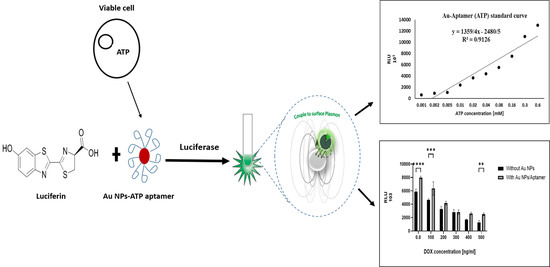
Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Expression and Purification of Luciferase Enzymes
2.3. Preparation of Gold Nanoparticles (Au NPs)
2.4. Preparation of Silver Nanoparticles (Ag NPs)
2.5. Preparation of Core–Shell Au–Ag Nanoparticles
2.6. Preparation of BSA–Au Nanoclusters (BSA-AuNCs)
2.7. Preparation of Gold Nanorods (GNRs)
2.8. Immobilization of Enzyme with Metal-NPs
2.9. Luciferase Activity Assay
2.10. UV/Visible Spectra
2.11. Particle Size Distribution and Zeta Potential
2.12. Transmission Electron Microscopy (TEM)
2.13. Fluorescence Spectra Measurement
2.14. Circular Dichroism (CD) Analysis
2.15. Bioluminescence Spectra (BL Spectra)
2.16. AuNP Stability Test
2.17. Adenosine Triphosphate (ATP) Assay
2.18. Cell Culture
3. Results
3.1. Nanoparticle Characterization
3.2. Nanoparticle Effect on Luciferase Activity
3.3. The Enzyme–Substrate Complexes Effect on SPR
3.4. Nanoparticle Effect on Structural Properties
3.5. AuNP Stability Test
3.6. ATP Assay
3.7. Cell Culture
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseinkhani, S. Molecular enigma of multicolor Bioluminescence of firefly luciferase. Cell. Mol. Life Sci. 2011, 68, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Bhuckory, S.; Kays, J.C.; Dennis, A.M. In Vivo Biosensing Using Resonance Energy Transfer. Biosensors 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Elward, J.M.; Irudayanathan, F.J.; Nangia, S.; Chakraborty, A. Optical Signature of Formation of Protein Corona in the firefly Luciferase-CdSe Quantum Dot Complex. J. Chem. Theory Comput. 2014, 10, 5224–5228. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chu, M. Self-illuminating quantum dots for highly sensitive in vivo real-time luminescent mapping of sentinel lymph nodes. Int. J. Nanomed. 2012, 7, 3433–3443. [Google Scholar]
- Yao, Z.; Zhang, B.S.; Prescher, J.A. Advances in bioluminescence imaging: New probes from old recipes. Curr. Opin. Chem. Biol. 2018, 45, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jin, J.; Liu, Y.; Li, J.; Tokatlian, T.; Lu, Z.; Segura, T.; Yuan, X.B.; Yang, X.; Lu, Y. Gold-Nanocrystal-Enhanced Bioluminescent Nanocapsules. ACS Nano 2014, 8, 9964–9969. [Google Scholar] [CrossRef]
- Yan, M.; Du, J.; Gu, Z.; Liang, M.; Hu, Y.; Zhang, W.; Priceman, S.; Wu, L.; Zhou, Z.H.; Liu, Z.; et al. A Novel Intracellular Protein Delivery Platform Based on Single-Protein Nanocapsules. Nat. Nanotechnol. 2010, 5, 48–53. [Google Scholar] [CrossRef]
- Gu, Z.; Yan, M.; Hu, B.; Joo, K.-I.; Biswas, A.; Huang, Y.; Lu, Y.; Wang, P.; Tang, Y. Protein Nanocapsule Weaved with Enzymatically Degradable Polymeric Network. Nano Lett. 2009, 9, 4533–4538. [Google Scholar] [CrossRef]
- Moradi, A.; Hosseinkhani, S.; Naderi-Manesh, H.; Sadeghizadeh, M.; Said Alipour, B. Effect of Charge Distribution in a Flexible Loop on the Bioluminescence Color of firefly Luciferases. Biochemistry 2009, 48, 575–582. [Google Scholar] [CrossRef]
- Safavi, A.; Emamzadeh, R.; Nazari, M.; Ehsani, M.; Zarkesh-Esfahani, S.H.; Rahgozar, S. Super RLuc8-sFv; a new luciferase-labeled probe for detection of human CD4+ cells. Mol. BioSyst 2017, 13, 470–475. [Google Scholar] [CrossRef]
- Eltzov, E.; Prilutsky, D.; Kushmaro, A.; Marks, R.S.; Geddes, C.D. Metal-enhanced bioluminescence: An approach for monitoring biological luminescent processes. Appl. Phys. Lett. 2009, 94, 083901. [Google Scholar] [CrossRef]
