Vital Signs Sensing Gown Employing ECG-Based Intelligent Algorithms
Abstract
1. Introduction
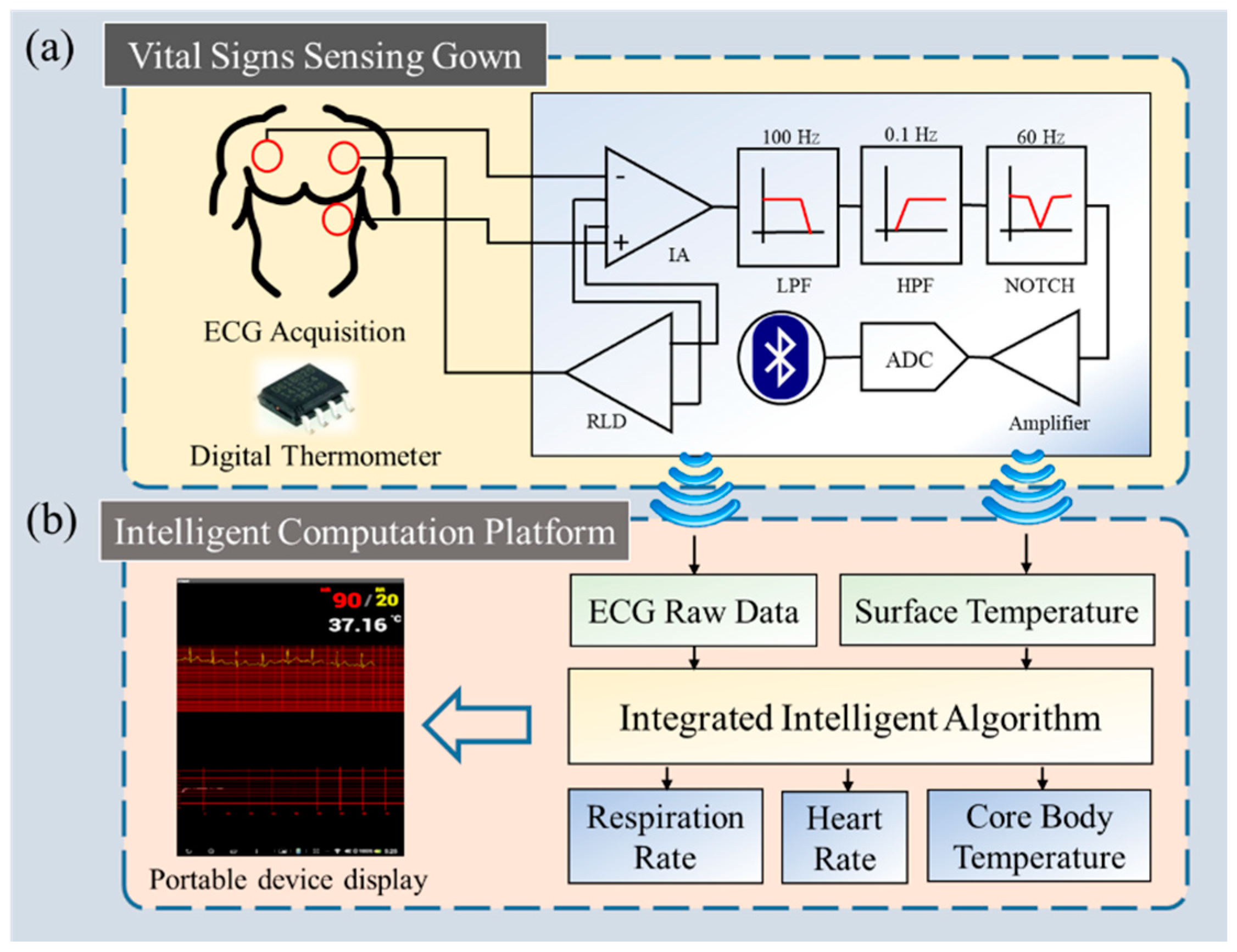
