GLAD Based Advanced Nanostructures for Diversified Biosensing Applications: Recent Progress
Abstract
1. Introduction
2. Recent Strategies for Fabrication of Nanostructures Using the GLAD Technique
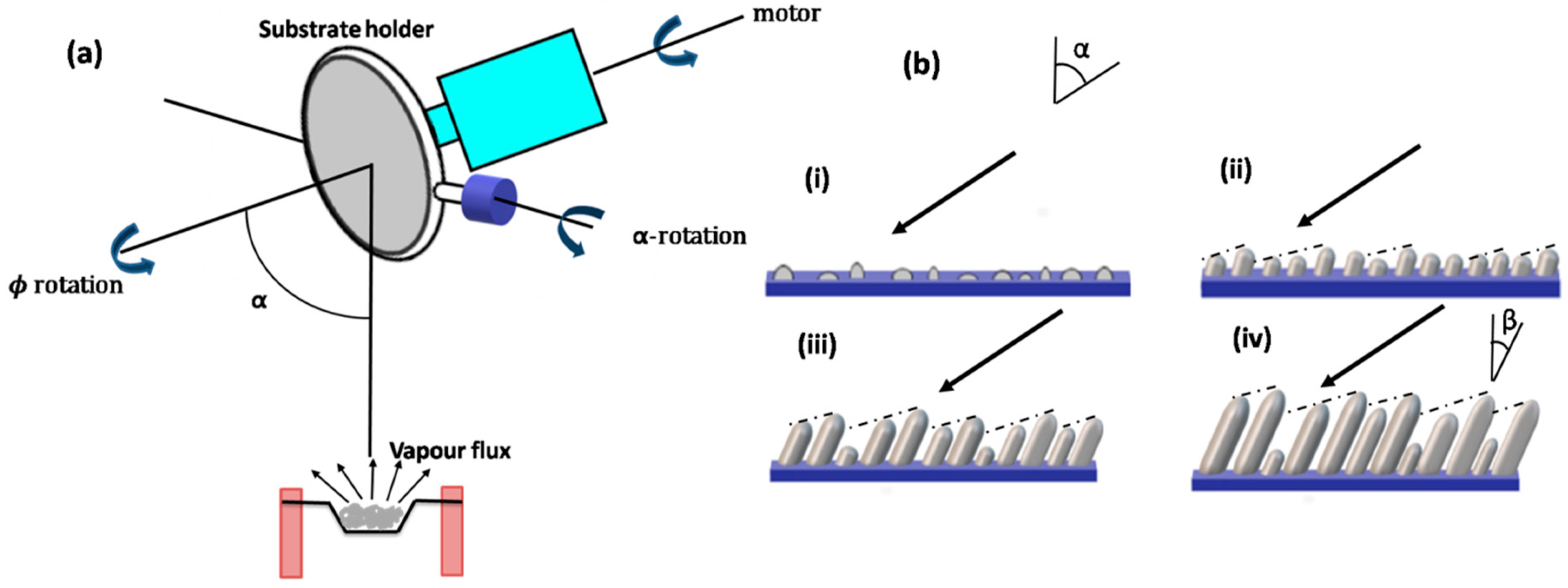
2.1. Setup Configuration of GLAD
2.2. Effect of Various Growth Parameters