- Vasquez, E.S.; Feugang, J.M.; Willard, S.T.; Ryan, P.L.; Walters, K.B. Bioluminescent magnetic nanoparticles as potential imaging agents for mammalian spermatozoa. Nanobiotechnol 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotech. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D. Surface Plasmon Enhanced, Coupled and Controlled Fluorescence; John Wiley & Sons, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Liu, J.; Peng, Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017, 55, 13–27. [Google Scholar] [CrossRef]
- Karim, Z.; Adnan, R.; Ansari, M.S. Low Concentration of Silver Nanoparticles Not Only Enhances the Activity of Horseradish Peroxidase but Alter the Structure Also. PLoS ONE 2012, 7, 41422. [Google Scholar] [CrossRef]
- Abhijith, K.S.; Sharma, R.; Ranjan, R.; Thakur, M.S. Facile Synthesis of gold-silver alloy nanoparticles for application in metal enhanced Bioluminescence. Photochem. Photobiol. Sci. 2014, 13, 986–991. [Google Scholar] [CrossRef]
- Abhijith, K.S.; Ragavan, K.V.; Thakur, M.S. Gold nanoparticles enhanced chemiluminescence—A novel approach for sensitive determination of aflatoxin-B1. Anal. Methods 2013, 5, 4838. [Google Scholar] [CrossRef]
- Golberg, K.; Elbaz, A.; McNeil, R.; Kushmaro, A.; Geddes, C.D.; Marks, R.S. Increased bioassay sensitivity of bioactive molecule discovery using metal-enhanced Bioluminescence. J. Nanopart. Res. 2014, 16, 2770. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Hosseinkhani, S.; Heydari, A.; Akbari, J. Simple and Rapid Immobilization of firefly luciferase on Functionalized Magnetic Nanoparticles; a Try to Improve Kinetic Properties and Stability. Biomacromolecular J. 2015, 1, 104–112. [Google Scholar]
- Belleti, E.; Bevilaqua, V.R.; Brito, A.M.M.; Modesto, D.A.; Lanfredi, A.J.C.; Viviani, V.R.; Nantes-Cardoso, I.L. Synthesis of bioluminescent gold nanoparticle–luciferase hybrid systems for technological applications. Photochem. Photobiol. Sci. 2021, 20, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.J.; Xu, K. Fluorescent metal nanoclusters: From Synthesis to Applications. Trends Anal. Chem. 2014, 58, 90–98. [Google Scholar] [CrossRef]
- Li, D.; Chen, Z.; Mei, X. Fluorescence enhancement for noble metal nanoclusters. Adv. Colloid Interface Sci. 2017, 250, 25–39. [Google Scholar] [CrossRef]
- Guevel, L.X.; Daum, N.; Schneider, M. Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology 2011, 22, 275103. [Google Scholar] [CrossRef]
- Ren, L.; Xu, P.; Zhang, P.; Qin, Z.; Zhang, Y.; Jiang, L. Label-free fluorescence aptasensor based on AuNPs and CQDs for the detection of ATP. AIP Adv. 2021, 11, 015316. [Google Scholar] [CrossRef]
- Huo, Y.; Qi, L.; Lv, X.-J.; Lai, T.; Zhang, J.; Zhang, Z.-Q. A sensitive aptasensor for colorimetric detection of adenosine triphosphate based on the protective effect of ATP-aptamer complexes on unmodified gold nanoparticles. Biosensors and Bioelectron. 2016, 78, 315–320. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, W.; Shen, X.; Xu, L.; Li, X.; Wang, R.; Liu, C.; Zhou, X. Colorimetric and visual determination of adenosine triphosphate using a boronic acid as the recognition element, and based on the deaggregation of gold nanoparticles. Microchim. Acta 2017, 184, 4305–4312. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, P.; Liu, J. Sensing Adenosine and ATP by Aptamers and Gold Nanoparticles: Opposite Trends of Color Change from Domination of Target Adsorption Instead of Aptamer Binding. ACS Sens. 2020, 5, 2885–2893. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the Synthesis of colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Chen, X.; Parker, G.S.; Zou, G.; Su, W.; Zhang, Q. β-Cyclodextrin functionalized silver nanoparticles for the naked-eye detection of aromatic isomers. ACS Nano 2010, 4, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Bai, S.; Hartmann, J.; Wang, D. Synthesis of Monodisperse Quasi-Spherical Gold Nanoparticles in Water via Silver(I)-Assisted Citrate Reduction. Langmuir 2010, 26, 3585–3589. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gue, W.; Wei, H. Protein- and Peptide-directed Approaches to Fluorescent Metal Nanoclusters. Isr. J. Chem. 2015, 55, 682–697. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Moghadam, T.T.; Ranjbar, B.; Khosro, K.; Etezad, S.M.; Khalifeh, K.; Ganjalikhany, M.R. the interaction of lysozyme with gold nanorods: Conformation and activity investigations. Int. J. Biol. Macromol. 2011, 49, 629–636. [Google Scholar] [CrossRef]
- Müller, I.; Niethammer, D.; Bruchelt, G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity. Int. J. Mol. Med. 1998, 1, 491–495. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorods-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B: Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Mathew, A.; Sajanlal, P.R.; Pradeep, T. A fifteen atom silver cluster confined in bovine serum albumin. J. Mater. Chem. 2011, 21, 11205. [Google Scholar] [CrossRef]
- Moghadam, T.T.; Ranjbar, B.; Khosro, K. Conformation and activity of lysozyme on binding to two types of gold nanorods: A comparative study. Int. J. Biol. Macromol. 2012, 51, 91–96. [Google Scholar] [CrossRef]
- Kakinen, A.; Ding, F.; Chen, P.; Mortimer, M.; Kahru, A.; Ke, P.C. The interaction of firefly luciferase and silver nanoparticles and its impact on enzyme activity. Nanotechnology 2013, 24, 345101. [Google Scholar] [CrossRef]
- Borghei, Y.S.; Hosseinkhani, S.; Ganjali, M.R. “Plasmonic Nanomaterials”: An emerging avenue in biomedical and biomedical engineering opportunities. J. Adv. Res. 2021, 39, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Wang, L.; Hou, X.; Liu, W.; Chen, C. Interaction of gold nanoparticles with proteins and cells. Sci. Technol. Adv. Mater. 2015, 16, 034610. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. Leaves and their synergistic effects with antibiotics. J. Radiat. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.A.; Medintz, I.L.; Claussen, J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef]
- Meng, H.-M.; Zhang, X.; Lv, Y.; Zhao, Z.; Wang, N.-N. DNA dendrimer: An efficient Nanocarrier of functional nucleic acids for intracellular molecular sensing. ACS Nano 2014, 8, 6171–6181. [Google Scholar] [CrossRef]
