Simple Staining of Cells on a Chip
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Cell Culture
2.3. Patient Samples
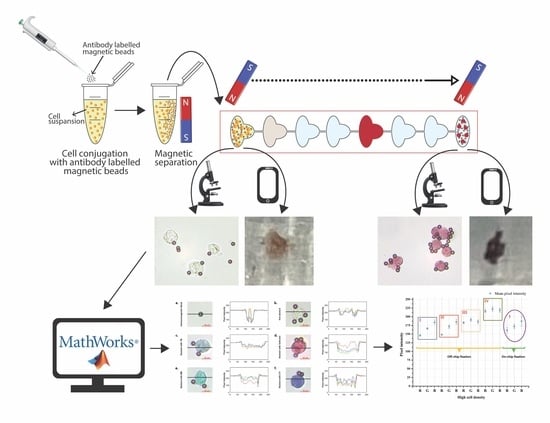
2.4. Preparation of Cell–Magnetic Bead Conjugates
2.5. Chip Fabrication
2.6. Surface Modification of the Chip
2.6.1. Cell Staining Procedure
2.6.2. Magnetic Force on Conjugate
2.6.3. Inspection and Analysis of Stained Cells in the Chip
3. Results and Discussion
3.1. Trypan Blue Concentration Experiments
3.2. Staining with Different Simple Stains
3.3. Control Experiments with Jurkat and MDA-MB-231 Cell Lines
3.4. Mobile Phone Image Analysis
3.5. Performance Analysis of the Chip
3.6. Patient Samples’ Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Leboffe, M.J.; Pierce, B.E. Microbiology: Laboratory Theory and Application: Essentials; Morton Publishing Company: Englewood, CO, USA, 2019; pp. 57–68. [Google Scholar]
- Dey, P. Basic and Advanced Laboratory Techniques in Histopathology and Cytology; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Welsh, C. Microbiology: A Laboratory Manual; Pearson Education: Upper Saddle River, NJ, USA, 2014; p. 541. [Google Scholar]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction; Pearson: Upper Saddle River, NJ, USA, 2018. [Google Scholar]
- Temiz, A. Genel Mikrobiyoloji Uygulama Teknikleri; Hatiboğlu Yayıncılık: Ankara, Türkiye, 2010. [Google Scholar]
- Wong, R.C.W.; Heung, S.S.Y.; Ho, Y.C.; Yip, K.T.; Que, T.L. Evaluation of PREVI Color Gram Automated Staining System on Positive Blood Culture Samples. Lab. Med. 2011, 42, 414–418. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Laguerre, V.; Packert, D.; Nakasone, A.; Moscinski, L. A Simple and Efficient Method for Preparing Cell Slides and Staining without Using Cytocentrifuge and Cytoclips. Int. J. Cell Biol. 2015, 2015, 813216. [Google Scholar] [CrossRef] [PubMed]
- Siguenza, N.; Jangid, A.; Strong, E.B.; Merriam, J.; Martinez, A.W.; Martinez, N.W. Micro-Staining Microbes: An Alternative to Traditional Staining of Microbiological Specimens Using Microliter Volumes of Reagents. J. Microbiol. Methods 2019, 164, 105654. [Google Scholar] [CrossRef] [PubMed]
- Castillo-León, J.; Svendsen, W.E. Lab-on-a-Chip Devices and Micro-Total Analysis Systems: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2014; p. 243. [Google Scholar]
- İçöz, K.; Mzava, O. Detection of Proteins Using Nano Magnetic Particle Accumulation-Based Signal Amplification. Appl. Sci. 2016, 6, 394. [Google Scholar] [CrossRef]
- İçöz, K.; Akar, Ü.; Ünal, E. Microfluidic Chip Based Direct Triple Antibody Immunoassay for Monitoring Patient Comparative Response to Leukemia Treatment. Biomed. Microdevices 2020, 22, 48. [Google Scholar] [CrossRef]
- Alapan, Y.; Kim, C.; Adhikari, A.; Gray, K.E.; Gurkan-Cavusoglu, E.; Little, J.A.; Gurkan, U.A. Sickle Cell Disease Biochip: A Functional Red Blood Cell Adhesion Assay for Monitoring Sickle Cell Disease. Transl. Res. 2016, 173, 74–91.e8. [Google Scholar] [CrossRef]
- Zhou, J.; Tu, C.; Liang, Y.; Huang, B.; Fang, Y.; Liang, X.; Ye, X. The Label-Free Separation and Culture of Tumor Cells in a Microfluidic Biochip. Cite This Anal. 2020, 145, 1706. [Google Scholar] [CrossRef]
- Wickramaratne, B.; Pappas, D. Tandem Microfluidic Chip Isolation of Prostate and Breast Cancer Cells from Simulated Liquid Biopsies Using CD71 as an Affinity Ligand. RSC Adv. 2020, 10, 32628–32637. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Gulino, K.; Zhang, Y.H.; Sabestien, A.; Chou, T.W.; Zhou, B.; Lin, Z.; Albert, I.; Lu, H.; Swaminathan, V.; et al. A Rapid and Label-Free Platform for Virus Capture and Identification from Clinical Samples. Proc. Natl. Acad. Sci. USA 2020, 117, 895–901. [Google Scholar] [CrossRef]
