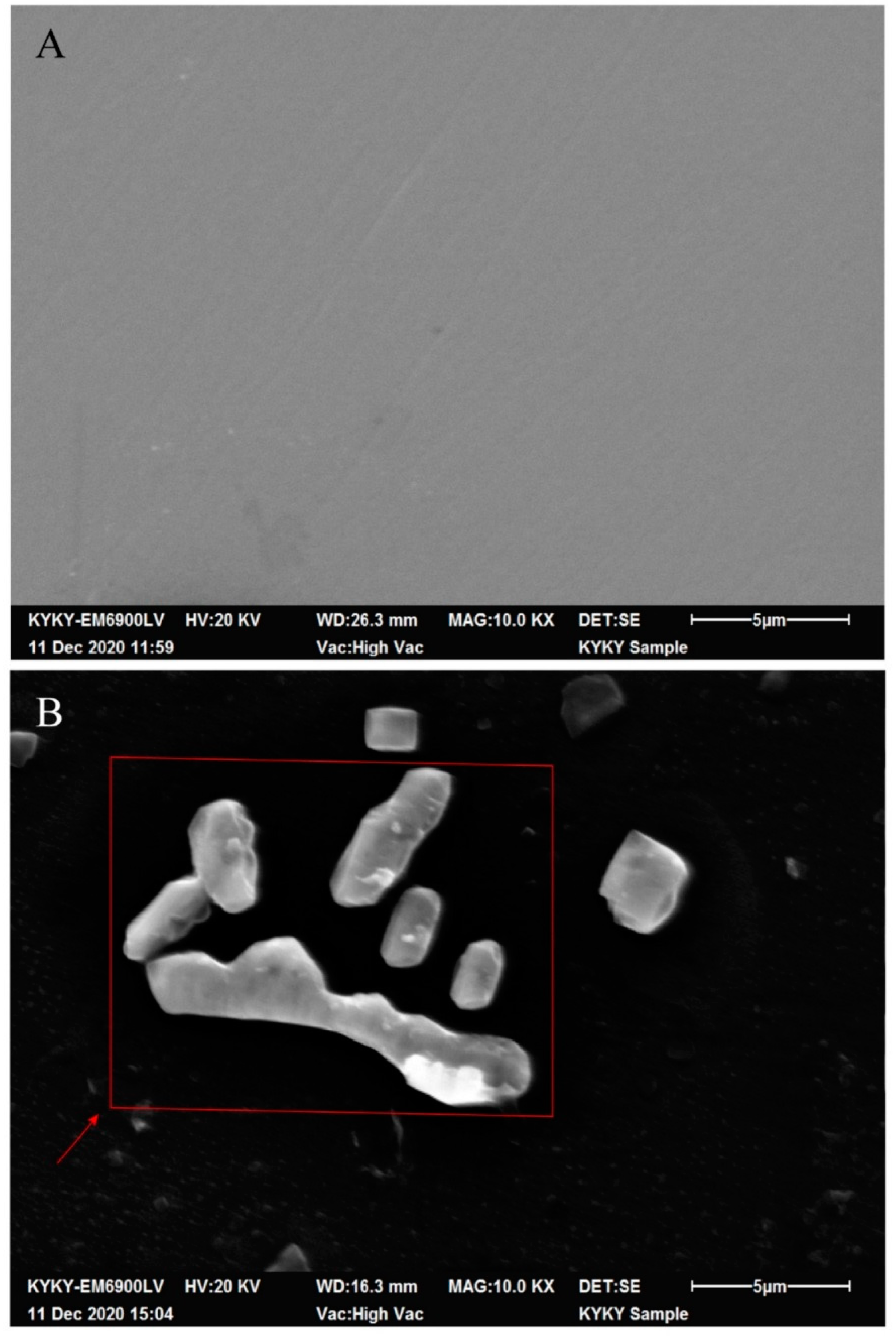
2.9. Scanning Electron Microscopy
2.10. Fulvic Acid Sample
2.11. Statistical Analysis
3. Results and Discussion
3.1. Establishment of the Detection Method
3.2. Quantitative Detection of E. coli by Monitoring i0 and A
3.3. Relationship between Bacterial Adsorption on the Electrode Surface and Bacterial Concentration
3.4. Influence of Humus on Test Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization; United Nations Children’s Fund (UNICEF). Progress on Drinking Water and Sanitation—2014 Update; World Health Organization: Geneva, Switzerland, 2012; Volume 2, p. 902. [Google Scholar] [CrossRef]
- World Health Organization. Hazard Characterization for Pathogens in Food and Water: Guidelines; Food & Agriculture Org; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Kim, S.U.; Jo, E.-J.; Mun, H.; Noh, Y.; Kim, M.-G. Ultrasensitive Detection of Escherichia coli O157:H7 by Immunomagnetic Separation and Selective Filtration with Nitroblue Tetrazolium/5-Bromo-4-chloro-3-indolyl Phosphate Signal Amplification. J. Agric. Food Chem. 2018, 66, 4941–4947. [Google Scholar] [CrossRef] [PubMed]
- Razmi, N.; Hasanzadeh, M.; Willander, M.; Nur, O. Recent Progress on the Electrochemical Biosensing of Escherichia coli O157:H7: Material and Methods Overview. Biosensors 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhu, F.; Guo, W.; Gu, H.; Zhao, J.; Yan, M.; Liu, S. Selective capture and rapid identification of E. coli O157:H7 by carbon nanotube multilayer biosensors and microfluidic chip-based LAMP. RSC Adv. 2017, 7, 30446–30452. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhao, S.; Mao, Y.; Fang, Z.; Lu, X.; Zeng, L. A sensitive lateral flow biosensor for Escherichia coli O157:H7 detection based on aptamer mediated strand displacement amplification. Anal. Chim. Acta 2015, 861, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wen, Z.; Hai-Xia, W.; Guang-Cheng, J.; Zheng, L. Application of Aptamer-Based Biosensor for Rapid Detection of Pathogenic Escherichia coli. Sensors 2018, 18, 2518. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Yu, Z.; Han, X.; Lai, R. Electrochemical aptamer-based sensors for food and water analysis: A review. Anal. Chim. Acta 2019, 1051, 1–23. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. Electrochemical biosensors for rapid detection of Escherichia coli O157:H7. Talanta 2017, 162, 511–522. [Google Scholar] [CrossRef]
- Deisingh, A.K.; Thompson, M. Strategies for the detection of Escherichia coli O157:H7 in foods. J. Appl. Microbiol. 2004, 96, 419–429. [Google Scholar] [CrossRef]
- Li, Z.; He, L.; He, N.; Shi, Z.; Wang, H.; Li, S.; Liu, H.; Li, X.; Dai, Y.; Wang, Z. Chemiluminescent Detect of E. coli O157:H7 Using Immunological Method Based on Magnetic Nanoparticles. J. Nanoence 2010, 10, 696. [Google Scholar] [CrossRef]