2. System Architecture
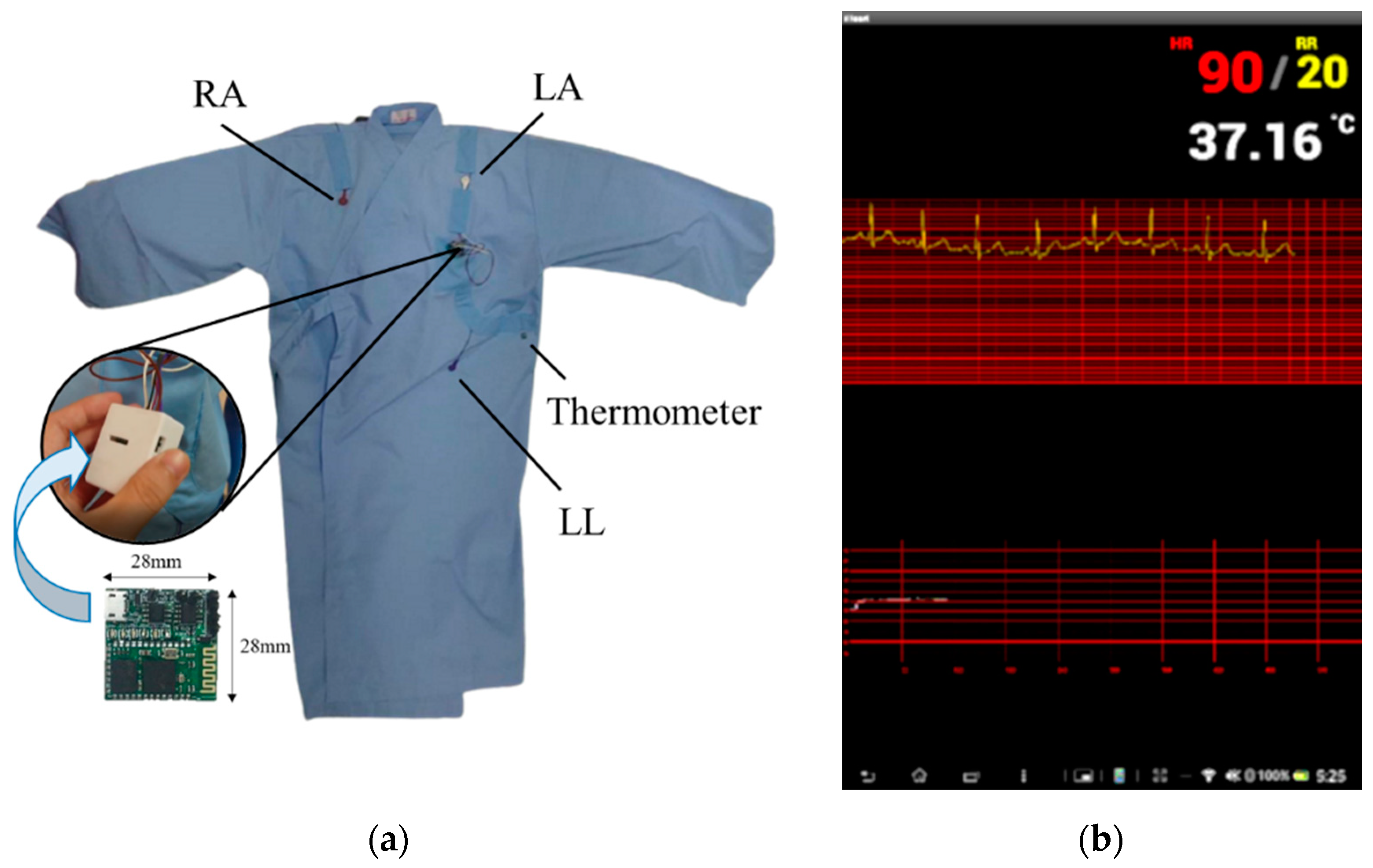
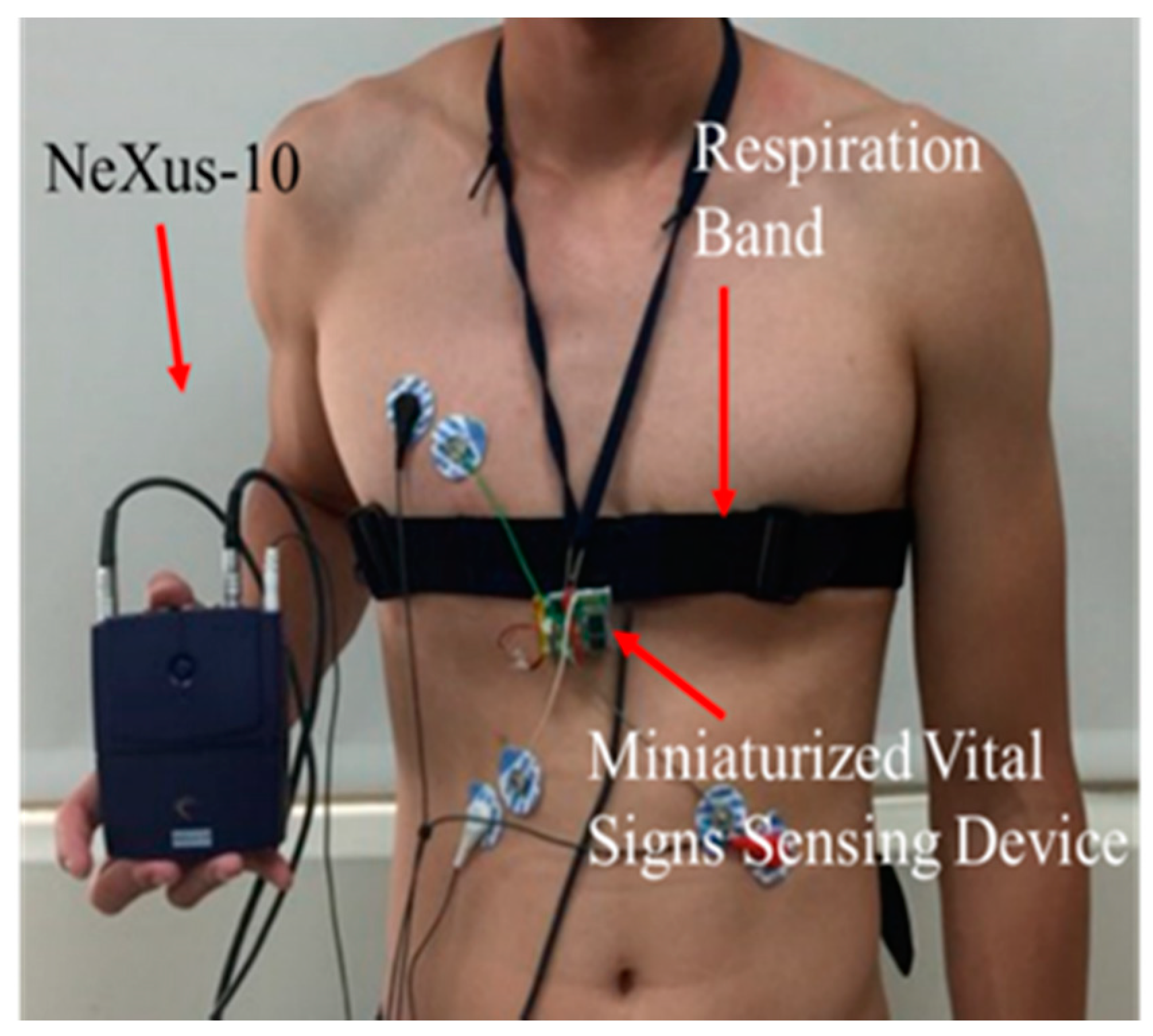
2.1. Vital Signs Sensing Gown
2.2. Intelligent Computation Platform
3. Integrated Intelligent Algorithm
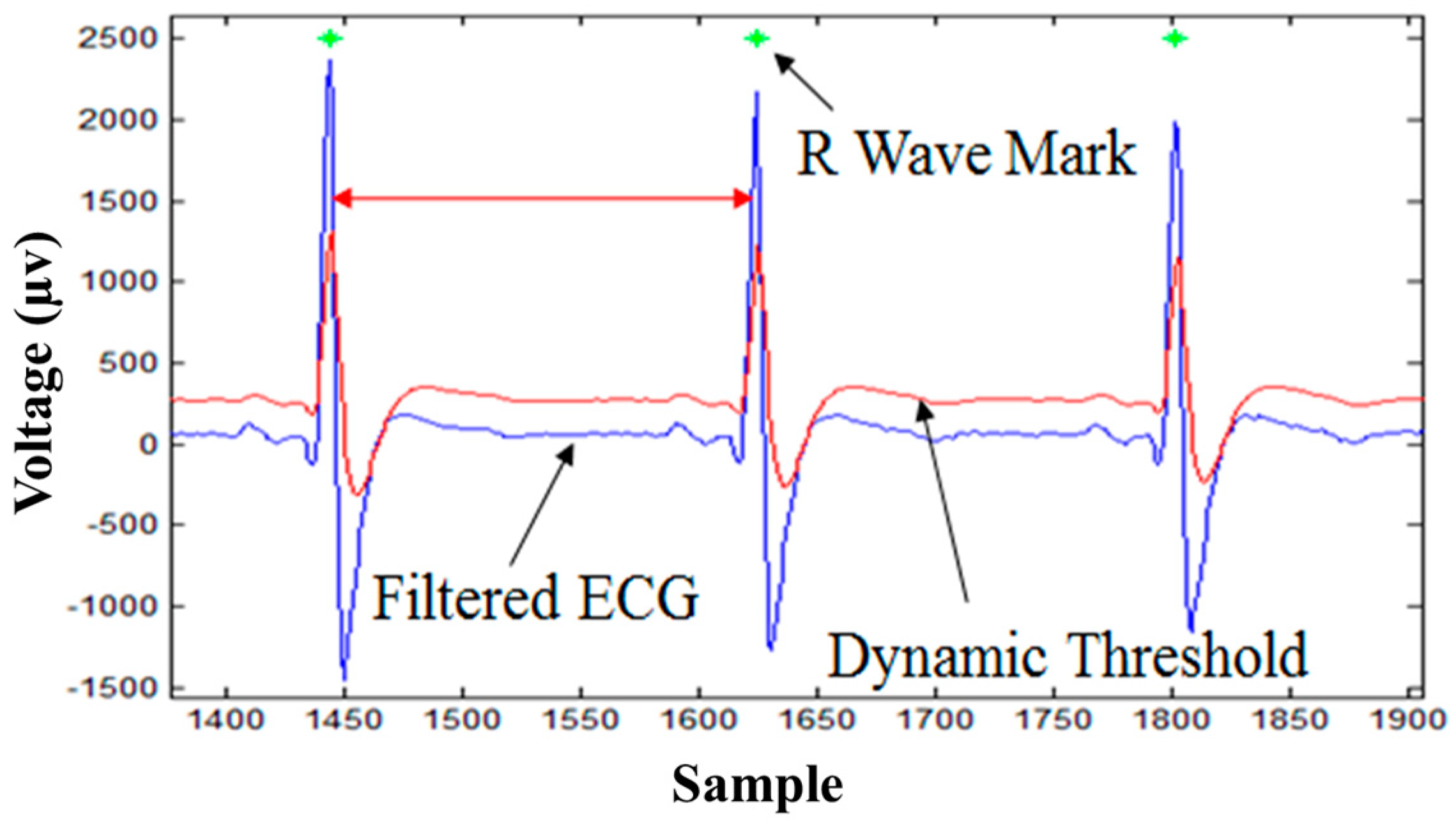
3.1. R-Wave Detection Algorithm