2.2.1. Tilt Angle
2.2.2. Azimuthal Rotation (ϕ)
2.3. GLAD-Based Nanostructures
3. Designing Substrates and Chips for Biosensing Applications
3.1. Low-Temperature Biosensing Chips and Arrays
3.2. Flexible Substrates
3.3. Multilayer Substrates
3.4. Patterned Substrates
3.5. Coated and Co-Deposited Nanostructured Thin-Film Substrates
3.6. Recyclable Chip and Substrates
3.7. Microfluidics-Based Substrates
4. Biosensing Applications of GLAD-Fabricated Nanostructures
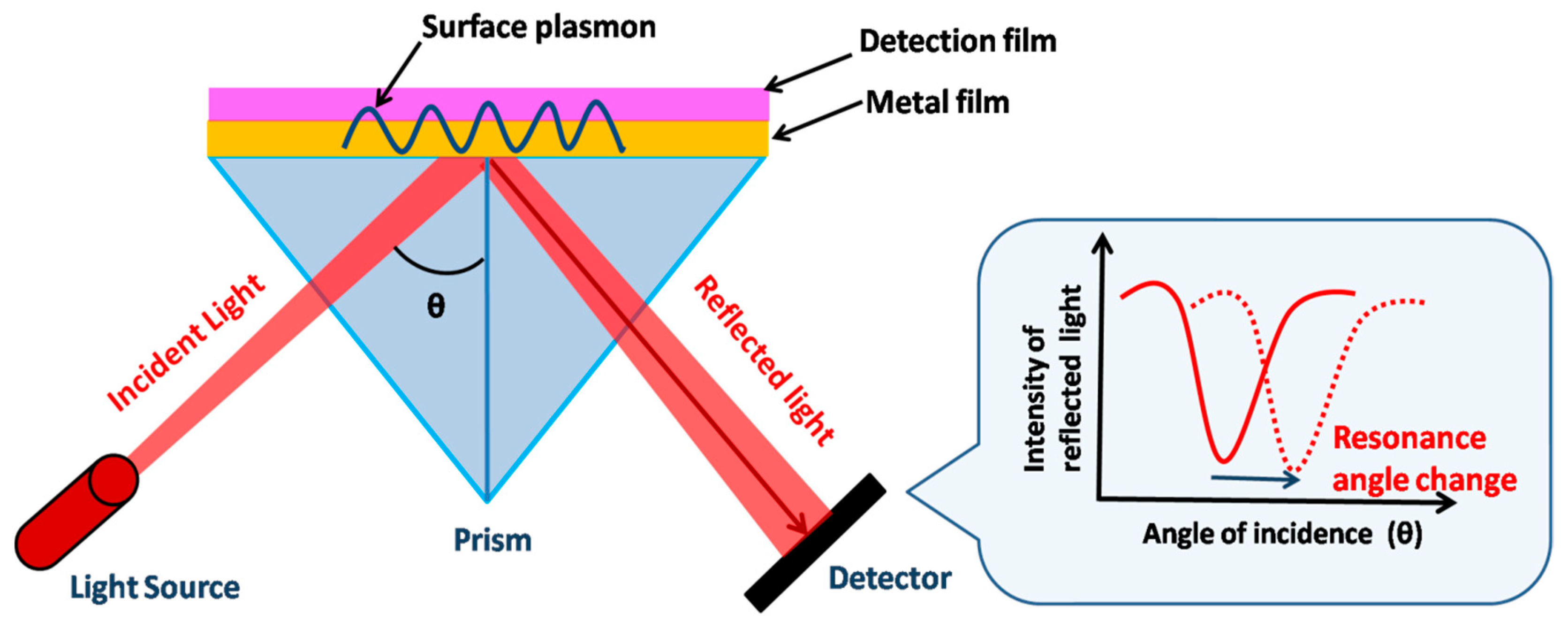
4.1. SPR-Based Detection
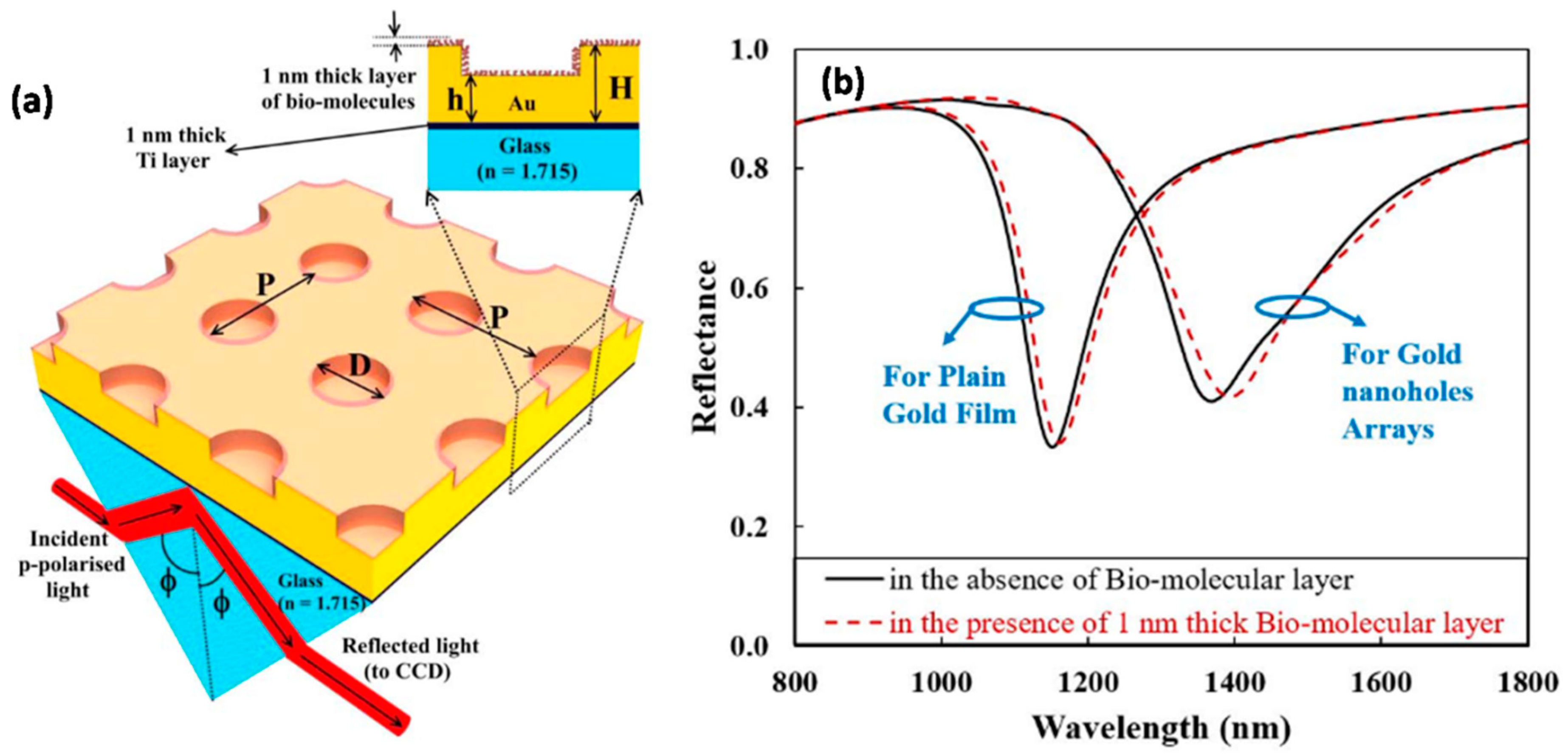
4.1.1. Bi-Material-Based SPR Enhancement
4.1.2. Nanocolumnar Thin-Film-Based SPR Chips
4.1.3. Microfluidics-Based SPR Chips
4.2. SERS-Based Biosensing
4.2.1. Detection of Pathogens
4.2.2. Detection of Biomarkers
4.2.3. Detection of microRNA and Neurotransmitters
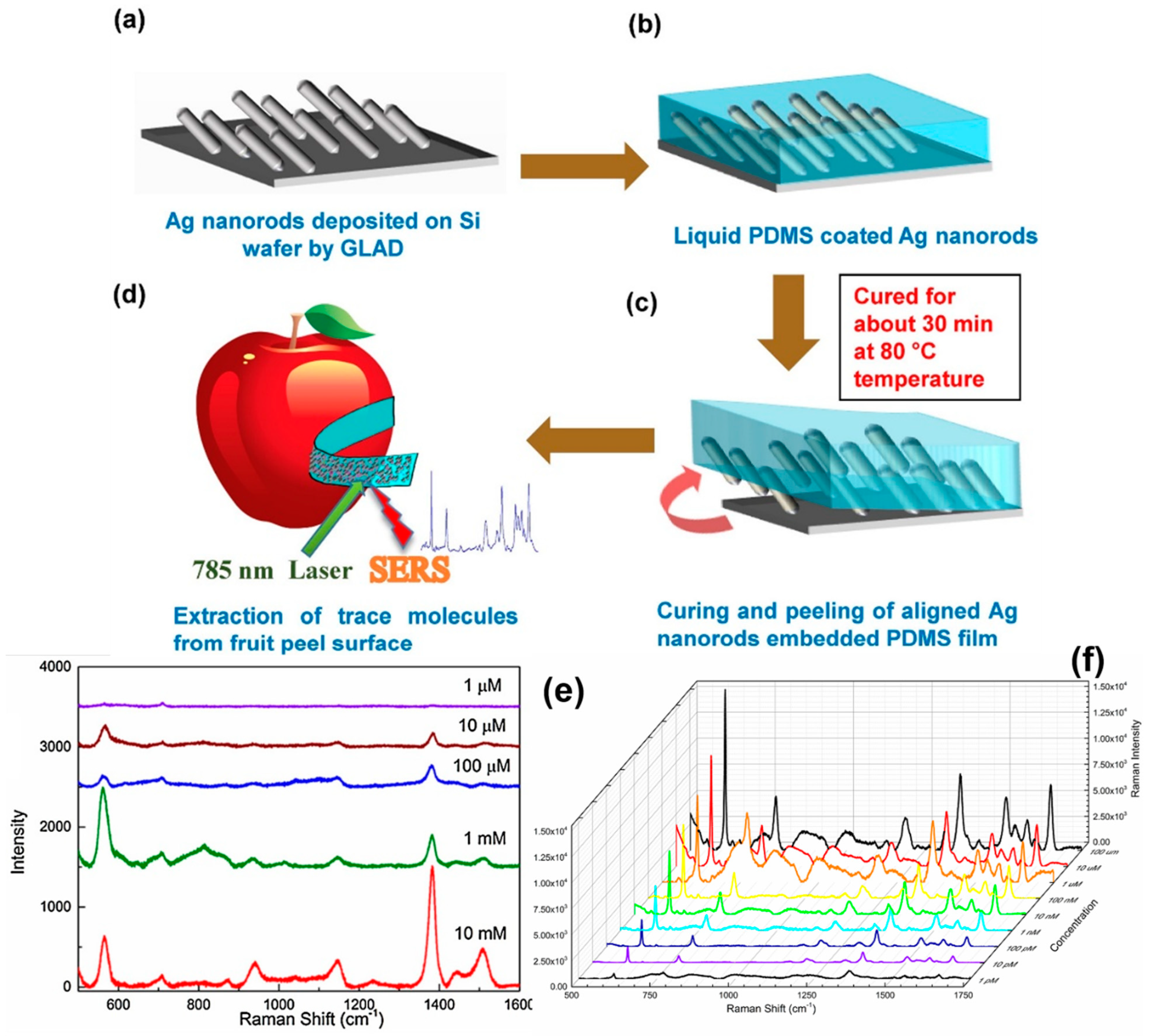
4.2.4. Detection of Pesticides and Chemicals
4.2.5. Detection of Drugs and Food Adulterants
4.3. Fluorescence-Based Biosensing
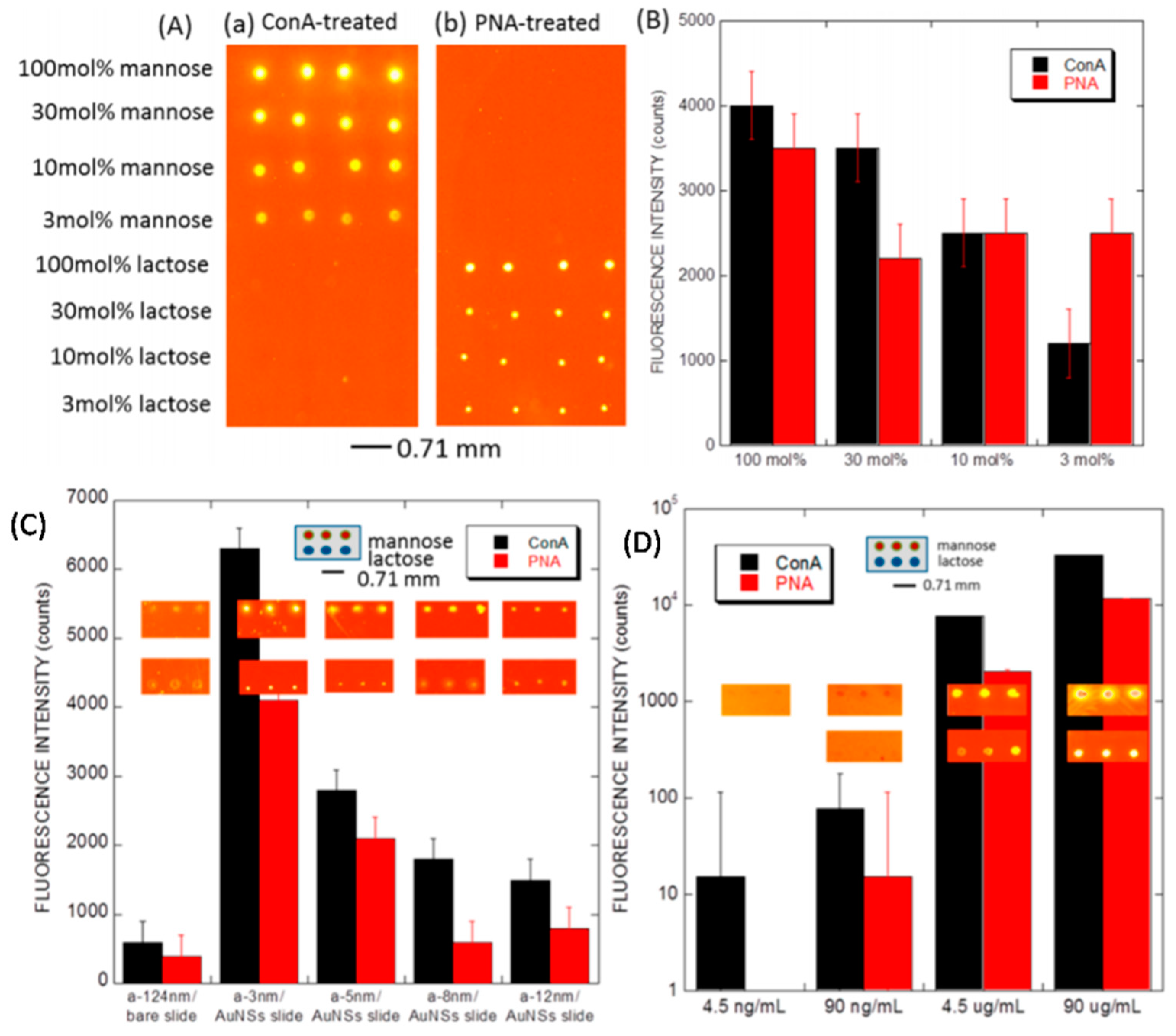
4.3.1. Protein- and DNA-Fluorescence-Enhanced Detection
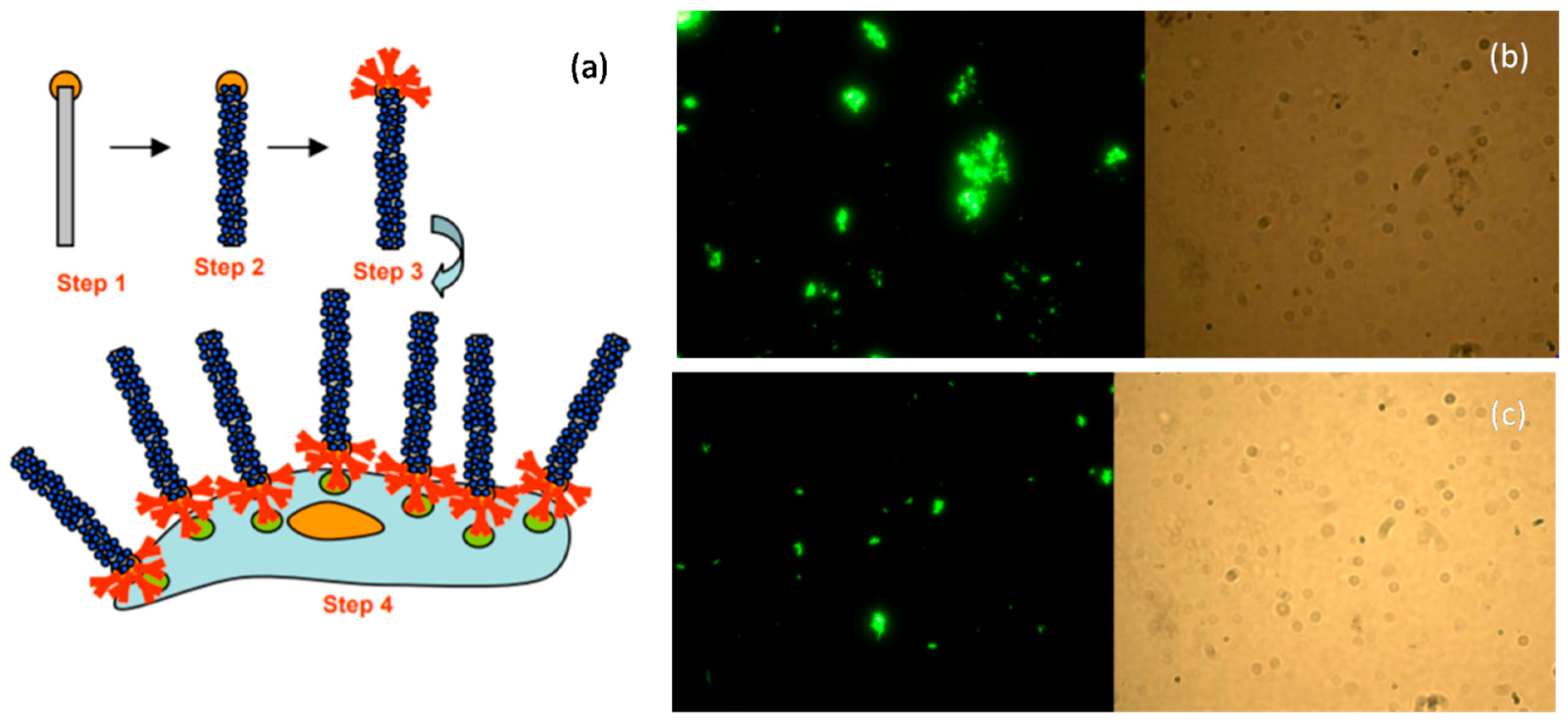
4.3.2. Detection of Pathogens
4.3.3. Single-Molecule and Tissue Detection
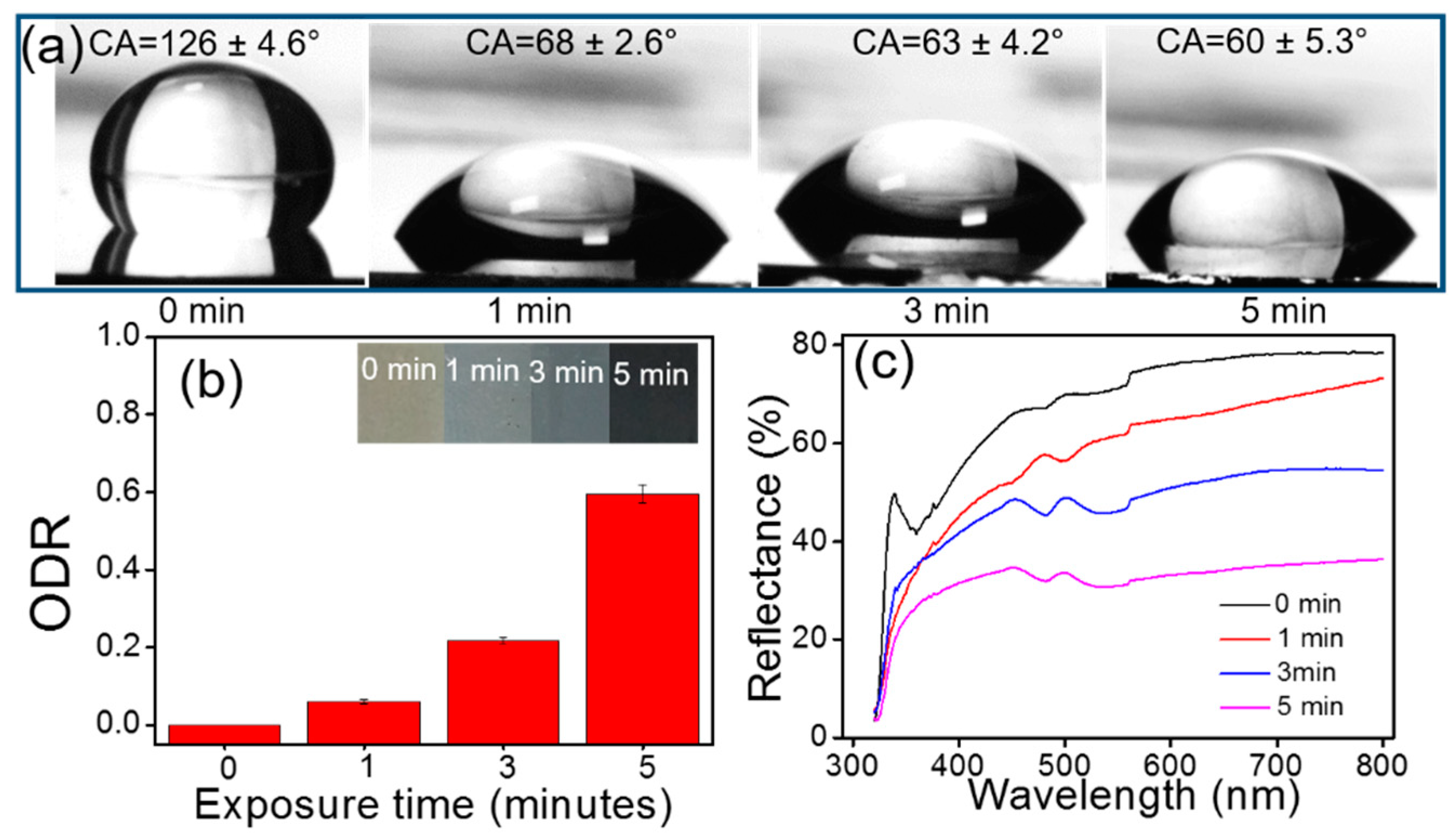
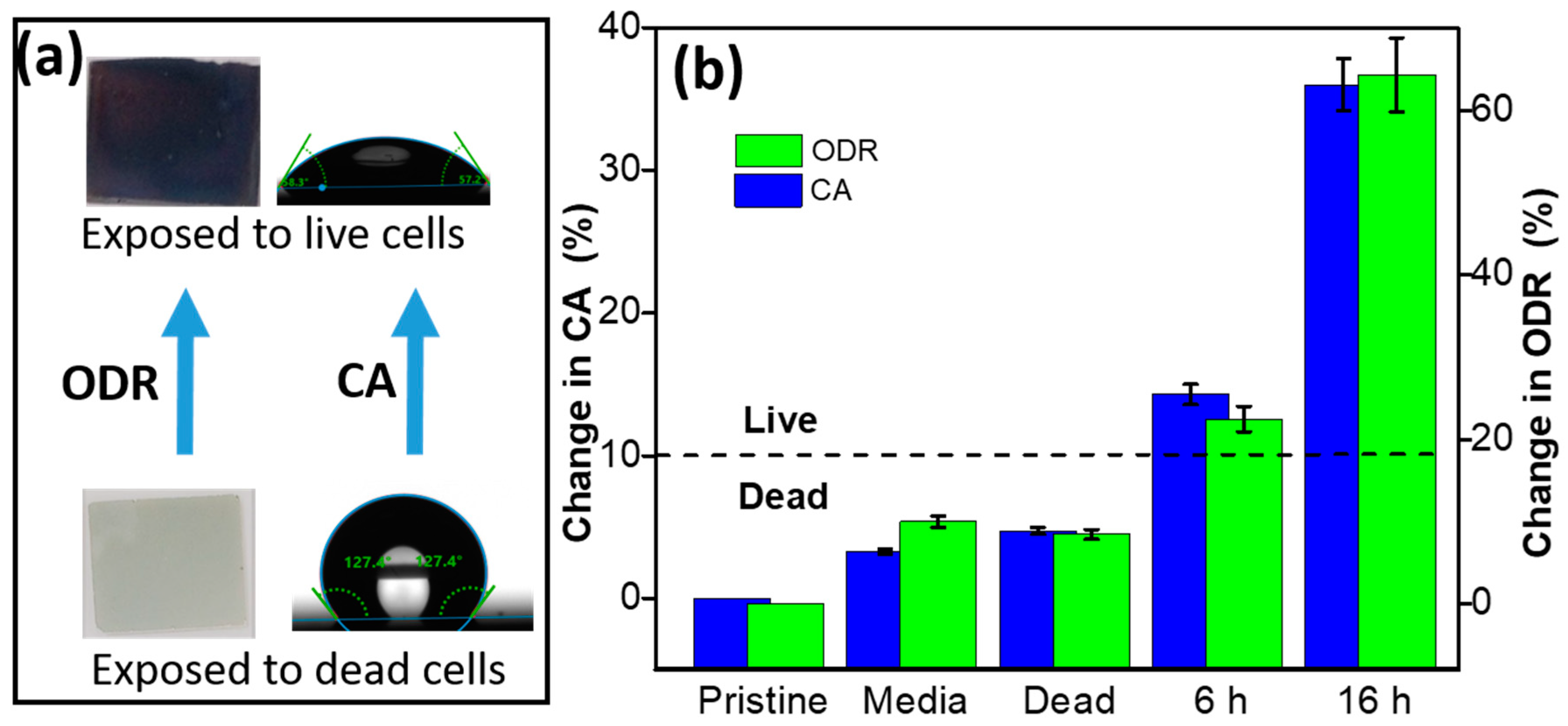
4.4. Colorimetric- and Wettability-Based Detection
4.5. Molecular Imaging
4.5.1. Fluorescence Imaging
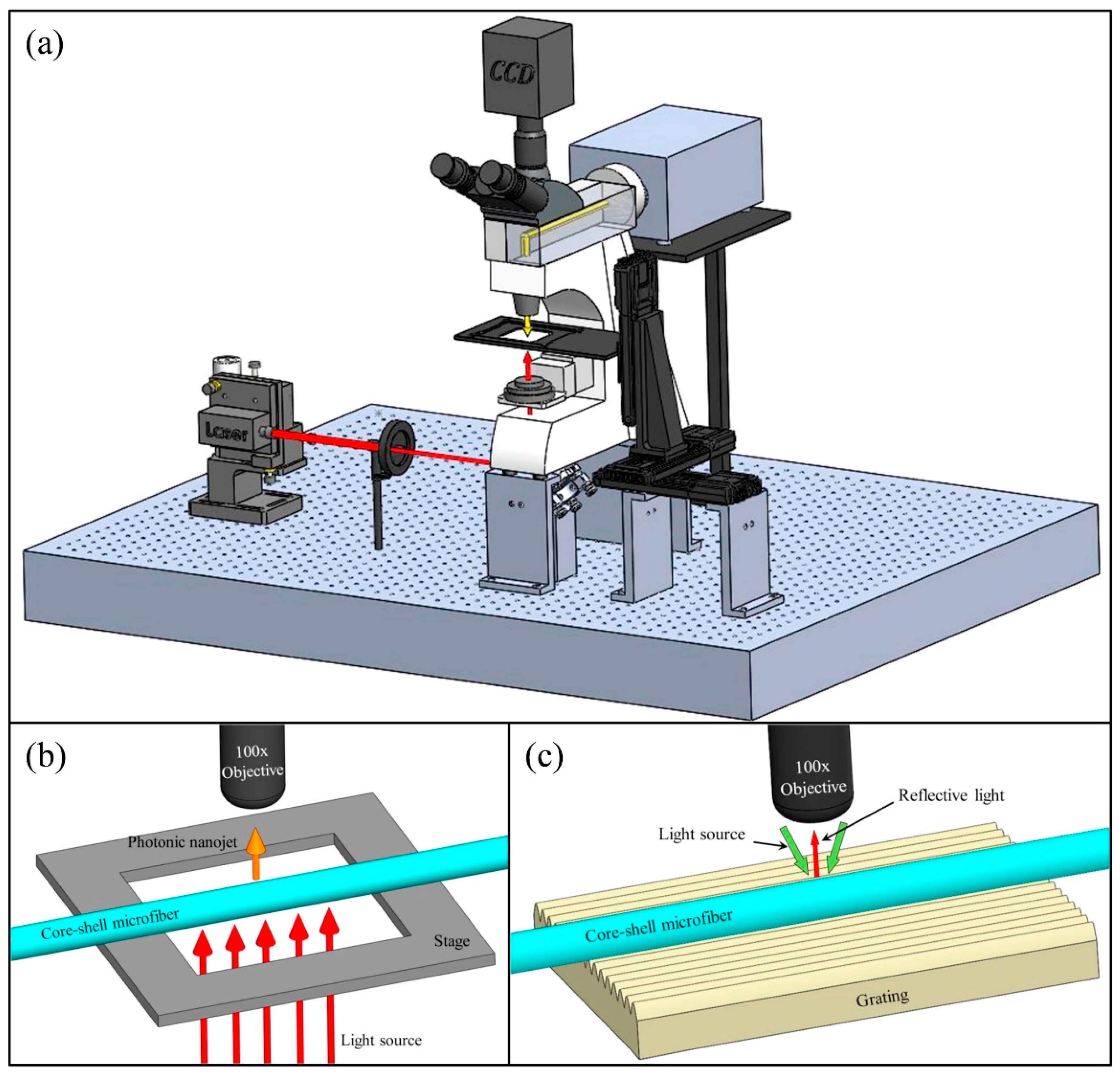
4.5.2. Super-Resolution Imaging
4.5.3. Photoacoustic Imaging (PA)
4.6. In Vivo Application of GLAD
4.7. Optical and Electrochemical GLAD-Based Sensors
5. Limitations of GLAD Technique
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jianrong, C.; Yuqing, M.; Nongyue, H.; Xiaohua, W. Nanotechnology and biosensors. Biotechnol. Adv. 2004, 22, 505–518. [Google Scholar] [CrossRef]
- Biosensors Nanotechnology—Google Books. Available online: https://books.google.co.in/books?hl=en&lr=&id=TG_rAwAAQBAJ&oi=fnd&pg=PA1916&dq=Silver+and+gold+based+nanostructures+for+biosensing&ots=L91KN0-F6r&sig=QFNHttYRTC-r0lIn1_poN68Blik#v=onepage&q=Silver+and+gold+based+nanostructures+for+biosensing&f=false (accessed on 8 July 2020).
- Psychoyios, V.N.; