A Hemin–Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Cell Culture
2.3. Synthesis and Characterization of Hemin-Functionalized Graphene Nanosheets
2.4. Preparation of DNA Probe HGNs
2.5. Generation of Magnetic Capture Probe
2.6. Detection of CEM Cells
3. Results and Discussion
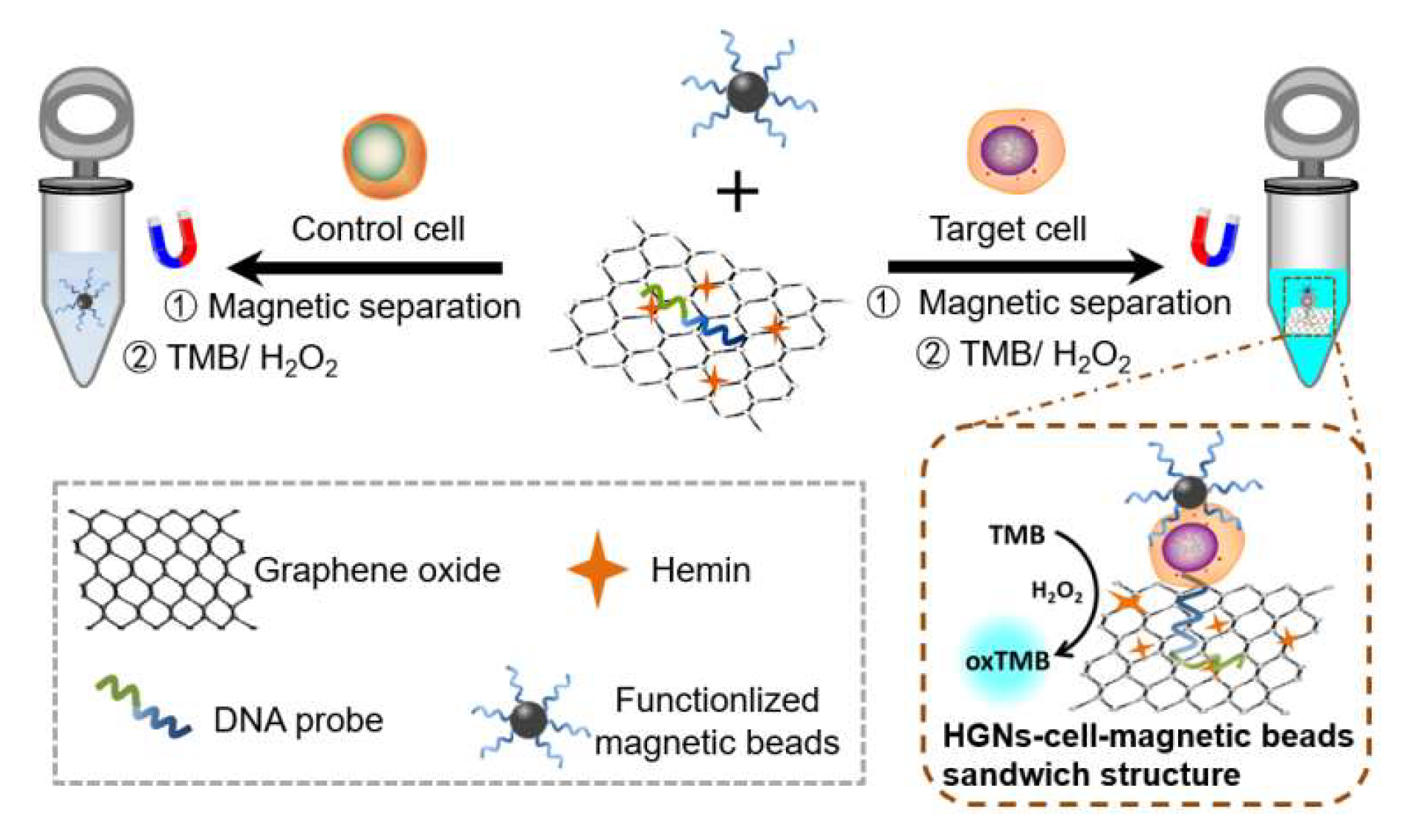
3.1. Principle of the HGN-Based Aptasensor for the Detection of Leukaemia Cells
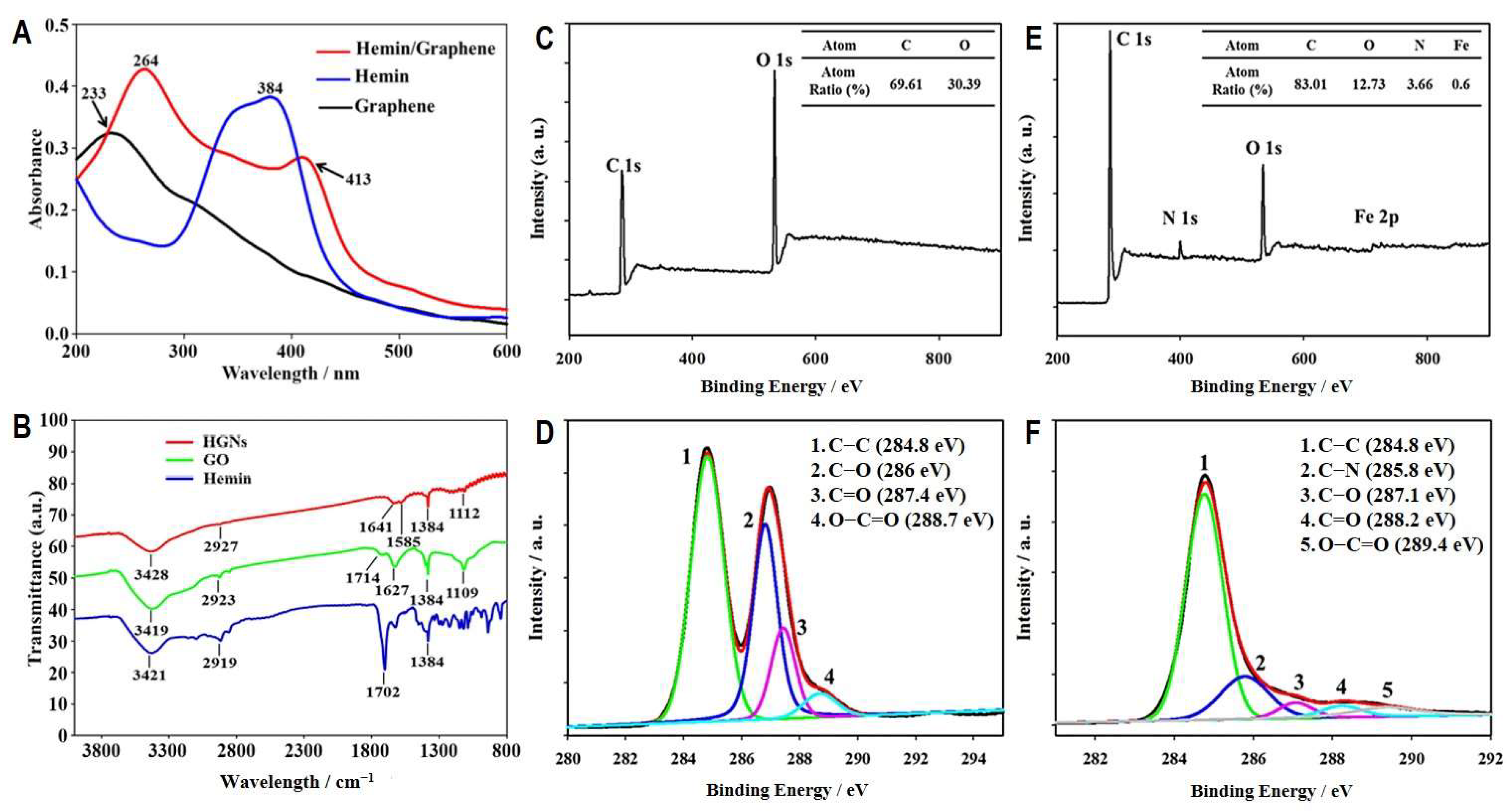
3.2. Preparation and Characterization of HGNs
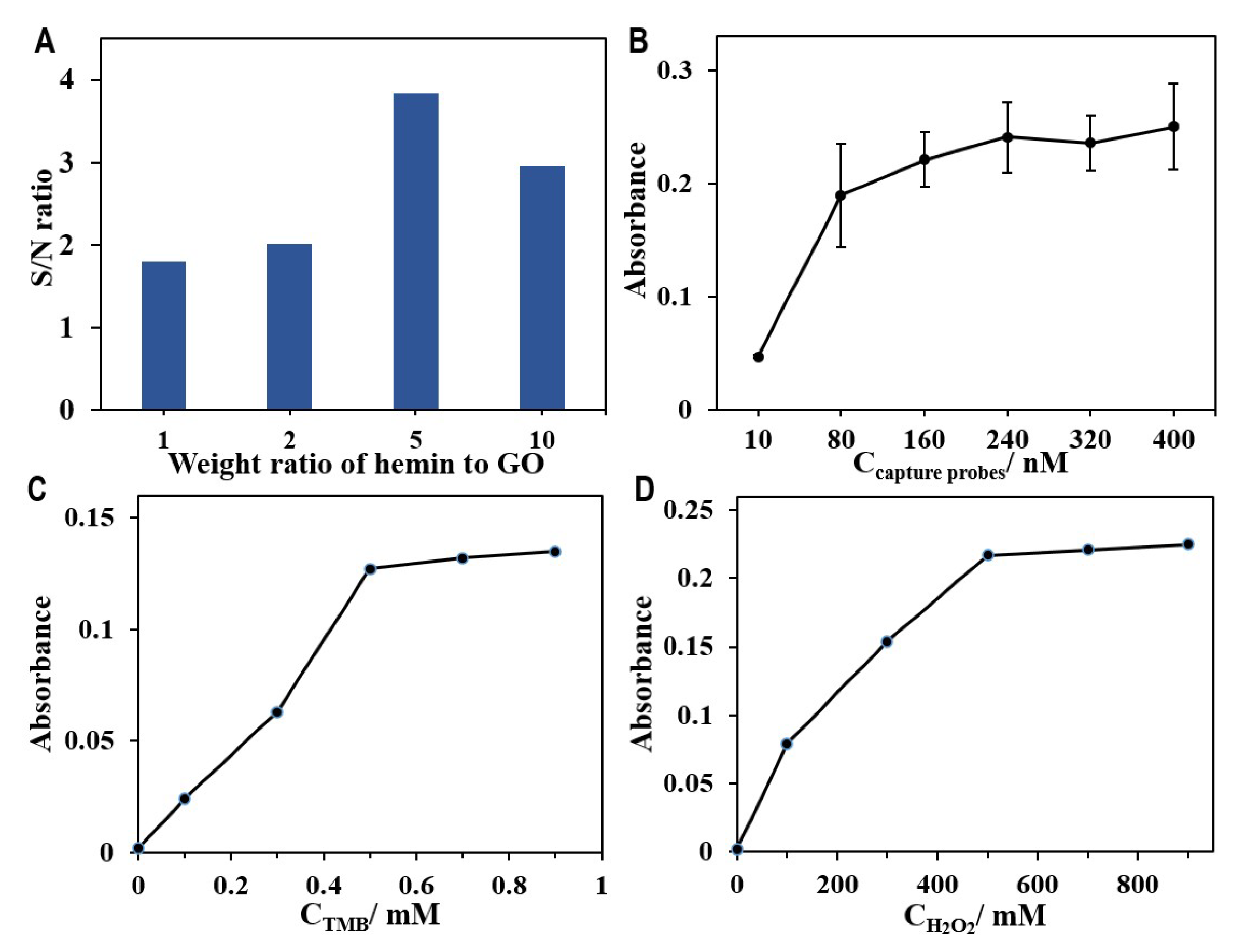
3.3. Optimization of Experimental Conditions for the Aptasensor
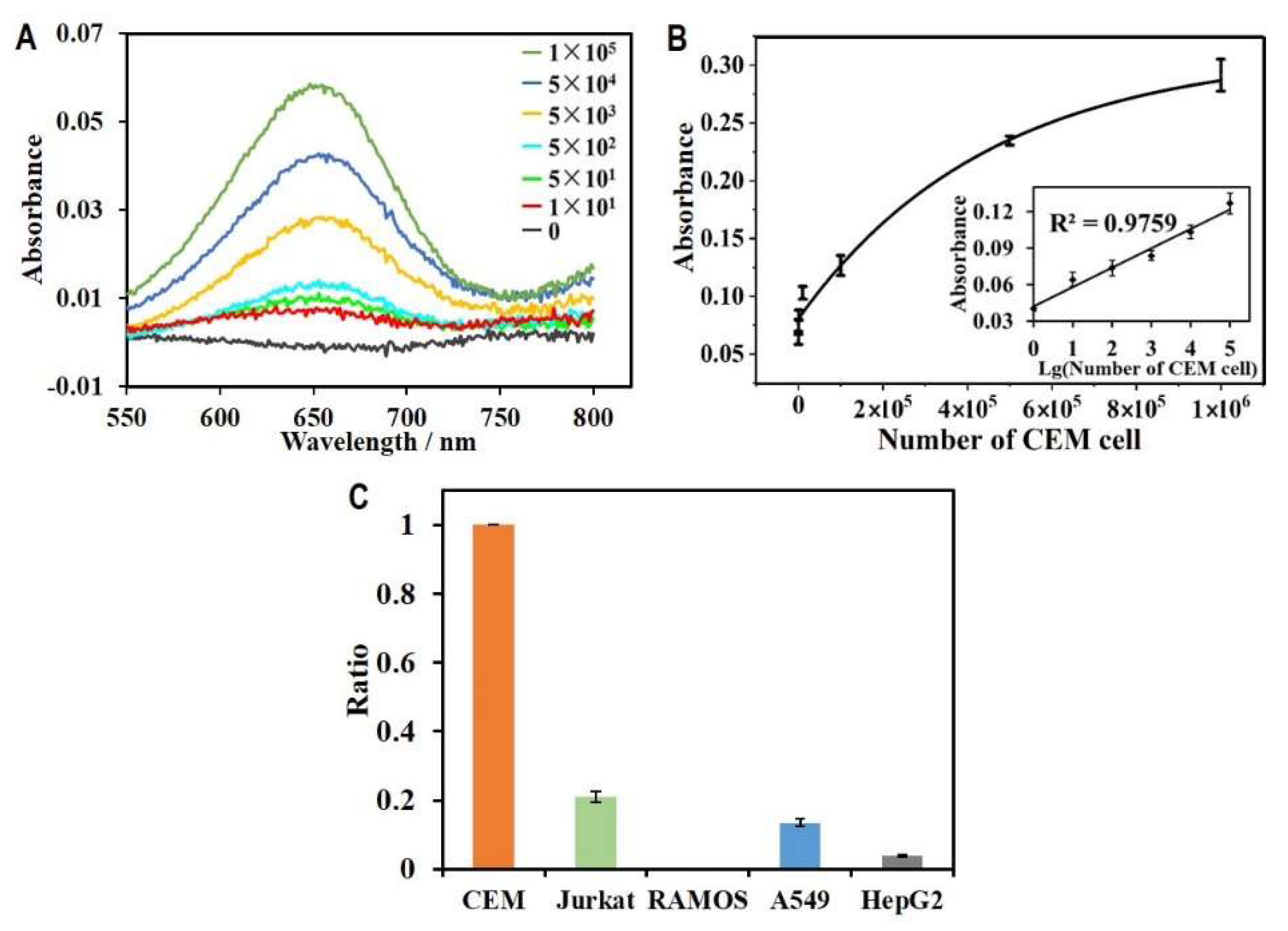
3.4. HGN-Based Aptasensor for Ultrasensitive Detection of CEM Cells
3.5. Specificity Assay of the HGN-Based Aptasensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, A.; Stephens, J.M.; Laskin, B.L.; Pashos, C.L.; Botteman, M.F. The burden and outcomes associated with four leukemias: AML, ALL, CLL and CML. Expert Rev. Anticancer Ther. 2003, 3, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Okikiolu, J.; Dillon, R.; Raj, K. Acute leukaemia. Medicine 2021, 49, 274–281. [Google Scholar] [CrossRef]
- Burnett, A.K.; Eden, O.B. The treatment of acute leukaemia. Lancet 1997, 349, 270–275. [Google Scholar] [CrossRef]
- Campos, L.; Guyotat, D.; Archimbaud, E.; Devaux, Y.; Treille, D.; Larese, A.; Maupas, J.; Gentilhomme, O.; Ehrsam, A.; Fiere, D. Surface marker expression in adult acute myeloid leukaemia: Correlations with initial characteristics, morphology and response to therapy. Br. J. Haematol. 1989, 72, 161–166. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2022, 97, 1236–1256. [Google Scholar] [CrossRef]