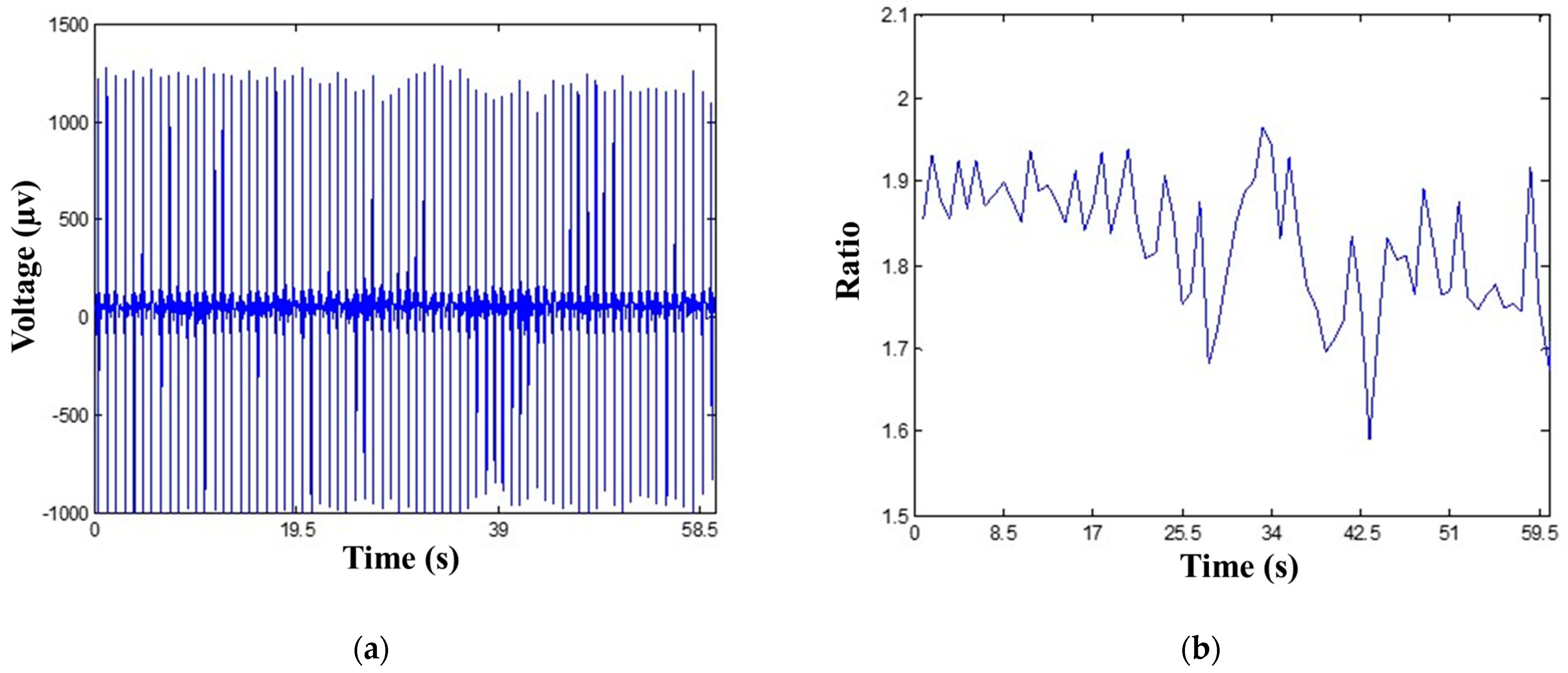
3.2. ECG-Derived Respiration Algorithm
- Employ the R-wave detection algorithm to determine R-wave amplitudes.