- Hunter, D.M.; Lim, D.V. Rapid detection and identification of bacterial pathogens by using an ATP bioluminescence immunoassay. J. Food Prot. 2010, 73, 739. [Google Scholar] [CrossRef]
- Velican, A.M.; Kamerzan, C.; Maruțescu, L.; Lambert, C.; Chifiriuc, M.C. The development of an analysis protocol based on flow cytometry for rapid detection of uropathogenic E. coli in artificially contaminated urine samples. Rom. Biotechnol. Lett. 2019, 24, 563–570. [Google Scholar] [CrossRef]
- Law, W.F.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneshvar, S.; Sudhakumari, C.C.; Balasubramanian, S.; Hridayesh, P. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ye, Z.; Ying, Y. New Trends in Impedimetric Biosensors for the Detection of Foodborne Pathogenic Bacteria. Sensors 2012, 12, 3449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroughi, M.M.; Jahani, S.; Aramesh-Broujeni, Z.; Dolatabad, M.R.; Shahbazkhani, K. Synthesis of 3D cubic of Eu3+/Cu2O with clover-like faces nanostructures and their application as an electrochemical sensor for determination of antiretroviral drug nevirapine. Ceram. Int. 2021, 47, 19727–19736. [Google Scholar] [CrossRef]
- Foroughi, M.M.; Noroozifar, M.; Khorasani-Motlagh, M. Simultaneous determination of hydroquinone and catechol using a modified glassy carbon electrode by ruthenium red/carbon nanotube. J. Iran. Chem. Soc. 2015, 12, 1139–1147. [Google Scholar] [CrossRef]
- Jandaghi, N.; Jahani, S.; Foroughi, M.M.; Kazemipour, M.; Ansari, M. Cerium-doped flower-shaped ZnO nano-crystallites as a sensing component for simultaneous electrochemical determination of epirubicin and methotrexate. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef]
- Foroughi, M.M.; Jahani, S.; Rajaei, M. Facile fabrication of 3D dandelion-like cobalt oxide nanoflowers and its functionalization in the first electrochemical sensing of oxymorphone: Evaluation of kinetic parameters at the surface electrode. J. Electrochem. Soc. 2019, 166, B1300. [Google Scholar] [CrossRef]
- Zhu, Y.; Jović, M.; Lesch, A.; Tissières Lovey, L.; Prudent, M.; Pick, H.; Girault, H.H. Immuno-Affinity Amperometric Detection of Bacterial Infections. Angew. Chem. Int. Ed. 2018, 57, 14942–14946. [Google Scholar] [CrossRef]
- Sepunaru, L.; Tschulik, K.; Batchelor-McAuley, C.; Gavish, R.; Compton, R.G. Electrochemical detection of single E. coli bacteria labeled with silver nanoparticles. Biomater. Sci. 2015, 3, 816–820. [Google Scholar] [CrossRef]
- Couto, R.A.; Chen, L.; Kuss, S.; Compton, R.G. Detection of Escherichia coli bacteria by impact electrochemistry. Analyst 2018, 143, 4840–4843. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S. The anodic film on Platinum Electrodes. Electroanal. Chem. 1967, 2, 112–189. [Google Scholar]
