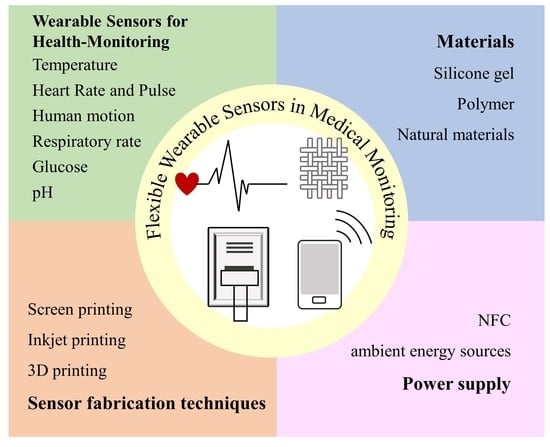
Flexible Wearable Sensors in Medical Monitoring
Abstract
:1. Introduction
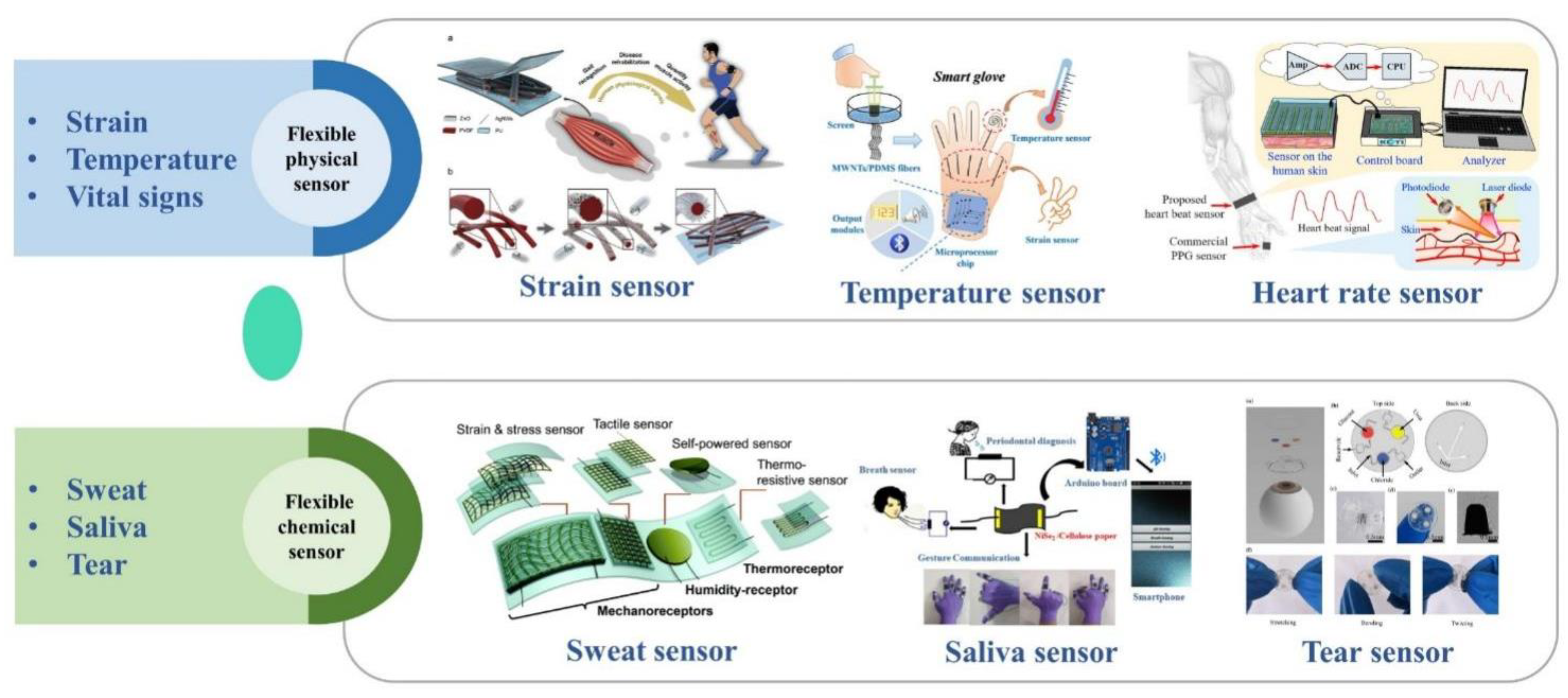
2. Application of Flexible Wearable Sensors for Health-Monitoring
2.1. Temperature
2.2. Heart Rate and Pulse

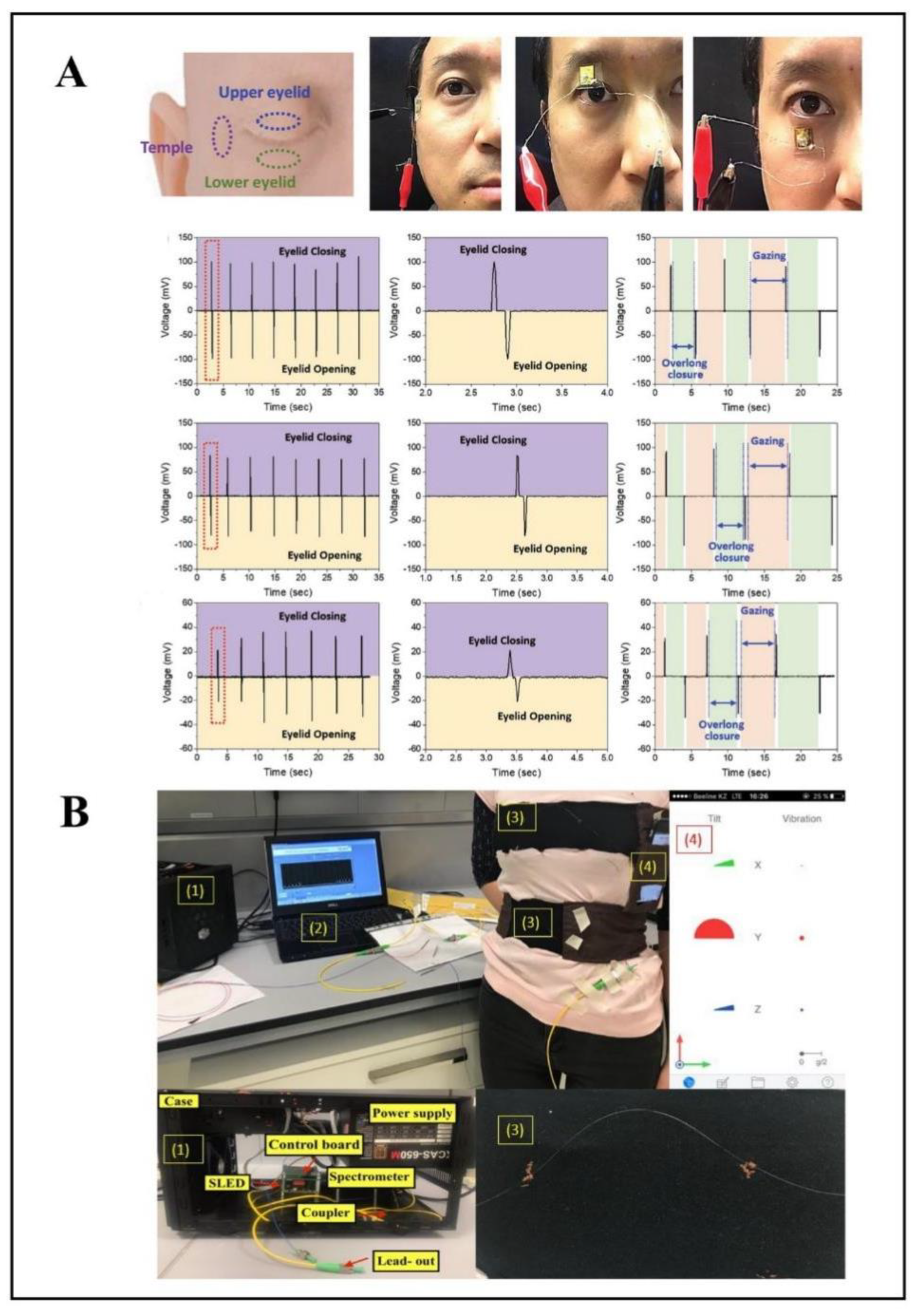
2.3. Human Motion
2.4. Respiratory Rate
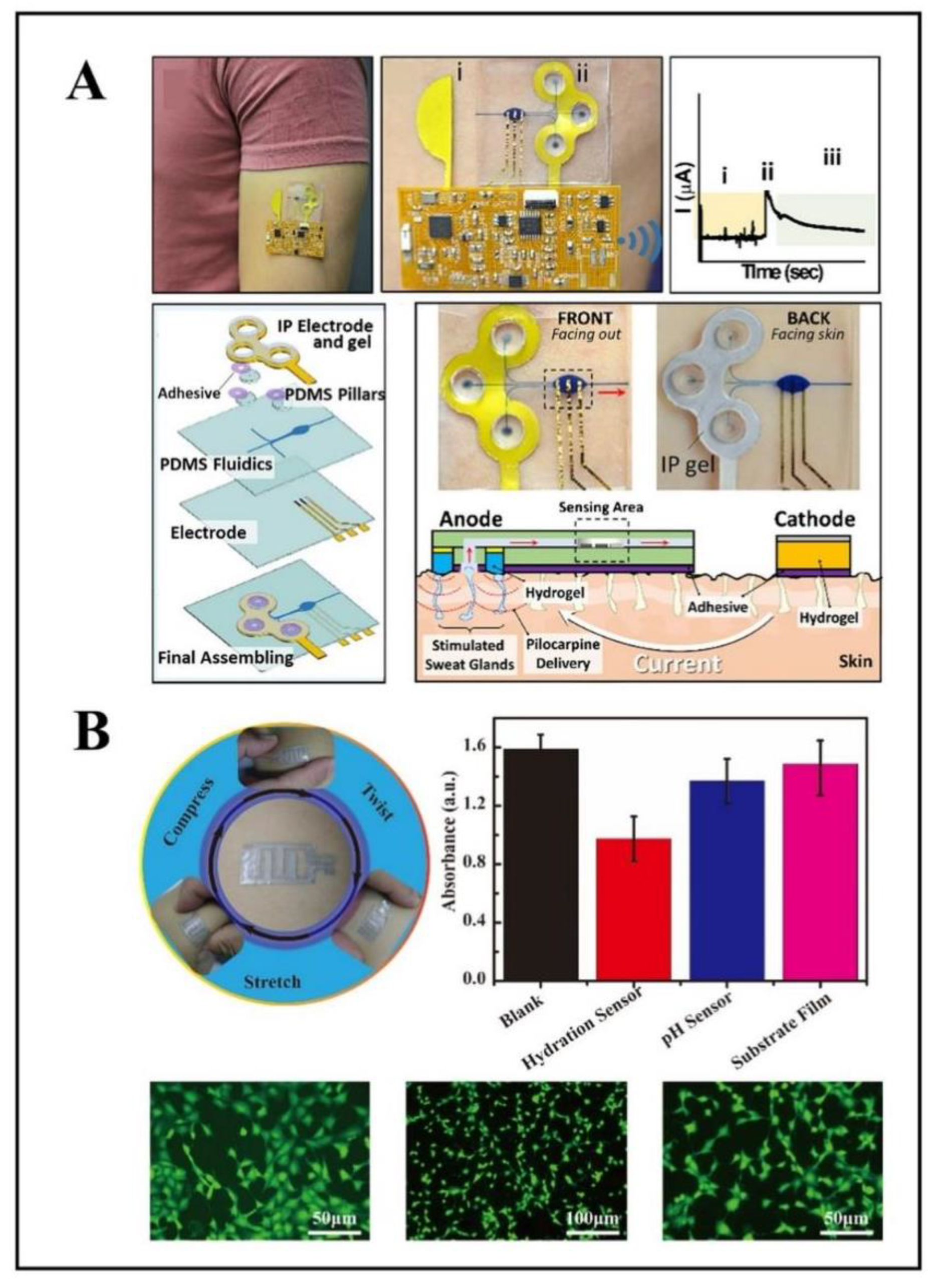
2.5. Glucose
2.6. pH
| Biological Signals | Sensors | Flexible Materials | Features | Refs |
|---|---|---|---|---|
| Temperature | temperature sensor array | PANI, Ecoflex | precise response time, high resistance sensitivity | [43] |
| temperature sensor array | PI, PDMS | excellent mechanical flexibility, visible transparency, self-power | [44] | |
| temperature sensors | PEI | ultrathin, flexible, lightweight | [46] | |
| Blood pressure | graphene electronic tattoos | graphene | self-adhesive, low-impedance, lightweight | [55] |
| piezo-composite ultrasonic sensor | PDMS | noninvasive, nonocclusive, calibration-free | [56] | |
| ECG | ECG electrode | natural leather | convenient, comfortable, flexible, wearable | [61] |
| Pulse | strain sensor | PANI | flexible, low cost, wearable, simple manufacturing process | [65] |
| pressure sensor arrays | PDMS, PET | flexible, wearable, multichannel | [66] | |
| Human motion | strain sensor | conductive textile | washable, lightweight, flexible, reusable wearable | [78] |
| triboelectric nanogenerator fabric sensor arrays | PDMS | flexible, stretchable, self-power | [79] | |
| eye-movement sensor | PDMS | highly-sensitive, skin-attachable, noninvasive | [81] | |
| Respiratory rate | fiber Bragg gratings sensors | PI, silicone | wearable, flexible | [88] |
| fiber Bragg gratings sensors | textiles | Wearable, multi-point sensing, | [89] | |
| Glucose | sweat sensor | agarose hydrogel | noninvasive, simple, in-situ analysis | [95] |
| iontophoresis integrated microfluidic epidermal biosensor | PDMS | soft, flexible, wearable, skin-mounted, non-invasive, | [96] | |
| pH | pH sensor | composite silk fibroin film | high flexibility, biocompatibility, air permeability, biodegradability | [102] |
| electrochemical pH Sensor | PET, PANI | skin-attachable, wearable, flexible | [103] |
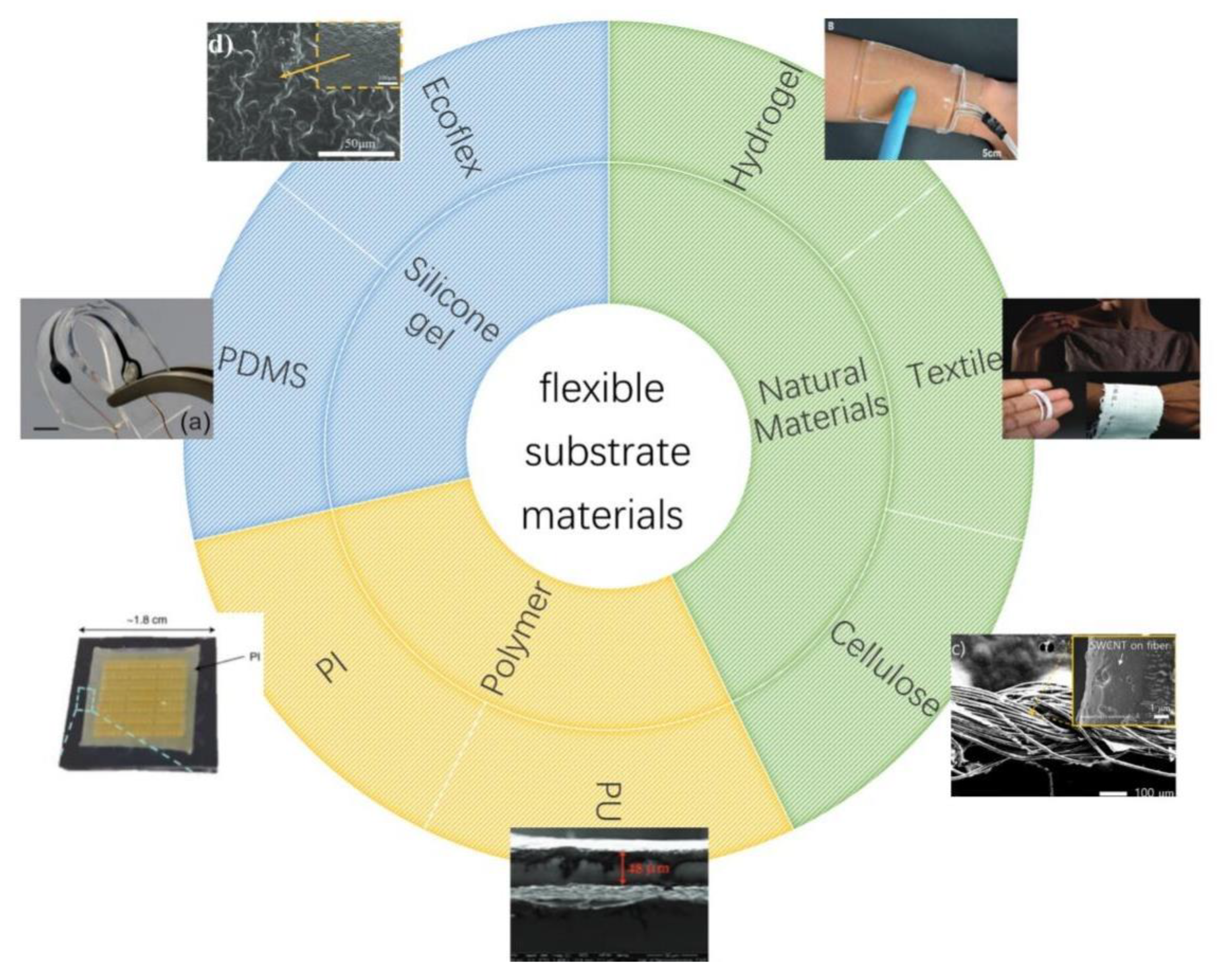
3. Materials

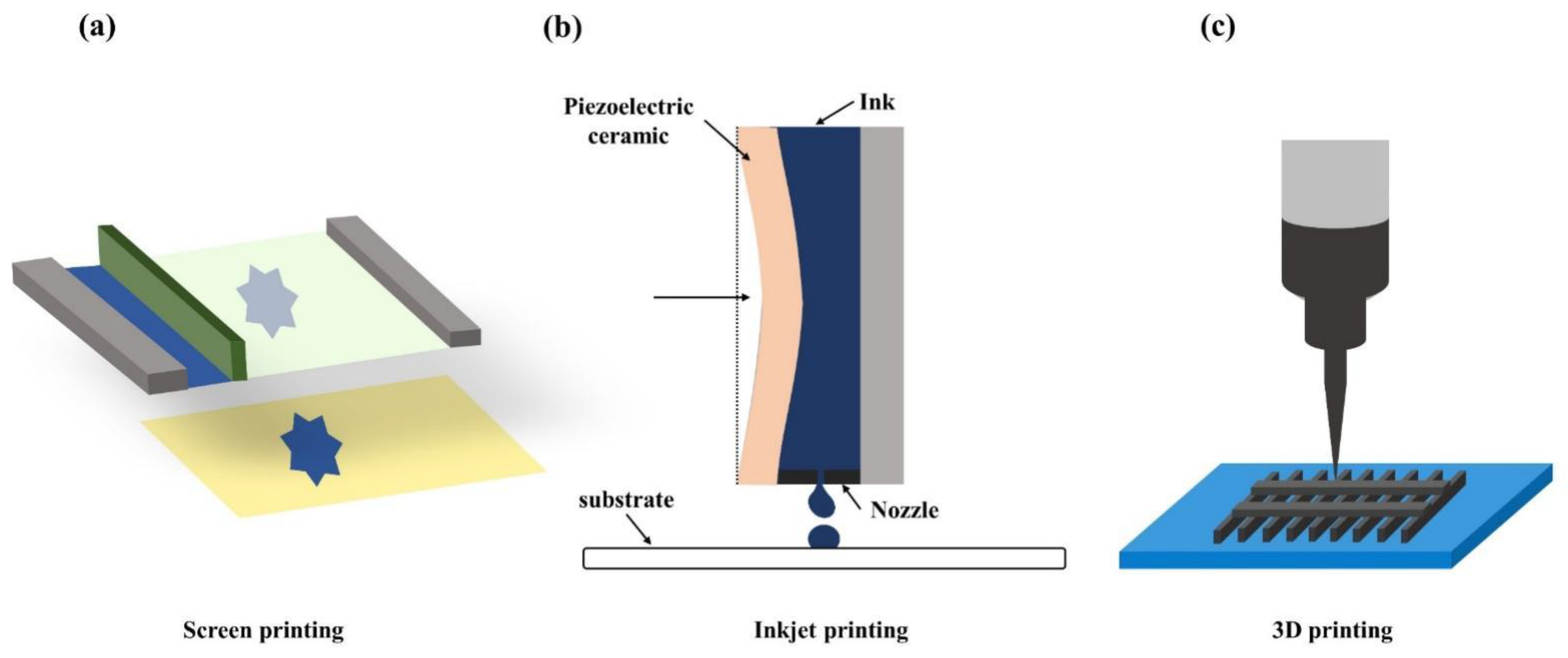
4. Sensor Fabrication Techniques
5. Power Supply
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libanori, A.; Chen, G.; Zhao, X.; Zhou, Y.; Chen, J. Smart Textiles for Personalized Healthcare. Nat. Electron. 2022, 5, 142–156. [Google Scholar] [CrossRef]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-Based Electrical Respiration Sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and Highly Sensitive Graphene Strain Sensors for Human Motion Monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar] [CrossRef]
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-Inspired Highly Stretchable and Conformable Matrix Networks for Multifunctional Sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantelopoulos, A.; Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Man Cybern. C 2010, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, J.; Wang, T.; Li, C.; Wei, Y.; Deng, X.; Chen, X. Emerging Intraoral Biosensors. J. Mater. Chem. B 2020, 8, 3341–3356. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Heo, J.S.; Hossain, M.F.; Kim, I. Challenges in Design and Fabrication of Flexible/Stretchable Carbon- and Textile-Based Wearable Sensors for Health Monitoring: A Critical Review. Sensors 2020, 20, 3927. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Bai, Y.; Zhang, J.; Liu, H.; Zhu, W. Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring. Sensors 2020, 20, 4009. [Google Scholar] [CrossRef]
- Shahandashti, P.F.; Pourkheyrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Highly Conformable Stretchable Dry Electrodes Based on Inexpensive Flex Substrate for Long-Term Biopotential (EMG/ECG) Monitoring. Sens. Actuators A Phys. 2019, 295, 678–686. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.-K.; Tok, J.B.-H.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef]
- Zhu, B.; Ling, Y.; Yap, L.W.; Yang, M.; Lin, F.; Gong, S.; Wang, Y.; An, T.; Zhao, Y.; Cheng, W. Hierarchically Structured Vertical Gold Nanowire Array-Based Wearable Pressure Sensors for Wireless Health Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 29014–29021. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Lin, G.; Feng, Y. Recent Progress in the Fabrication of Flexible Materials for Wearable Sensors. Biomater. Sci. 2022, 10, 614–632. [Google Scholar] [CrossRef]
- Yang, T.; Pan, H.; Tian, G.; Zhang, B.; Xiong, D.; Gao, Y.; Yan, C.; Chu, X.; Chen, N.; Zhong, S.; et al. Hierarchically Structured PVDF/ZnO Core-Shell Nanofibers for Self-Powered Physiological Monitoring Electronics. Nano Energy 2020, 72, 104706. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, C.; Liu, S.; Huang, L.; Fang, T.; Li, J.X.; Xu, F.; Li, F. Smart Glove Integrated with Tunable MWNTs/PDMS Fibers Made of a One-Step Extrusion Method for Finger Dexterity, Gesture, and Temperature Recognition. ACS Appl. Mater. Interfaces 2020, 12, 23764–23773. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Kim, W.; Park, K.B.; Kim, K.; Seo, S. Flexible Heartbeat Sensor for Wearable Device. Biosens. Bioelectron. 2017, 94, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wu, P. Adaptable Polyionic Elastomers with Multiple Sensations and Entropy-Driven Actuations for Prosthetic Skins and Neuromuscular Systems. Mater. Horiz. 2019, 6, 538–545. [Google Scholar] [CrossRef]
- Veeralingam, S.; Sahatiya, P.; Kadu, A.; Mattela, V.; Badhulika, S. Direct, One-Step Growth of NiSe 2 on Cellulose Paper: A Low-Cost, Flexible, and Wearable with Smartphone Enabled Multifunctional Sensing Platform for Customized Noninvasive Personal Healthcare Monitoring. ACS Appl. Electron. Mater. 2019, 1, 558–568. [Google Scholar] [CrossRef]
- Yang, X.; Yao, H.; Zhao, G.; Ameer, G.A.; Sun, W.; Yang, J.; Mi, S. Flexible, Wearable Microfluidic Contact Lens with Capillary Networks for Tear Diagnostics. J. Mater. Sci. 2020, 55, 9551–9561. [Google Scholar] [CrossRef]
- Bhide, A.; Muthukumar, S.; Saini, A.; Prasad, S. Simultaneous Lancet-Free Monitoring of Alcohol and Glucose from Low-Volumes of Perspired Human Sweat. Sci. Rep. 2018, 8, 6507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, E.; Hwang, B.-U.; Kim, D.; Kim, B.-Y.; Lee, N.-E. Stretchable, Transparent, Ultrasensitive, and Patchable Strain Sensor for Human–Machine Interfaces Comprising a Nanohybrid of Carbon Nanotubes and Conductive Elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef]
- Zhan, Z.; Lin, R.; Tran, V.-T.; An, J.; Wei, Y.; Du, H.; Tran, T.; Lu, W. Paper/Carbon Nanotube-Based Wearable Pressure Sensor for Physiological Signal Acquisition and Soft Robotic Skin. ACS Appl. Mater. Interfaces 2017, 9, 37921–37928. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, F.; Huang, D.; Gao, X.; Di, C.; Zhu, D. Flexible Suspended Gate Organic Thin-Film Transistors for Ultra-Sensitive Pressure Detection. Nat. Commun. 2015, 6, 6269. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Tee, B.C.-K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.L.; Wong, H.-S.P.; McConnell, M.V.; Bao, Z. Continuous Wireless Pressure Monitoring and Mapping with Ultra-Small Passive Sensors for Health Monitoring and Critical Care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [Green Version]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Flexible Electronics: Wearable, Human-Interactive, Health-Monitoring, Wireless Devices Fabricated by Macroscale Printing Techniques (Adv. Funct. Mater. 22/2014). Adv. Funct. Mater. 2014, 24, 3298. [Google Scholar] [CrossRef]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.-G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.-H.; Bao, Z. Highly Skin-Conformal Microhairy Sensor for Pulse Signal Amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable Amplified Lead Zirconate Titanate Sensors with Enhanced Piezoelectric Response for Cutaneous Pressure Monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- AitMou, Y.; Elgendy, M.; Jan, S.; Lucas, A.M.; Elzein, A.; Bermak, A. Smart Wearable Sensing Platform with Wireless Communication and Embedded Processing for Health Monitoring Applications. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha, Qatar, 19–20 March 2018; Hamad bin Khalifa University Press (HBKU Press): Doha, Qatar, 2018; Volume 2018. Issue 2. [Google Scholar]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Wang, F.-X.; Li, J.-J.; Peng, H.-D.; Yan, J.-H.; Pan, G.-B. Wearable Wide-Range Strain Sensors Based on Ionic Liquids and Monitoring of Human Activities. Sensors 2017, 17, 2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skafjeld, A.; Iversen, M.; Holme, I.; Ribu, L.; Hvaal, K.; Bente, K. A Pilot Study Testing the Feasibility of Skin Temperature Monitoring to Reduce Recurrent Foot Ulcers in Patients with Diabetes—A Randomized Controlled Trial. BMC Endocr. Disord. 2015, 15, 55. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Philip, J.; Jayakumar, T.; Raj, B.; Rao, P.N.S.; Varalakshmi, M.; Mohan, V. Correlation between Plantar Foot Temperature and Diabetic Neuropathy: A Case Study by Using an Infrared Thermal Imaging Technique. J. Diabetes Sci. Technol. 2010, 4, 1386–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.L.; Byrne, C.; Lee, J.K. Human Thermoregulation and Measurement of Body Temperature in Exercise and Clinical Settings. Ann. Acad. Med. Singap. 2008, 37, 347–353. [Google Scholar]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the Thermal Regulation of Immunity: The Immune System Feels the Heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Barcroft, H.; Edholm, O.G. The Effect of Temperature on Blood Flow and Deep Temperature in the Human Forearm. J. Physiol. 1943, 102, 5–20. [Google Scholar] [CrossRef]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S.; et al. Ultrathin Conformal Devices for Precise and Continuous Thermal Characterization of Human Skin. Nat. Mater 2013, 12, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Jia, H.; He, Y.; Liao, S.; Wang, Y. Ultrafast Paper Thermometers Based on a Green Sensing Ink. ACS Sens. 2017, 2, 449–454. [Google Scholar] [CrossRef]
- Matzeu, G.; Pucci, A.; Savi, S.; Romanelli, M.; Di Francesco, F. A Temperature Sensor Based on a MWCNT/SEBS Nanocomposite. Sens. Actuators A Phys. 2012, 178, 94–99. [Google Scholar] [CrossRef]
- Shih, W.-P.; Tsao, L.-C.; Lee, C.-W.; Cheng, M.-Y.; Chang, C.; Yang, Y.-J.; Fan, K.-C. Flexible Temperature Sensor Array Based on a Graphite-Polydimethylsiloxane Composite. Sensors 2010, 10, 3597–3610. [Google Scholar] [CrossRef]
- Rodgers, M.M.; Pai, V.M.; Conroy, R.S. Recent Advances in Wearable Sensors for Health Monitoring. IEEE Sens. J. 2015, 15, 3119–3126. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, Y.H.; Park, H.; Jin, S.W.; Jeong, Y.R.; Yun, J.; You, I.; Zi, G.; Ha, J.S. Stretchable Active Matrix Temperature Sensor Array of Polyaniline Nanofibers for Electronic Skin. Adv. Mater. 2016, 28, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, Z.; Guo, H.; Cheng, X.; Zhang, H. Self-Powered Flexible and Transparent Smart Patch for Temperature Sensing. Appl. Phys. Lett. 2020, 116, 043902. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.; Bermak, A. Recent Developments in Printing Flexible and Wearable Sensing Electronics for Healthcare Applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, H.; Chen, Y.; Cao, Y.; Ma, Y.; Feng, X. Ultrathin Flexible Inorganic Device for Long-Term Monitoring of Light and Temperature. IEEE Trans. Electron Devices 2021, 68, 3558–3561. [Google Scholar] [CrossRef]
- Nassar, J.M.; Mishra, K.; Lau, K.; Aguirre-Pablo, A.A.; Hussain, M.M. Recyclable Nonfunctionalized Paper-Based Ultralow-Cost Wearable Health Monitoring System. Adv. Mater. Technol. 2017, 2, 1600228. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Regional Office for Europe. Global Atlas on Cardiovascular Disease Prevention and Control: Published by the World Health Organization in Collaboration with the World Heart Federation and the World Stroke Organization; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2011; ISBN 978-92-4-156437-3. [Google Scholar]
- Myerburg, R.J.; Junttila, M.J. Sudden Cardiac Death Caused by Coronary Heart Disease. Circulation 2012, 125, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible Wearable Sensors for Cardiovascular Health Monitoring. Adv. Healthc. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef]
- Haji, S.A.; Movahed, A. Right Ventricular Infarction-Diagnosis and Treatment. Clin. Cardiol. 2000, 23, 473–482. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Mancia, G. Arterial Stiffness. J. Hypertens. 1999, 17, 1–4. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Alpert, B.; Mieke, S.; Asmar, R.; Atkins, N.; Eckert, S.; Frick, G.; Friedman, B.; Graßl, T.; Ichikawa, T.; et al. A Universal Standard for the Validation of Blood Pressure Measuring Devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension 2018, 71, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Vischer, A.S.; Burkard, T. Principles of Blood Pressure Measurement—Current Techniques, Office vs Ambulatory Blood Pressure Measurement. In Hypertension: From Basic Research to Clinical Practice; Advances in Experimental Medicine and, Biology; Islam, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 956, pp. 85–96. ISBN 978-3-319-44250-1. [Google Scholar]