- Apply Equation (2) to extract the respiration signals from R-wave amplitude curves.
- Derive the amount of respiration oscillations to calculate RR.
3.3. Core Body Temperature Algorithm
- Calculate HR by the R-wave detection algorithm.
- Calculate RR by the EDR algorithm.
- Apply Equation (3) to implement core body temperature.
4. Experimental Design and Materials
4.1. Subjects
4.2. Clinical Experimental Design for Algorithm Validation
5. Experimental Results
5.1. R-Wave Detection Algorithm Results
5.2. ECG-Derived Respiration Algorithm Results
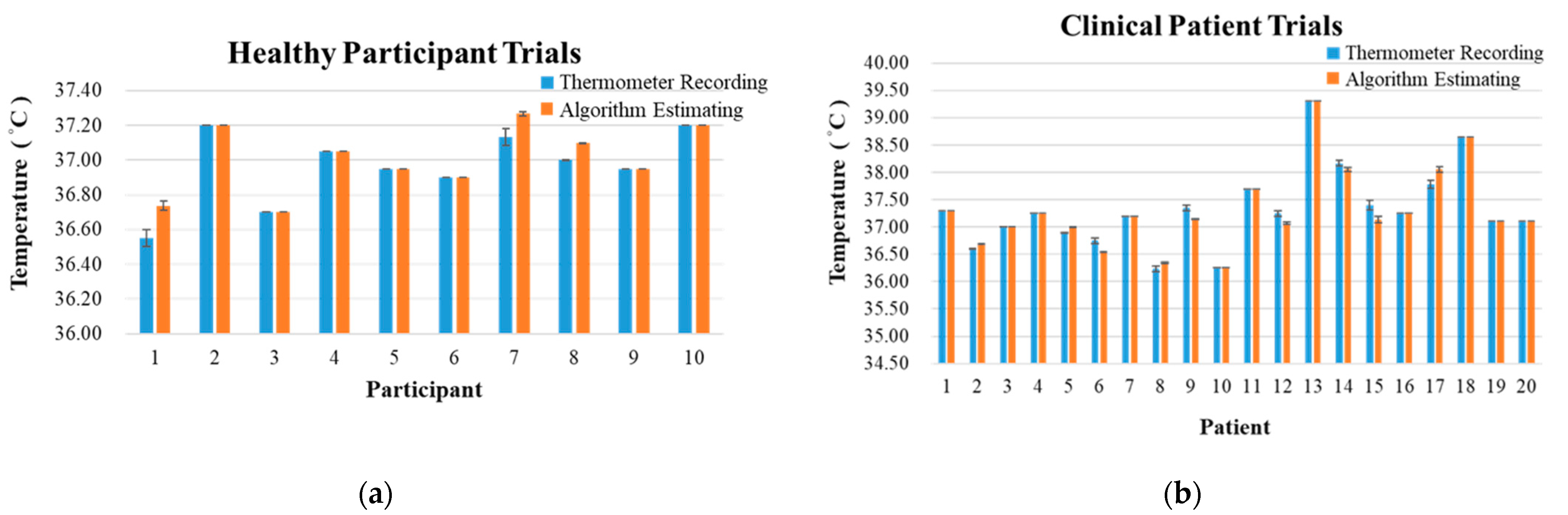
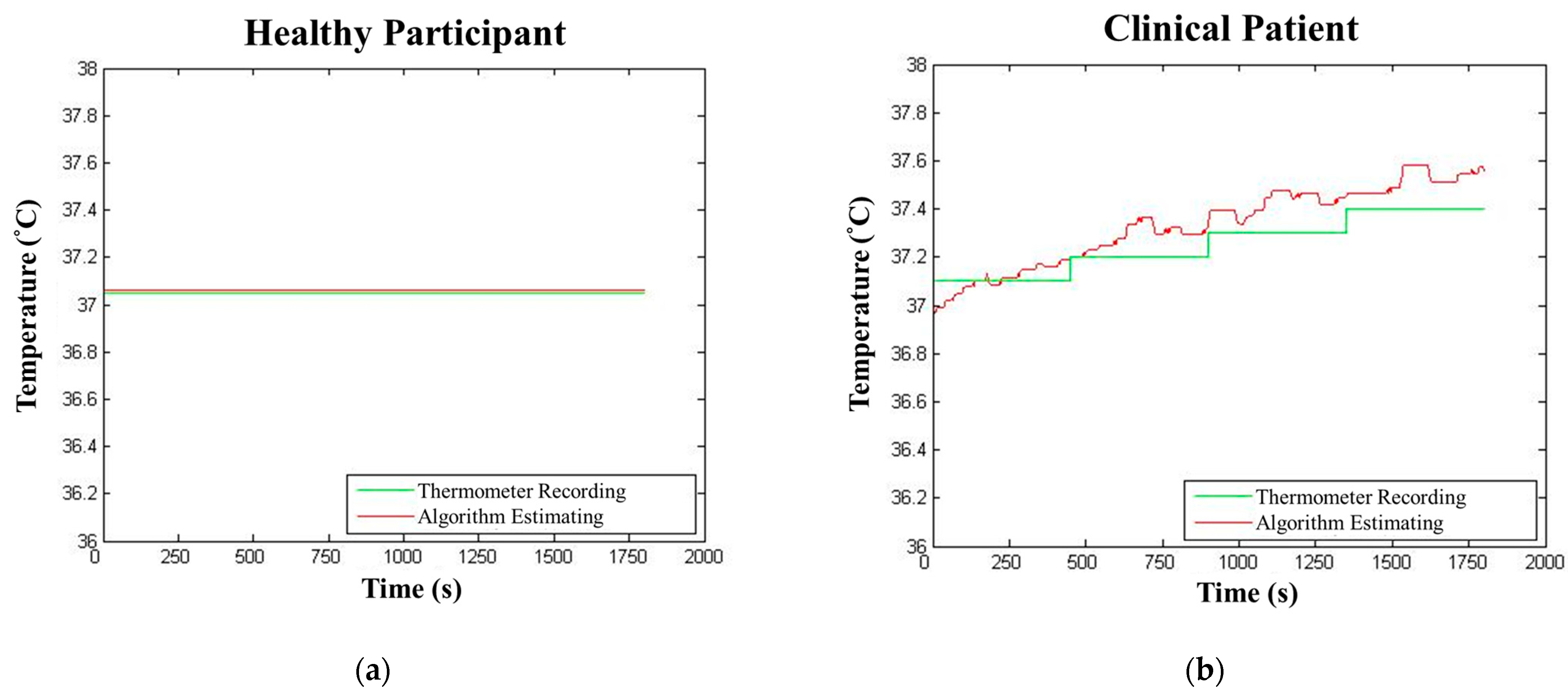
5.3. Core Body Temperature Algorithm Results
6. Discussion
- Wireless: The communication between the miniaturized circuit and portable devices is wireless (Bluetooth/Wi-Fi), which enhanced the convenience in applying this device to either healthy participants or clinical patients, inside or outside of hospitals.
- Mobility: The circuit on the sensing gown is small-sized, lightweight, and power-saving compared to current vital signs sensing devices. The proposed device is low-cost, convenient, and most importantly, as accurate as the devices that were commonly used in clinical environments. Furthermore, the circuit is compatible not only with the proposed sensing gown, but with other designs of vital signs sensing systems.
- Real-time: With the simplicity that the proposed algorithms exhibited, the values transmitted by the device and derived from the algorithms can be displayed on portable devices in real-time without any delay.
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neuman, M.R. Measurement of Vital Signs: Temperature [Tutorial]. IEEE Pulse 2010, 1, 40–49. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, J.; Di, Y. A study of human skin and surface temperatures in stable and unstable thermal environments. J. Therm. Biol. 2013, 38, 440–448. [Google Scholar] [CrossRef]
- Onur, O.E.; Guneysel, O.; Akoglu, H.; Aydin, Y.D.; Denizbasi, A. Oral, axillary, and tympanic temperature measurements in older and younger adults with or without fever. Eur. J. Emerg. Med. 2008, 15, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; Hayward, C.; Avolio, A.; O’rourke, M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 1989, 80, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Gavela, J.F.; Harris, M. Combining Algorithms in Automatic Detection of QRS Complexes in ECG Signals. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 468–475. [Google Scholar] [CrossRef]
- Kohler, B.U.; Hennig, C.; Orglmeister, R. The principles of software QRS detection. IEEE Eng. Med. Biol. Mag. 2002, 21, 42–57. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. In IEEE Transactions on Biomedical Engineering; IEEE: Piscataway, NJ, USA, 1985; Volume BME-32, pp. 230–236. [Google Scholar] [CrossRef]
- Pahlm, O.; Sörnmo, L. Software QRS detection in ambulatory monitoring—A review. Med. Biol. Eng. Comput. 1984, 22, 289–297. [Google Scholar] [CrossRef]
- Friesen, G.M.; Jannett, T.C.; Jadallah, M.A.; Yates, S.L.; Quint, S.R.; Nagle, H.T. A comparison of the noise sensitivity of nine QRS detection algorithms. IEEE Trans. Biomed. Eng. 1990, 37, 85–98. [Google Scholar] [CrossRef]
- AL-Khalidi, F.Q.; Saatchi, R.; Burke, D.; Elphick, H.; Tan, S. Respiration rate monitoring methods: A review. Pediatric Pulmonol. 2011, 46, 523–529. [Google Scholar] [CrossRef]
- Moody, G.B.; Mark, R.G.; Bump, M.A.; Weinstein, J.S.; Berman, A.D.; Mietus, J.E.; Goldberger, A. Clinical Validation of the ECG-Derived Respiration (EDR) Technique. Comput. Cardiol. 1986, 13, 507–510. [Google Scholar]
- Varon, C.; Morales, J.; Lázaro, J.; Orini, M.; Deviaene, M.; Kontaxis, S.; Testelmans, D.; Buyse, B.; Borzée, P.; Sörnmo, L. A Comparative Study of ECG-derived Respiration in Ambulatory Monitoring using the Single-lead ECG. Sci. Rep. 2020, 10, 5704. [Google Scholar] [CrossRef] [PubMed]
- Nazeran, H.; Behbehani, K.; Fu-Chung, Y.; Ray, P. Reconstruction of respiratory patterns from electrocardiographic signals. In Proceedings of the 2nd International Conference on Bioelectromagnetism (Cat. No.98TH8269), Melbourne, Australia, 15–18 February 1998; pp. 183–184. [Google Scholar]
- Arunachalam, S.P.; Brown, L.F. Real-time estimation of the ECG-derived respiration (EDR) signal using a new algorithm for baseline wander noise removal. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 5681–5684. [Google Scholar]
- Mazzanti, B.; Lamberti, C.; De Bie, J. Validation of an ECG-derived respiration monitoring method. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003; pp. 613–616. [Google Scholar]
- Lee, P.-I.; Hsueh, P.-R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J. Microbiol. Immunol. Infect. 2020, 53, 365. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-H.; Chen, T.-C.; Chien, H.-C.; Yang, C.-J.; Chen, Y.-H. Measurement of body temperature to prevent pandemic COVID-19 in hospitals in Taiwan: Repeated measurement is necessary. J. Hosp. Infect. 2020, 105, 360–361. [Google Scholar] [CrossRef]
- Tharakan, S.; Nomoto, K.; Miyashita, S.; Ishikawa, K. Body temperature correlates with mortality in COVID-19 patients. Crit. Care 2020, 24, 298. [Google Scholar] [CrossRef] [PubMed]
- Hands, C.; Reid, E.; Meredith, P.; Smith, G.B.; Prytherch, D.R.; Schmidt, P.E.; Featherstone, P.I. Patterns in the recording of vital signs and early warning scores: Compliance with a clinical escalation protocol. BMJ Qual. Saf. 2013, 22, 719–726. [Google Scholar] [CrossRef]
- McGaughey, J.; O’Halloran, P.; Porter, S.; Blackwood, B. Early warning systems and rapid response to the deteriorating patient in hospital: A systematic realist review. J. Adv. Nurs. 2017, 73, 2877–2891. [Google Scholar] [CrossRef]
- Biel, L.; Pettersson, O.; Philipson, L.; Wide, P. ECG analysis: A new approach in human identification. IEEE Trans. Instrum. Meas. 2001, 50, 808–812. [Google Scholar] [CrossRef]
- Kang-Ping, L.; Chang, W.H. A technique for automated arrythmia detection of Holter ECG. In Proceedings of the 17th International Conference of the Engineering in Medicine and Biology Society, Montreal, QC, Canada, 20–25 September 1995; Volume 181, pp. 183–184. [Google Scholar]
- Chun-Lung, C.; Kang-Ping, L.; Te-Ho, T.; Tsai, K.; Chang, W.H. Validation of automated arrhythmia detection for Holter ECG. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol.20 Biomedical Engineering towards the Year 2000 and Beyond (Cat. No. 98CH36286), Hong Kong, China, 29 October–1 November 1998; Volume 101, pp. 101–103. [Google Scholar]
- Bernardi, L.; Rossi, M.; Fratino, P.; Finardi, G.; Mevio, E.; Orlandi, C. Relationship between phasic changes in human skin blood flow and autonomic tone. Microvasc. Res. 1989, 37, 16–27. [Google Scholar] [CrossRef]
- Davies, P.; Maconochie, I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg. Med. J. 2009, 26, 641. [Google Scholar] [CrossRef]
- Niedermann, R.; Wyss, E.; Annaheim, S.; Psikuta, A.; Davey, S.; Rossi, R.M. Prediction of human core body temperature using non-invasive measurement methods. Int. J. Biometeorol. 2014, 58, 7–15. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Moody, G.B.; Mark, R.G. The impact of the MIT-BIH arrhythmia database. IEEE Eng. Med. Biol. Mag. 2001, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]



| NeXus-10 Recording | Algorithm Estimating | |
|---|---|---|
| Mean R–R interval ± SD (ms) | 757.0 ± 40.3 | 757.0 ± 37.6 |
| Mean heart rate ± SD (bpm) | 79.47 ± 4.28 | 79.44 ± 3.98 |
| Relative error of heart rate ± SD (%) | 0.45 ± 0.64 | |
| NeXus-10 Recording | Algorithm Estimating | ||
|---|---|---|---|
| Mean R–R interval ± SD (ms) | Adults | 820.7 ± 87.7 | 821.5 ± 87.8 |
| Children | 451.6 ± 44.18 | 458.0 ± 41.7 | |
| Mean heart rate ± SD (bpm) | Adults | 73.89 ± 8.32 | 73.81 ± 8.31 |
| Children | 133.87 ± 11.64 | 131.89 ± 10.78 | |
| Relative error of heart rate ± SD (%) | Adults | 0.17 ± 0.10 | |
| Children | 1.41 ± 2.22 | ||
| NeXus-10 Recording | Algorithm Estimating | |
|---|---|---|
| Mean respiration rate ± SD (breaths/min) | 18.28 ± 1.34 | 17.85 ± 1.02 |
| Relative error of respiration rate ± SD (%) | 2.38 ± 3.58 | |
| NeXus-10 Recording | Algorithm Estimating | |
|---|---|---|
| Mean respiration rate ± SD (breaths/min.) | 21.50 ± 7.35 | 20.56 ± 7.56 |
| Relative error of respiration rate ± SD (%) | 5.52 ± 9.83 | |
| Braun IRT-4020 (Actual Recording) | Algorithm Estimating | |
|---|---|---|
| Core body temperature ± SD (°C) | 36.96 ± 0.21 | 37.01 ± 0.19 |
| RMSE ± SD (°C) | 0.04 ± 0.07 | |
| Bias ± SD (°C) | −0.04 ± 0.07 | |
| Braun IRT-4020 (Actual Recording) | Algorithm Estimating | |
|---|---|---|
| Core body temperature ± SD (°C) | 37.33 ± 0.74 | 37.31 ± 0.74 |
| RMSE ± SD (°C) | 0.08 ± 0.11 | |
| Bias ± SD (°C) | 0.02 ± 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, L.-W.; Chang, Y.; Lin, B.-K.; Lin, D.-S. Vital Signs Sensing Gown Employing ECG-Based Intelligent Algorithms. Biosensors 2022, 12, 964. https://doi.org/10.3390/bios12110964
Ko L-W, Chang Y, Lin B-K, Lin D-S. Vital Signs Sensing Gown Employing ECG-Based Intelligent Algorithms. Biosensors. 2022; 12(11):964. https://doi.org/10.3390/bios12110964
Chicago/Turabian StyleKo, Li-Wei, Yang Chang, Bo-Kai Lin, and Dar-Shong Lin. 2022. "Vital Signs Sensing Gown Employing ECG-Based Intelligent Algorithms" Biosensors 12, no. 11: 964. https://doi.org/10.3390/bios12110964
APA StyleKo, L.-W., Chang, Y., Lin, B.-K., & Lin, D.-S. (2022). Vital Signs Sensing Gown Employing ECG-Based Intelligent Algorithms. Biosensors, 12(11), 964. https://doi.org/10.3390/bios12110964