- Allen, J.B.; Larry, R.F. Electrochemical Methods Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Zhao, B.; Randall, A.A.; Taylor, J.S. Drinking water heterotrophic plate count: Monitoring method precision. Fresenius Environ. Bull. 2012, 21, 1202–1211. [Google Scholar]
- Kim, T.G.; Knudsen, G.R. Comparison of plate count, microscopy, and DNA quantification methods to quantify a biocontrol fungus. Appl. Soil Ecol. 2016, 98, 285–288. [Google Scholar] [CrossRef]
- Adler:, J.; Shi, W. Galvanotaxis in Bacteria. Cold Spring Harb. Symp. Quant. Biol. 1988, 53, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, W.; Papadopoulos, K.D. Electrokinetic movement of Escherichia coli in capillaries. Environ. Microbiol. 2010, 1, 99–102. [Google Scholar] [CrossRef]
- Ronspees, A.T.; Thorgaard, S.N. Blocking electrochemical collisions of single E. coli and B. subtilis bacteria at ultramicroelectrodes elucidated using simultaneous fluorescence microscopy. Electrochim. Acta 2018, 278, 412–420. [Google Scholar] [CrossRef]
- Hanchang, Z.; Xiaobing, Z.; Zhaorong, W. Glassy carbon electrode modified by Electrochemical Pretreatment. Anal. Instrum. 1997, 1, 2–7. [Google Scholar]
- Nagaoka, T.; Katayama, M.; Fujimoto, M.; Nakao, H.; Ogura, K.; Okada, T. Electrochemically pretreated glassy carbon as a chromatographic sensor for cationic and redox species. Anal. Chim. Acta 1994, 285, 135–141. [Google Scholar] [CrossRef]
- Fang, D.; Gao, G.; Yang, Y.; Wang, Y.; Gao, L.; Zhi, J. Redox Mediator-Based Microbial Biosensors for Acute Water Toxicity Assessment: A Critical Review. ChemElectroChem 2020, 7, 2513–2526. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, B.K.; Kang, M.; Park, J.H. Label-Free Detection of Single Living Bacteria via Electrochemical Collision Event. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareilleux, A.; Sicard, N. Lethal Effects of Electric Current on Escherichia coli. Appl. Microbiol. 1970, 19, 421–424. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Zhang, Y. Theory of Electrochemical Kinetics for Perovskite Solar Cells: Fitting Current–Voltage Curves. Russ. J. Electrochem. 2019, 55, 1299–1304. [Google Scholar] [CrossRef]
- Liu, K.; Duan, X.; Yuan, M.; Xu, Y.; Gao, T.; Li, Q.; Zhang, X.; Huang, M.; Wang, J. How to fit a response current-concentration curve? A semi-empirical investigation of non-enzymatic glucose sensor based on PANI-modified nickel foam. J. Electroanal. Chem. 2019, 840, 384–390. [Google Scholar] [CrossRef]
- Boika, A.; Thorgaard, S.N.; Bard, A.J. Monitoring the electrophoretic migration and adsorption of single insulating nanoparticles at ultramicroelectrodes. J. Phys. Chem. B 2013, 117, 4371. [Google Scholar] [CrossRef]