- Kiesslich, T.; Oberdanner, C.B.; Krammer, B.; Plaetzer, K. Fast and reliable determination of intracellular ATP from cells cultured in 96- well microplates. J. Biochem. Biophys. Methods 2003, 57, 247–251. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Martín, J. Determination of ATP via the photochemical generation of hydrogen peroxide using flow injection luminol chemiluminescence detection. Anal. Bioanal. Chem. 2003, 377, 189–194. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Zhou, C.; Fang, X. Aptamer-based ATP assay using a luminescent light switching complex. Anal. Chem. 2005, 77, 3542–3546. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Bi, S. Design of molecular beacons as signaling probes for adenosine triphosphate detection in cancer cells based on chemiluminescence resonance energy transfer. Anal. Chem. 2009, 81, 8695–8701. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Chen, S.; Jiang, Y.; Zhao, Y.; Zhao, S. Mass amplifying quantum dots in a fluorescence polarization-based aptasensor for ATP. Micro. Chim. Acta 2012, 180, 203–209. [Google Scholar] [CrossRef]
- Aslan, K.; Geddes, C.D. Metal-enhanced chemiluminescence: Advanced chemiluminescence concepts for the 21st century. Chem. Soc. Rev 2009, 38, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.H.; Aslan, K.; Malyn, S.N.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced chemiluminescence: Radiating plasmons generated from chemically induced electronic excited states. Appl. Phys. Lett. 2006, 88, 173104. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.G.; Ferreira, A.M.; Frota, O.P.; Rigotti, M.A.; Andrade, D.; Brizzotti, N.S.; Peresi, J.T.M.; Castilho, E.M.; de Almeida, M.T.G. Effectiveness of ATP bioluminescence assay for presumptive identification of microorganisms in hospital water sources. BMC Infect. Dis. 2017, 17, 485. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Dallolio, L.; Raggi, A.; Mazzetti, M.; Lorusso, G.; Zanni, A.; Farruggia, P.; Leoni, E. ATP bioluminescence assay for evaluating cleaning practices in operating theatres: Applicability and limitations. BMC Infect. Dis. 2018, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.; Hosseinkhani, S.; Ahmadian, S.; Nazari, M. Design and introduction of a disulfide bridge in firefly luciferase: Increase of thermostability and decrease of pH sensitivity. Photochem. Photobiol. Sci. 2010, 9, 1167–1177. [Google Scholar] [CrossRef]
- Amini-Bayat, Z.; Hossinkhani, S.; Jafari, R.; Khajeh, K.H. Relationship between stability and flexibility in the most flexible region of Photinus pyralis luciferase. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 350–358. [Google Scholar] [CrossRef]
- Nazari, M.; Hosseinkhani, S. Design of disulfide bridge as an alternative mechanism for color shift in firefly luciferase and development of secreted luciferase. Photochem. Photobiol. Sci. 2011, 10, 1203–1215. [Google Scholar] [CrossRef]
- Alipour, B.S.; Hossinkhani, S.; Ardestani, S.K.; Moradi, A. The effective role of positive charge saturation in bioluminescence color and thermostability of firefly luciferase. Photochem. Photobiol. Sci. 2009, 8, 847–855. [Google Scholar] [CrossRef]
- Kheirabadi, M.; Sharafian, Z.; Nadri-Manesh, H.; Heineman, U.; GohlKe, U.; Hosseinkhani, S. Crystal structure of native and a mutant of Lampyris turkestanicus luciferase implicate in bioluminescence color shift. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 2729–2735. [Google Scholar] [CrossRef]
- Lohrasbi-Njad, A.; Torkzadeh-Mahani, M.; Hossinkhani, S. Hydrophobin-1 promotes thermostability of firefly luciferase. FEBS J. 2016, 283, 2494–2507. [Google Scholar] [CrossRef]
- Yousefi, F.; Ataei, F.; Mortazavi, M.; Hossinkhani, S. Bifunctional role of leucine 300 of firefly luciferase in structural rigidity. Int. J. Biol. Macromol. 2017, 101, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.R.; Hossinkhani, S.; Ghiasi, P.; Akbari, J.; Heydari, A. Magnetic nanoparticles supported ionic liquids improve firefly luciferase properties. Appl. BioChem. Biotchnol. 2014, 172, 3116–3127. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Salehi, N.; Saboury, A.A.; Hosseinkhani, S.; Karimi-Jafari, M.H.; Firouzi, R.; Rzaei-Ghaleh, N.; Moosavi-Movahedi, A.A. Histidine substitution in the most flexible fragments of firefly luciferase modifies its thermal stability. Arch. Biochem. Biophys. 2017, 629, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-movahedi, A.A.; Saboury, A.A.; Hossinkhani, S.; Lohrasbi-Nejad, A.; Habibi-Rzaei, M.; Maghami, P.; Atri, M.; Fotouhi, L. Protein Stability, Folding, Disaggregation and Etiology of Conformational Malfunctions. Biomacromol. J. 2016, 2, 8–20. [Google Scholar]









| Samples | α-Helix (%) | β-Strand (%) |
|---|---|---|
| Fluc | 84.27 | 1.24 |
| Fluc-AgNPs | 67.45 | 3.24 |
| Rluc | 47.93 | 8.19 |
| Rluc-Au-AgNPs | 43.94 | 10.56 |
| Mfluc | 19.69 | 31.52 |
| Mfluc-GNRs | 12.96 | 38.78 |
| Size | Zeta | |||||||
|---|---|---|---|---|---|---|---|---|
| NPs | Rluc | Fluc | Mfluc | NPs | Rluc | Fluc | Mfluc | |
| Au NPs | 29.0 | 183.0 | 290.0 | 72.0 | −28.0 | +13.0 | +6.0 | −7.0 |
| Ag NPs | 13.5 | 82.0 | 44.0 | 153.3 | −24.0 | −9.0 | −6.0 | −8.0 |
| Au-Ag NPs | 3.0 | 55.0 | 412.0 | 80.12 | −33.0 | −9.0 | −6.0 | −9.0 |
| BSA-Au NCs | 31.0 | 119.0 | 98.0 | 52.04 | −39.0 | −12.0 | −4.0 | −6.0 |
| GNRs | 49.0 | 589.0 | 400 | 1529.0 | +15.0 | −1.0 | +2.0 | −5.0 |
| Enzymes | - | 68.0 | 61.0 | 69.0 | - | −2.0 | −9.0 | −4.0 |
| Methods/Materials Used | Analytical Ranges/LODs (μM) | Samples | References |
|---|---|---|---|
| Luminescence/luciferase, luciferin | 5 nM–100 μM | Cell extract | [47] |
| Luminescence/hexokinase, glucose, luminol | 0.30–80/70 | pharmaceuticals, milk, and soils | [48] |
| Luminescence/aptamer, [Ru(phen)2(dppz)]2+ | 1–100 nM/1 nM | - | [49] |
| Luminescence/aptamer, peroxidase, luminol | 1–10/0.1 | Cancer cell extract | [50] |
| Chemiluminescence/luminol-H2O2- HRP-fluorescein | 1–10/0.32 | Cancer cell extract | [50] |
| Fluorescence polarization/QD-DNA | 10–350/3.7 | Serum | [51] |
| Bioluminescent/luciferase, luciferin, aptamer, Au NPs | 1–600/5 | Cancer cell extract | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, E.; Nikkhah, M.; Hosseinkhani, S. Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection. Biosensors 2022, 12, 918. https://doi.org/10.3390/bios12110918
Karimi E, Nikkhah M, Hosseinkhani S. Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection. Biosensors. 2022; 12(11):918. https://doi.org/10.3390/bios12110918
Chicago/Turabian StyleKarimi, Elham, Maryam Nikkhah, and Saman Hosseinkhani. 2022. "Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection" Biosensors 12, no. 11: 918. https://doi.org/10.3390/bios12110918
APA StyleKarimi, E., Nikkhah, M., & Hosseinkhani, S. (2022). Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection. Biosensors, 12(11), 918. https://doi.org/10.3390/bios12110918