- Kireev, D.; Sel, K.; Ibrahim, B.; Kumar, N.; Akbari, A.; Jafari, R.; Akinwande, D. Continuous Cuffless Monitoring of Arterial Blood Pressure via Graphene Bioimpedance Tattoos. Nat. Nanotechnol. 2022, 17, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Chen, M.; Sim, H.K.; Zhu, Y.; Jiang, X. Noninvasive and Nonocclusive Blood Pressure Monitoring via a Flexible Piezo-Composite Ultrasonic Sensor. IEEE Sens. J. 2021, 21, 2642–2650. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.-P.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Oresko, J.J.; Jin, Z.; Cheng, J.; Huang, S.; Sun, Y.; Duschl, H.; Cheng, A.C. A Wearable Smartphone-Based Platform for Real-Time Cardiovascular Disease Detection Via Electrocardiogram Processing. IEEE Trans. Inform. Technol. Biomed. 2010, 14, 734–740. [Google Scholar] [CrossRef]
- Ou, K.-L.; Chang, C.-C.; Chang, W.-J.; Lin, C.-T.; Chang, K.-J.; Huang, H.-M. Effect of Damping Properties on Fracture Resistance of Root Filled Premolar Teeth: A Dynamic Finite Element Analysis. Int. Endod. J. 2009, 42, 694–704. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry Electrodes for Electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.; Gou, L.; Zou, Y. A Novel Wearable Flexible Dry Electrode Based on Cowhide for ECG Measurement. Biosensors 2021, 11, 101. [Google Scholar] [CrossRef]
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-Organic Optoelectronic Sensor for Pulse Oximetry. Nat. Commun. 2014, 5, 5745. [Google Scholar] [CrossRef] [Green Version]
- Sim, J.K.; Ahn, B.; Doh, I. A Contact-Force Regulated Photoplethysmography (PPG) Platform. AIP Adv. 2018, 8, 045210. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the Central Blood Pressure Waveform via a Conformal Ultrasonic Device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pradana Rachim, V.; Baek, J.-H.; Lee, S.Y.; Park, S.-M. A Flexible Patch-Type Strain Sensor Based on Polyaniline for Continuous Monitoring of Pulse Waves. IEEE Access 2020, 8, 152105–152115. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Wu, Z.; Zhang, Y.; Lin, J.; Chen, T.; Liu, H.; Wang, F.; Sun, L. Wearable Multichannel Pulse Condition Monitoring System Based on Flexible Pressure Sensor Arrays. Microsyst. Nanoeng. 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Gaïtis, A.; Sato, J.; Miyazawa, K.; Muraki, K.; Shiwaku, R.; Takeda, Y.; Matsui, H.; Kumaki, D.; Domingues Dos Santos, F.; et al. Low Operating Voltage and Highly Pressure-Sensitive Printed Sensor for Healthcare Monitoring with Analogic Amplifier Circuit. ACS Appl. Electron. Mater. 2019, 1, 246–252. [Google Scholar] [CrossRef]
- Cavanagh, P.R.; Lipsky, B.A.; Bradbury, A.W.; Botek, G. Treatment for Diabetic Foot Ulcers. Lancet 2005, 366, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.L.; Solomont, J.; Kowall, N.; Hausdorff, J.M. Influence of Executive Function on Locomotor Function: Divided Attention Increases Gait Variability in Alzheimer’s Disease. J. Am. Geriatr. Soc. 2003, 51, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Lai, X.; Wang, J.; Tang, H.; Ren, H.; Yang, Y.; Jin, Q.; Zhang, L.; Yu, R.; Ma, G.; et al. Multi-Shelled Hollow Micro-/Nanostructures. Chem. Soc. Rev. 2015, 44, 6749–6773. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable Silicon Nanoribbon Electronics for Skin Prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lou, Z.; Jiang, K.; Shen, G. Bio-Multifunctional Smart Wearable Sensors for Medical Devices. Adv. Intell. Syst. 2019, 1, 1900040. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire–Elastomer Nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef]
- Abdul Razak, A.H.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot Plantar Pressure Measurement System: A Review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait Partitioning Methods: A Systematic Review. Sensors 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Bautista, J.A.; Huerta-Ruelas, J.A.; Chaparro-Cardenas, S.L.; Hernandez-Zavala, A. A Review in Detection and Monitoring Gait Disorders Using In-Shoe Plantar Measurement Systems. IEEE Rev. Biomed. Eng. 2017, 10, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, S.; Wang, Y.; Fan, X.; Ding, L.; Xuan, S.; Gong, X. Conductive Shear Thickening Gel/Polyurethane Sponge: A Flexible Human Motion Detection Sensor with Excellent Safeguarding Performance. Compos. Part A Appl. Sci. Manuf. 2018, 112, 197–206. [Google Scholar] [CrossRef]
- Di Tocco, J.; Carnevale, A.; Presti, D.L.; Bravi, M.; Bressi, F.; Miccinilli, S.; Sterzi, S.; Longo, U.G.; Denaro, V.; Schena, E.; et al. Wearable Device Based on a Flexible Conductive Textile for Knee Joint Movements Monitoring. IEEE Sens. J. 2021, 21, 26655–26664. [Google Scholar] [CrossRef]
- He, M.; Du, W.; Feng, Y.; Li, S.; Wang, W.; Zhang, X.; Yu, A.; Wan, L.; Zhai, J. Flexible and Stretchable Triboelectric Nanogenerator Fabric for Biomechanical Energy Harvesting and Self-Powered Dual-Mode Human Motion Monitoring. Nano Energy 2021, 86, 106058. [Google Scholar] [CrossRef]
- Shu, Q.; Xu, Z.; Liu, S.; Wu, J.; Deng, H.; Gong, X.; Xuan, S. Magnetic Flexible Sensor with Tension and Bending Discriminating Detection. Chem. Eng. J. 2022, 433, 134424. [Google Scholar] [CrossRef]
- Kim, N.; Chen, J.; Wang, W.; Moradnia, M.; Pouladi, S.; Kwon, M.; Kim, J.; Li, X.; Ryou, J. Highly-Sensitive Skin-Attachable Eye-Movement Sensor Using Flexible Nonhazardous Piezoelectric Thin Film. Adv. Funct. Mater. 2021, 31, 2008242. [Google Scholar] [CrossRef]
- Min, S.D.; Yun, Y.; Shin, H. Simplified Structural Textile Respiration Sensor Based on Capacitive Pressure Sensing Method. IEEE Sens. J. 2014, 14, 3245–3251. [Google Scholar] [CrossRef]
- Atalay, O.; Kennon, W.R.; Demirok, E. Weft-Knitted Strain Sensor for Monitoring Respiratory Rate and Its Electro-Mechanical Modeling. IEEE Sens. J. 2015, 15, 110–122. [Google Scholar] [CrossRef]
- Allison, R.D.; Holmes, E.L.; Nyboer, J. Volumetric Dynamics of Respiration as Measured by Electrical Impedance Plethysmography. J. Appl. Physiol. 1964, 19, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, N.; Ge, J.; Wang, X.; Fok, M.P. Stretchable Fiber-Bragg-Grating-Based Sensor. Opt. Lett. 2018, 43, 2503. [Google Scholar] [CrossRef] [PubMed]
- Mihailov, S.J. Fiber Bragg Grating Sensors for Harsh Environments. Sensors 2012, 12, 1898–1918. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Di Tocco, J.; Massaroni, C.; Schena, E.; Formica, D. Wearable Systems Based on Fiber Bragg Grating Sensors for Respiratory Monitoring: Design, Fabrication, Open Challenges, and Future Directions. In Proceedings of the 2022 IEEE International Workshop on Metrology for Industry 4.0 & IoT (MetroInd4.0&IoT), Trento, Italy, 7–9 June 2022; pp. 377–381. [Google Scholar]
- Tocco, J.D.; Presti, D.L.; Zaltieri, M.; D’Alesio, G.; Filosa, M.; Massari, L.; Aliperta, A.; Rienzo, M.D.; Carrozza, M.C.; Ferrarin, M.; et al. A Wearable System Based on Flexible Sensors for Unobtrusive Respiratory Monitoring in Occupational Settings. IEEE Sens. J. 2021, 21, 14369–14378. [Google Scholar] [CrossRef]
- Issatayeva, A.; Beisenova, A.; Tosi, D.; Molardi, C. Fiber-Optic Based Smart Textiles for Real-Time Monitoring of Breathing Rate. Sensors 2020, 20, 3408. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global NCD Target: Improve Access to Technologies and Medicines to Treat NCDs; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kyun Kim, H.; et al. Wearable Smart Sensor Systems Integrated on Soft Contact Lenses for Wireless Ocular Diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [Green Version]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Heng, W. Emerging Wearable Flexible Sensors for Sweat Analysis. Bio-Des. Manuf. 2022, 5, 64–84. [Google Scholar] [CrossRef]
- Nagamine, K.; Mano, T.; Nomura, A.; Ichimura, Y.; Izawa, R.; Furusawa, H.; Matsui, H.; Kumaki, D.; Tokito, S. Noninvasive Sweat-Lactate Biosensor Emplsoying a Hydrogel-Based Touch Pad. Sci. Rep. 2019, 9, 10102. [Google Scholar] [CrossRef] [Green Version]
- Bolat, G.; De la Paz, E.; Azeredo, N.F.; Kartolo, M.; Kim, J.; de Loyola e Silva, A.N.; Rueda, R.; Brown, C.; Angnes, L.; Wang, J.; et al. Wearable Soft Electrochemical Microfluidic Device Integrated with Iontophoresis for Sweat Biosensing. Anal. Bioanal. Chem. 2022, 414, 5411–5421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Zhang, T. Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiolo, L.; Mirabella, S.; Maita, F.; Alberti, A.; Minotti, A.; Strano, V.; Pecora, A.; Shacham-Diamand, Y.; Fortunato, G. Flexible PH Sensors Based on Polysilicon Thin Film Transistors and ZnO Nanowalls. Appl. Phys. Lett. 2014, 105, 093501. [Google Scholar] [CrossRef]
- Durbin, R.P. Letter: Acid Secretion by Gastric Mucous Membrane. Am. J. Physiol. 1975, 229, 1726. [Google Scholar] [CrossRef]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible Potentiometric PH Sensors for Wearable Systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Zhang, F.; Chen, C.; Zhang, Y.; Wu, R.; Ma, L.; Lin, C.; Guo, W.; Liu, X.Y. Wearable Hydration and PH Sensor Based on Protein Film for Healthcare Monitoring. Chem. Pap. 2021, 75, 4927–4934. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.; Liu, G.; Lin, H.; Bao, Y.; Han, D.; Wang, W.; Ma, Y.; Zhang, B.; Niu, L. Superhydrophobic Functionalized Ti3C2Tx MXene-Based Skin-Attachable and Wearable Electrochemical PH Sensor for Real-Time Sweat Detection. Anal. Chem. 2022, 94, 7319–7328. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable Flexible Sweat Sensors for Healthcare Monitoring: A Review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Progress and Challenges in Fabrication of Wearable Sensors for Health Monitoring. Sens. Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Kim, S.; Zhang, X.; Daugherty, R.; Lee, E.; Kunnen, G.; Allee, D.R.; Forsythe, E.; Chae, J. Design and Implementation of Electrostatic Micro-Actuators in Ultrasonic Frequency on a Flexible Substrate, PEN (Polyethylene Naphthalate). Sens. Actuators A Phys. 2013, 195, 198–205. [Google Scholar] [CrossRef]
- Lee, C.; Jug, L.; Meng, E. High Strain Biocompatible Polydimethylsiloxane-Based Conductive Graphene and Multiwalled Carbon Nanotube Nanocomposite Strain Sensors. Appl. Phys. Lett. 2013, 102, 183511. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of Advanced Polydimethylsiloxane-Based Functional Materials: Bulk Modifications and Surface Functionalizations. Chem. Eng. J. 2021, 408, 127262. [Google Scholar] [CrossRef]
- Dinh, T.; Phan, H.-P.; Nguyen, T.-K.; Qamar, A.; Foisal, A.R.M.; Nguyen Viet, T.; Tran, C.-D.; Zhu, Y.; Nguyen, N.-T.; Dao, D.V. Environment-Friendly Carbon Nanotube Based Flexible Electronics for Noninvasive and Wearable Healthcare. J. Mater. Chem. C 2016, 4, 10061–10068. [Google Scholar] [CrossRef]
- Ding, H.; Wen, Z.; Qin, E.; Yang, Y.; Zhang, W.; Yan, B.; Wu, D.; Shi, Z.; Tian, Y.; Li, X. Influence of the Pore Size on the Sensitivity of Flexible and Wearable Pressure Sensors Based on Porous Ecoflex Dielectric Layers. Mater. Res. Express 2019, 6, 066304. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-Free and Label-Free Diagnostics of Glucose in Sweat Using Zinc Oxide Based Flexible Bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Meet the Editorial Board and Advisory Board of Materials Chemistry Frontiers. Mater. Chem. Front. 2017, 1, 12–23. [CrossRef]
- Ji, D.; Li, T.; Hu, W.; Fuchs, H. Recent Progress in Aromatic Polyimide Dielectrics for Organic Electronic Devices and Circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef]
- Kim, T.A.; Kim, H.S.; Lee, S.S.; Park, M. Single-Walled Carbon Nanotube/Silicone Rubber Composites for Compliant Electrodes. Carbon 2012, 50, 444–449. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.; Chen, H.; De Marco, N.; Yang, Y. Recent Progress in Materials and Devices toward Printable and Flexible Sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef]
- Zhou, S.; Nyholm, L.; Strømme, M.; Wang, Z. Cladophora Cellulose: Unique Biopolymer Nanofibrils for Emerging Energy, Environmental, and Life Science Applications. Acc. Chem. Res. 2019, 52, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, L. Nanocellulose toward Advanced Energy Storage Devices: Structure and Electrochemistry. Acc. Chem. Res. 2018, 51, 3154–3165. [Google Scholar] [CrossRef]
- Fu, Q.; Cui, C.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Emerging Cellulose-Derived Materials: A Promising Platform for the Design of Flexible Wearable Sensors toward Health and Environment Monitoring. Mater. Chem. Front. 2021, 5, 2051–2091. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous Self-Healing Silk Fibroin Injectable Hydrogels Formed via Surfactant-Free Hydrophobic Association. ACS Appl. Mater. Interfaces 2020, 12, 1628–1639. [Google Scholar] [CrossRef]
- Kurra, N.; Kulkarni, G.U. Pencil-on-Paper: Electronic Devices. Lab Chip 2013, 13, 2866. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Badhulika, S. Low Cost, Flexible and Biodegradable Touch Sensor Fabricated by Solvent-Free Processing of Graphite on Cellulose Paper. Sens. Actuators B Chem. 2017, 242, 857–864. [Google Scholar] [CrossRef]
- Seyedin, S.; Zhang, P.; Naebe, M.; Qin, S.; Chen, J.; Wang, X.; Razal, J.M. Textile Strain Sensors: A Review of the Fabrication Technologies, Performance Evaluation and Applications. Mater. Horiz. 2019, 6, 219–249. [Google Scholar] [CrossRef]
- Lee, H.-R.; Kim, C.-C.; Sun, J.-Y. Stretchable Ionics—A Promising Candidate for Upcoming Wearable Devices. Adv. Mater. 2018, 30, 1704403. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Gu, X.; Xu, Y.; Shen, J.; Zhu, D.; Chao, J.; Fan, C.; Wang, L. Two-Dimensional Nanomaterials for Biosensing Applications. TrAC Trends Anal. Chem. 2019, 119, 115610. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical Sensor and Biosensor Platforms Based on Advanced Nanomaterials for Biological and Biomedical Applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cheng, C.; Liu, Z.; Yuan, W.; Wu, X.; Lu, Y.; Low, S.S.; Liu, J.; Zhu, L.; Ji, D.; et al. Battery-Free and Wireless Epidermal Electrochemical System with All-Printed Stretchable Electrode Array for Multiplexed In Situ Sweat Analysis. Adv. Mater. Technol. 2019, 4, 1800658. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.; Li, J. Graphene-Based Materials in Electrochemistry. Chem. Soc. Rev. 2010, 39, 3157. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cote, L.J.; Tung, V.C.; Tan, A.T.L.; Goins, P.E.; Wu, J.; Huang, J. Graphene Oxide Nanocolloids. J. Am. Chem. Soc. 2010, 132, 17667–17669. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Huang, Y.; Ma, Y.; Wang, Y.; Chen, Y. Size-Controlled Synthesis of Graphene Oxide Sheets on a Large Scale Using Chemical Exfoliation. Carbon 2009, 47, 3365–3368. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhu, H. Functionalized and Environment-Friendly Carbon Materials for Flexible and Wearable Electronic Devices. In Proceedings of the International Conference on Optoelectronic Materials and Devices (ICOMD 2021), Guangzhou, China, 16 February 2022; Lu, Y., Gu, Y., Chen, S., Eds.; SPIE: Bellingham, WA, USA, 2022; p. 80. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, K.; Lou, Z.; Chen, D.; Shen, G. Recent Developments in Graphene-Based Tactile Sensors and E-Skins. Adv. Mater. Technol. 2018, 3, 1700248. [Google Scholar] [CrossRef]
- Tao, L.-Q.; Tian, H.; Liu, Y.; Ju, Z.-Y.; Pang, Y.; Chen, Y.-Q.; Wang, D.-Y.; Tian, X.-G.; Yan, J.-C.; Deng, N.-Q.; et al. An Intelligent Artificial Throat with Sound-Sensing Ability Based on Laser Induced Graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Son, D.; Kim, J.; Lee, Y.B.; Song, J.-K.; Choi, S.; Lee, D.J.; Kim, J.H.; Lee, M.; Hyeon, T.; et al. Transparent and Stretchable Interactive Human Machine Interface Based on Patterned Graphene Heterostructures. Adv. Funct. Mater. 2015, 25, 375–383. [Google Scholar] [CrossRef]
- Du, Y.; Xiao, P.; Yuan, J.; Chen, J. Research Progress of Graphene-Based Materials on Flexible Supercapacitors. Coatings 2020, 10, 892. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gong, K.; Li, Y.; Ding, B.; Li, L.; Xu, Y.; Wang, R.; Li, L.; Zhang, G.; Lin, S. Flexible 2D Materials beyond Graphene: Synthesis, Properties, and Applications. Small 2022, 18, 2105383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Liu, Y.; Liu, L.; Xu, Q.; Liu, H.; Wang, W.; Chen, L. Ultra-Stretchable Monofilament Flexible Sensor with Low Hysteresis and Linearity Based on MWCNTs/Ecoflex Composite Materials. Macro Mater. Eng. 2021, 306, 2100113. [Google Scholar] [CrossRef]
- Daus, A.; Vaziri, S.; Chen, V.; Köroğlu, Ç.; Grady, R.W.; Bailey, C.S.; Lee, H.R.; Schauble, K.; Brenner, K.; Pop, E. High-Performance Flexible Nanoscale Transistors Based on Transition Metal Dichalcogenides. Nat. Electron. 2021, 4, 495–501. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, B.; Chen, Y.; Feng, X. Breathable and Stretchable Temperature Sensors Inspired by Skin. Sci. Rep. 2015, 5, 11505. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Moon, D.-I.; Seol, M.-L.; Kim, B.; Han, J.-W.; Meyyappan, M. Wearable UV Sensor Based on Carbon Nanotube-Coated Cotton Thread. ACS Appl. Mater. Interfaces 2018, 10, 40198–40202. [Google Scholar] [CrossRef] [PubMed]
- Satharasinghe, A.; Hughes-Riley, T.; Dias, T. Photodiodes Embedded within Electronic Textiles. Sci. Rep. 2018, 8, 16205. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-C.; Lee, H.-H.; Oh, K.H.; Sun, J.-Y. Highly Stretchable, Transparent Ionic Touch Panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef]
- Baccanari, D.; Phillips, A.; Smith, S.; Sinski, D.; Burchall, J. Purification and Properties of Escherichia Coli Dihydrofolate Reductase. Biochemistry 1975, 14, 5267–5273. [Google Scholar] [CrossRef]
- Khan, S.; Tinku, S.; Lorenzelli, L.; Dahiya, R.S. Flexible Tactile Sensors Using Screen-Printed P(VDF-TrFE) and MWCNT/PDMS Composites. IEEE Sens. J. 2015, 15, 3146–3155. [Google Scholar] [CrossRef]
- Maddipatla, D.; Narakathu, B.B.; Atashbar, M. Recent Progress in Manufacturing Techniques of Printed and Flexible Sensors: A Review. Biosensors 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Mukhopadhyay, S.C. Wearable Flexible Sensors: Fabrication and Characterization; Wearable Sensors 28; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Pereira, A.N.; Noushin, T.; Tabassum, S. A Wearable, Multiplexed Sensor for Real-Time and In-Situ Monitoring of Wound Biomarkers. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October–3 November 2021; pp. 1–4. [Google Scholar]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Marinou, A.; Saunders, R.; Casson, A.J. Flexible Inkjet Printed Sensors for Behind-the-Ear SSVEP EEG Monitoring. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar]
- Wu, Y.; Zeng, Y.; Chen, Y.; Li, C.; Qiu, R.; Liu, W. Photocurable 3D Printing of High Toughness and Self-Healing Hydrogels for Customized Wearable Flexible Sensors. Adv. Funct. Mater. 2021, 8, 2107202. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and Correlative Sweat Analysis Using High-Throughput Microfluidic Sensing Patches toward Decoding Sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.M.V.; Kim, N.; Gu, Y.; Bandodkar, A.J.; You, J.; Kumar, R.; Kurniawan, J.F.; Xu, S.; Wang, J. Merging of Thin- and Thick-Film Fabrication Technologies: Toward Soft Stretchable “Island–Bridge” Devices. Adv. Mater. Technol. 2017, 2, 1600284. [Google Scholar] [CrossRef]
- Kim, J.; Ma, H.; Cha, H.; Lee, H.; Sung, J.; Seo, M.; Oh, P.; Park, M.; Cho, J. A Highly Stabilized Nickel-Rich Cathode Material by Nanoscale Epitaxy Control for High-Energy Lithium-Ion Batteries. Energy Environ. Sci. 2018, 11, 1449–1459. [Google Scholar] [CrossRef]
- Song, W.-J.; Lee, S.; Song, G.; Park, S. Stretchable Aqueous Batteries: Progress and Prospects. ACS Energy Lett. 2019, 4, 177–186. [Google Scholar] [CrossRef]
- Garnier, B.; Mariage, P.; Rault, F.; Cochrane, C.; Koncar, V. Textile NFC Antenna for Power and Data Transmission across Clothes. Smart Mater. Struct. 2020, 29, 085017. [Google Scholar] [CrossRef]
- Rosa, B.M.G.; Anastasova-Ivanova, S.; Yang, G.Z. NFC-Powered Flexible Chest Patch for Fast Assessment of Cardiac, Hemodynamic, and Endocrine Parameters. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1603–1614. [Google Scholar] [CrossRef]
- Ha, M.; Lim, S.; Ko, H. Wearable and Flexible Sensors for User-Interactive Health-Monitoring Devices. J. Mater. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhu, R.; Li, G. Self-Powered Electronic Skin with Multisensory Functions Based on Thermoelectric Conversion. Adv. Mater. Technol. 2020, 5, 2000419. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, H.; Gao, Z.; Liu, J.; Zhang, F.; Wen, Z.; Sun, X. Energy Autonomous Electronic Skin with Direct Temperature-Pressure Perception. Nano Energy 2022, 98, 107273. [Google Scholar] [CrossRef]
- Jin, H.; Abu-Raya, Y.S.; Haick, H. Advanced Materials for Health Monitoring with Skin-Based Wearable Devices. Adv. Healthc. Mater. 2017, 6, 1700024. [Google Scholar] [CrossRef]
- Park, Y.; Shin, Y.-E.; Park, J.; Lee, Y.; Kim, M.P.; Kim, Y.-R.; Na, S.; Ghosh, S.K.; Ko, H. Ferroelectric Multilayer Nanocomposites with Polarization and Stress Concentration Structures for Enhanced Triboelectric Performances. ACS Nano 2020, 14, 7101–7110. [Google Scholar] [CrossRef]
- Thap, T.; Yoon, K.-H.; Lee, J. Graphite Based Electrode for ECG Monitoring: Evaluation under Freshwater and Saltwater Conditions. Sensors 2016, 16, 542. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Liu, B.; Li, H.; Li, M.; Song, Y.; Wang, R.; Wang, T.; Zhang, H. Flexible Wearable Sensors in Medical Monitoring. Biosensors 2022, 12, 1069. https://doi.org/10.3390/bios12121069
Yuan Y, Liu B, Li H, Li M, Song Y, Wang R, Wang T, Zhang H. Flexible Wearable Sensors in Medical Monitoring. Biosensors. 2022; 12(12):1069. https://doi.org/10.3390/bios12121069
Chicago/Turabian StyleYuan, Yingying, Bo Liu, Hui Li, Mo Li, Yingqiu Song, Runze Wang, Tianlu Wang, and Hangyu Zhang. 2022. "Flexible Wearable Sensors in Medical Monitoring" Biosensors 12, no. 12: 1069. https://doi.org/10.3390/bios12121069