- Lebègue, E.; Costa, N.L.; Louro, R.O.; Barrière, F. Communication—Electrochemical Single Nano-Impacts of Electroactive Shewanella Oneidensis Bacteria onto Carbon Ultramicroelectrode. J. Electrochem. Soc. 2020, 167, 105501. [Google Scholar] [CrossRef]
- Ye, S.-J.; He, Z.-H.; Hong, G.-F.; Tian, Z.-S.; Shi, X.-Y. Influence of Chemical Disinfectant on Zeta Potential of Free Bacterial Surface in Sewage Treatment System. Chin. J. Disinfect. 2006, 5, 415–418. [Google Scholar]
- Guner, A.; Cevik, E.; Senel, M.; Alpsoy, L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef]
- Fanjun, Z.; Guangying, Z.; Wenchao, D. A non-enzymatic electrochemical immunoassay for quantitative detection of Escherichia coli O157:H7 using Au@Pt and graphene. Anal. Biochem. 2018, 559, 34–43. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, C.M.; Soni, A.; Tiwari, I.; Rosillo-Lopez, M.; Salzmann, C.G.; Malhotra, B.D.; Sumana, G. Electrochemical genosensor based on carboxylated graphene for detection of water-borne pathogen. Sens. Actuators B Chem. 2018, 275, 312–321. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, G.; Dou, W. An electrochemical immunoassay for Escherichia coli O157:H7 using double functionalized Au@Pt/SiO2 nanocomposites and immune magnetic nanoparticles. Talanta Int. J. Pure Appl. Anal. Chem. 2018, 182, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Chaterjee, B.; Kapil, A.; Kumar, T. Aptamer-NanoZyme mediated sensing platform for the rapid detection of Escherichia coli in fruit juice. Sens. Bio-Sens. Res. 2020, 27, 100313. [Google Scholar] [CrossRef]
- Guanyue, G.; Dengchao, W.; Brocenschi, R.F.; Jin-Fang, Z.; Mirkin, M.V. Toward the Detection and Identification of Single Bacteria by Electrochemical Collision Technique. Anal. Chem. 2018, 90, acsanalchem8b03043. [Google Scholar] [CrossRef]
- Szumski, M.; Buszewski, B. Effect of Applied Voltage on Viability of Bacteria during Separation under Electrophoretic Conditions. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2689–2698. [Google Scholar] [CrossRef]
- Daya, K.S.; Tirumalai, P.S.; Alocilja, E. Impedance and Magnetohydrodynamic Measurements for Label Free Detection and Differentiation of E. coli and S. aureus Using Magnetic Nanoparticles. IEEE Trans. Nanobioscience 2018, 17, 443–448. [Google Scholar] [CrossRef]
- Thorgaard, S.N.; Jenkins, S.; Tarach, A.R. Influence of Electroosmotic Flow on Stochastic Collisions at Ultramicroelectrodes. Anal. Chem. 2020, 92, 12663–12669. [Google Scholar] [CrossRef]
- Esmaeili, H.; Ebrahimi, A.; Hajian, M.; Pourzamani, H.R. Kinetic and isotherm studies of humic acid adsorption onto iron oxide magnetic nanoparticles in aqueous solutions. Int. J. Environ. Health Eng. 2012, 1, 33. [Google Scholar]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Nagao, S.; Aramaki, T.; Fujitake, N.; Kodama, H.; Tanaka, T.; Ochiai, S.; Uchida, M.; Shibata, Y.; Yamamoto, M. Variation of Δ14C and δ13C Values of Dissolved Humic and Fulvic Acids in the Tokachi River System in Northern Japan. Radiocarbon 2013, 55, 1007–1016. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Hwang, G.; Han, Y.; Choi, S.Q.; Cho, S.; Kim, H. Bacterial inactivation by ultrasonic waves: Role of ionic strength, humic acid, and temperature. Water Air Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- Mirzavand, R.; Honari, M.M.; Laribi, B.; Khorshidi, B.; Sadrzadeh, M.; Mousavi, P. An unpowered sensor node for real-time water quality assessment (humic acid detection). Electronics 2018, 7, 231. [Google Scholar] [CrossRef] [Green Version]
- Mathelié-Guinlet, M.; Cohen-Bouhacina, T.; Gammoudi, I.; Martin, A.; Beven, L.; Delville, M.H.; Grauby-Heywang, C. Silica Nanoparticles-Assisted Electrochemical Biosensor for the Rapid, Sensitive and Specific Detection of Escherichia coli. Sens. Actuators B Chem. 2019, 292, 314–320. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhang, X.; He, F. Rapid Detection of Escherichia Coli Based on 16 S Rdna Nanogap Network Electrochemical Biosensor. Biosens. Bioelectron. 2018, 118, 9–15. [Google Scholar] [CrossRef]
- Wang, D.; Hinkley, T.; Chen, J.; Talbert, J.N.; Nugen, S.R. Phage Based Electrochemical Detection of Escherichia Coli in Drinking Water Using Affinity Reporter Probes. Analyst 2019, 144, 1345–1352. [Google Scholar] [CrossRef]
- Mo, X.; Wu, Z.; Huang, J.; Zhao, G.; Dou, W. A Sensitive and Regenerative Electrochemical Immunosensor for Quantitative Detection of Escherichia Coli O157:H7 Based on Stable Polyaniline Coated Screen-Printed Carbon Electrode and Rgo-Nr-Au@Pt. Analytical Methods 2019, 11, 1475–1482. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Li, C.; Yang, X.; Wang, S.; Zhang, X.; Zhao, C.; Xue, B.; Gao, C.; Zhou, H.; Yang, Y.; et al. Rapid Quantitative Detection of Live Escherichia coli Based on Chronoamperometry. Biosensors 2022, 12, 845. https://doi.org/10.3390/bios12100845
Cao Z, Li C, Yang X, Wang S, Zhang X, Zhao C, Xue B, Gao C, Zhou H, Yang Y, et al. Rapid Quantitative Detection of Live Escherichia coli Based on Chronoamperometry. Biosensors. 2022; 12(10):845. https://doi.org/10.3390/bios12100845
Chicago/Turabian StyleCao, Zhuosong, Chenyu Li, Xiaobo Yang, Shang Wang, Xi Zhang, Chen Zhao, Bin Xue, Chao Gao, Hongrui Zhou, Yutong Yang, and et al. 2022. "Rapid Quantitative Detection of Live Escherichia coli Based on Chronoamperometry" Biosensors 12, no. 10: 845. https://doi.org/10.3390/bios12100845