- Ladines-Castro, W.; Barragán-Ibañez, G.; Luna-Pérez, M.A.; Santoyo-Sánchez, A.; Collazo-Jaloma, J.; Mendoza-García, E.; Ramos-Peñafiel, C.O. Morphology of leukaemias. Rev. Med. Hosp. Gen. 2016, 79, 107–113. [Google Scholar] [CrossRef]
- Alexander, T.B.; Orgel, E. Mixed Phenotype Acute Leukemia: Current Approaches to Diagnosis and Treatment. Curr. Oncol. Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Bain, B.J.; Béné, M.C. Morphological and Immunophenotypic Clues to the WHO Categories of Acute Myeloid Leukaemia. Acta Haematol. 2019, 141, 232–244. [Google Scholar] [CrossRef]
- Soverini, S.; De Santis, S.; Martelli, M.; Monaldi, C.; Castagnetti, F.; Gugliotta, G.; Papayannidis, C.; Mancini, M.; Bruno, S.; Venturi, C.; et al. Droplet digital PCR for the detection of second-generation tyrosine kinase inhibitor-resistant BCR::ABL1 kinase domain mutations in chronic myeloid leukemia. Leukemia 2022, 36, 2250–2260. [Google Scholar] [CrossRef]
- Fang, X.; Liu, C.; Cheng, X.; Wang, Y.; Yang, Y. A spectral imaging array biosensor and its application in detection of leukemia cell. Sens. Actuators B 2011, 156, 760–764. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Datta, M.; Malhotra, B.D. Prospects of Nanomaterials in Biosensors. Anal. Lett. 2008, 41, 159–209. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, B.D.; Ali, M.A. Chapter 1—Nanomaterials in Biosensors: Fundamentals and Applications. In Nanomaterials for Biosensors; Malhotra, B.D., Ali, M.A., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–74. [Google Scholar]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-M.; Wu, G.-H.; Jiang, Y.-Q.; Wang, Y.-R.; Chen, X. Graphene and graphene-based nanomaterials: The promising materials for bright future of electroanalytical chemistry. Analyst 2011, 136, 4631–4640. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.; Wang, L.; Lv, M.; Tang, Z.; Fan, C. Graphene-based nanomaterials in biosystems. Nano Res. 2019, 12, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kochmann, S.; Hirsch, T.; Wolfbeis, O.S. Graphenes in chemical sensors and biosensors. TrAC, Trends Anal. Chem. 2012, 39, 87–113. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, H.; Wang, Z.; Wang, W.; Ajmal, M.; Xiao, F.; Chen, X.; Liu, H. Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Microchimica Acta 2019, 186, 61. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Jiang, S.; Qu, Y.; Su, Q.; Cheng, R.; Dubin, S.; Chiu, C.-Y.; Kaner, R.; Huang, Y.; Duan, X. Graphene-Supported Hemin as a Highly Active Biomimetic Oxidation Catalyst. Angew. Chem. Int. Ed. 2012, 51, 3822–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Deng, L.; Li, J.; Guo, S.; Wang, E.; Dong, S. Hemin−Graphene Hybrid Nanosheets with Intrinsic Peroxidase-like Activity for Label-free Colorimetric Detection of Single-Nucleotide Polymorphism. ACS Nano 2011, 5, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhong, H.; Zheng, S.; Deng, P.; Li, N.; Yun, W.; Yang, L. A visual detection of bisphenol A based on peroxidase-like activity of hemin–graphene composites and aptamer. Anal. Methods 2018, 10, 2450–2455. [Google Scholar] [CrossRef]
- Bi, S.; Zhao, T.; Jia, X.; He, P. Magnetic graphene oxide-supported hemin as peroxidase probe for sensitive detection of thiols in extracts of cancer cells. Biosens. Bioelectron. 2014, 57, 110–116. [Google Scholar] [CrossRef]
- Liu, G.; Guan, X.; Li, B.; Zhou, H.; Kong, N.; Wang, H. Hemin-graphene oxide-gold nanoflower-assisted enhanced electrochemiluminescence immunosensor for determination of prostate-specific antigen. Microchimica Acta 2022, 189, 297. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Y.; Ni, Y.; Kokot, S. Spectrophotometric analysis of phenols, which involves a hemin–graphene hybrid nanoparticles with peroxidase-like activity. J. Hazard. Mater. 2014, 266, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, S.-J.; Min, D.-H. Emerging Approaches for Graphene Oxide Biosensor. Anal. Chem. 2017, 89, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [Green Version]
- Herr, J.K.; Smith, J.E.; Medley, C.D.; Shangguan, D.; Tan, W. Aptamer-Conjugated Nanoparticles for Selective Collection and Detection of Cancer Cells. Anal. Chem. 2006, 78, 2918–2924. [Google Scholar] [CrossRef]
- Teng, Y.; Shi, J.; Pong, P.W.T. Sensitive and Specific Colorimetric Detection of Cancer Cells Based on Folate-Conjugated Gold–Iron-Oxide Composite Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 7421–7431. [Google Scholar] [CrossRef]
- Shi, H.; Li, D.; Xu, F.; He, X.; Wang, K.; Ye, X.; Tang, J.; He, C. A label-free activatable aptamer probe for colorimetric detection of cancer cells based on binding-triggered in situ catalysis of split DNAzyme. Analyst 2014, 139, 4181–4184. [Google Scholar] [CrossRef]
- Ho, L.-C.; Wu, W.-C.; Chang, C.-Y.; Hsieh, H.-H.; Lee, C.-H.; Chang, H.-T. Aptamer-Conjugated Polymeric Nanoparticles for the Detection of Cancer Cells through “Turn-On” Retro-Self-Quenched Fluorescence. Anal. Chem. 2015, 87, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, J.; Chen, H.; Zhang, S.; Kong, J. A label-free and high-efficient GO-based aptasensor for cancer cells based on cyclic enzymatic signal amplification. Biosens. Bioelectron. 2017, 91, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, A.; Ravan, H.; Mohammadi, A.; Hosseinzadeh, E.; Norouzi, M.; Fozooni, T. Aptamer–integrated DNA nanoassembly: A simple and sensitive DNA framework to detect cancer cells. Anal. Chim. Acta 2018, 1017, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, Q.; Yang, M. Multivalent Duplexed-Aptamer Networks Regulated a CRISPR-Cas12a System for Circulating Tumor Cell Detection. Anal. Chem. 2021, 93, 12921–12929. [Google Scholar] [CrossRef]
- Lei, J.; Shi, L.; Liu, W.; Li, B.; Jin, Y. Portable and sensitive detection of cancer cells via a handheld luminometer. Analyst 2022, 147, 3219–3224. [Google Scholar] [CrossRef]

| Methods/Strategy | Linear Range | Detection Limit/Sensitivity | Ref. |
|---|---|---|---|
| A label-free activatable aptamer probe-based colorimetric detection | 3300–26,900 cells | 3300 cells | [35] |
| Aptamer-conjugated polymeric (1,3-phenylenediamine resin) nanoparticles fluorescent detection | 1500–3 × 104 cells | 44 cells mL−1 | [36] |
| Graphene oxide-based aptasensor fluorescent detection | 50–105 cells | 25 cells | [37] |
| Aptamer-integrated DNA nanoassembly colorimetric detection | 175–1.5 × 104 cells | 175 cells | [38] |
| Multivalent duplexed-aptamer network fluorescent detection | 50–106 cells mL−1 | 26 cells mL−1 | [39] |
| Cell-triggered cyclic strand displacement reaction chemiluminescent detection | 100–5 × 104 cells mL−1 | 85 cells mL−1 | [40] |
| HGN-based colorimetric aptasensor | 10–105 cells | 10 cells | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Zhang, L.; Lai, L.; Zhu, W.; Hu, C. A Hemin–Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment. Biosensors 2022, 12, 1070. https://doi.org/10.3390/bios12121070
Su J, Zhang L, Lai L, Zhu W, Hu C. A Hemin–Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment. Biosensors. 2022; 12(12):1070. https://doi.org/10.3390/bios12121070
Chicago/Turabian StyleSu, Jing, Liqiang Zhang, Luogen Lai, Wufu Zhu, and Chong Hu. 2022. "A Hemin–Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment" Biosensors 12, no. 12: 1070. https://doi.org/10.3390/bios12121070
APA StyleSu, J., Zhang, L., Lai, L., Zhu, W., & Hu, C. (2022). A Hemin–Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment. Biosensors, 12(12), 1070. https://doi.org/10.3390/bios12121070