- Sakamoto, C.; Yamaguchi, N.; Nasu, M. Rapid and Simple Quantification of Bacterial Cells by Using a Microfluidic Device. Appl. Environ. Microbiol. 2005, 71, 1117–1121. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Lai, C.; Li, L.; Wu, H.; Wang, Y.; Shi, X. Fabrication of a Hydroxyapatite-PDMS Microfluidic Chip for Bone-Related Cell Culture and Drug Screening. Bioact. Mater. 2021, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Qin, J.; Shi, W.; Liu, X.; Lin, B. Cell-Based High Content Screening Using an Integrated Microfluidic Device. Lab Chip 2007, 7, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Wu, M.; Zhang, X.; Liu, W.; Zhou, Y.; Jia, C.; Cong, H.; Chen, X.; Zhao, J. EGFR Mutation Detection of Lung Circulating Tumor Cells Using a Multifunctional Microfluidic Chip. Talanta 2021, 225, 122057. [Google Scholar] [CrossRef] [PubMed]
- Phurimsak, C.; Tarn, M.D.; Peyman, S.A.; Greenman, J.; Pamme, N. On-Chip Determination of c-Reactive Protein Using Magnetic Particles in Continuous Flow. Anal. Chem. 2014, 86, 10552–10559. [Google Scholar] [CrossRef] [PubMed]
- Narayanamurthy, V.; Jeroish, Z.E.; Bhuvaneshwari, K.S.; Bayat, P.; Premkumar, R.; Samsuri, F.; Yusoff, M.M. Advances in Passively Driven Microfluidics and Lab-on-Chip Devices: A Comprehensive Literature Review and Patent Analysis. RSC Adv. 2020, 10, 11652–11680. [Google Scholar] [CrossRef]
- Phurimsak, C.; Yildirim, E.; Tarn, M.D.; Trietsch, S.J.; Hankemeier, T.; Pamme, N.; Vulto, P. Phaseguide Assisted Liquid Lamination for Magnetic Particle-Based Assays. Lab Chip 2014, 14, 2334–2343. [Google Scholar] [CrossRef]
- Gjergjizi, B.; Çoğun, F.; Yıldırım, E.; Eryılmaz, M.; Selbes, Y.; Sağlam, N.; Tamer, U. SERS-Based Ultrafast and Sensitive Detection of Luteinizing Hormone in Human Serum Using a Passive Microchip. Sens. Actuators B Chem. 2018, 269, 314–321. [Google Scholar] [CrossRef]
- Dogan, Ü.; Kasap, E.N.; Sucularli, F.; Yildirim, E.; Tamer, U.; Cetin, D.; Suludere, Z.; Boyaci, I.H.; Ertas, N. Multiplex Enumeration of: Escherichia Coli and Salmonella Enteritidis in a Passive Capillary Microfluidic Chip. Anal. Methods 2020, 12, 3788–3796. [Google Scholar] [CrossRef]
- Kasap, E.N.; Doğan, Ü.; Çoğun, F.; Yıldırım, E.; Boyacı, İ.H.; Çetin, D.; Suludere, Z.; Tamer, U.; Ertaş, N. Fast Fluorometric Enumeration of E. Coli Using Passive Chip. J. Microbiol. Methods 2019, 164, 105680. [Google Scholar] [CrossRef]
- Mzava, O.; Tas, Z.; İçöz, K. Magnetic Micro/Nanoparticle Flocculation-Based Signal Amplification for Biosensing. Int. J. Nanomedicine 2016, 11, 2619–2631. [Google Scholar] [CrossRef]
- Icoz, K.; Soylu, M.C.; Canikara, Z.; Unal, E. Quartz-Crystal Microbalance Measurements of CD19 Antibody Immobilization on Gold Surface and Capturing B Lymphoblast Cells: Effect of Surface Functionalization. Electroanalysis 2018, 30, 834–841. [Google Scholar] [CrossRef]
- Icoz, K.; Iverson, B.D.; Savran, C. Noise Analysis and Sensitivity Enhancement in Immunomagnetic Nanomechanical Biosensors. Appl. Phys. Lett. 2008, 93, 103902. [Google Scholar] [CrossRef]
- Chan, B.D.; Mateen, F.; Chang, C.L.; Icoz, K.; Savran, C.A. A Compact Manually Actuated Micromanipulator. J. Microelectromechanical Syst. 2012, 21, 7–9. [Google Scholar] [CrossRef]










| Difference from Background for: | Color Bands | ||
|---|---|---|---|
| R | G | B | |
| Bead | 125 | 120 | 140 |
| Unstained cell | 15 | 15 | 30 |
| Cell stained with TB | 50 | 40 | 40 |
| Cell stained with Safranin | 25 | 135 | 100 |
| Cell stained with MB | 100 | 100 | 45 |
| Cell stained with CV | 100 | 115 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosker, F.B.; Aydin, O.; Icoz, K. Simple Staining of Cells on a Chip. Biosensors 2022, 12, 1013. https://doi.org/10.3390/bios12111013
Kosker FB, Aydin O, Icoz K. Simple Staining of Cells on a Chip. Biosensors. 2022; 12(11):1013. https://doi.org/10.3390/bios12111013
Chicago/Turabian StyleKosker, Fatma Betul, Omer Aydin, and Kutay Icoz. 2022. "Simple Staining of Cells on a Chip" Biosensors 12, no. 11: 1013. https://doi.org/10.3390/bios12111013
APA StyleKosker, F. B., Aydin, O., & Icoz, K. (2022). Simple Staining of Cells on a Chip. Biosensors, 12(11), 1013. https://doi.org/10.3390/bios12111013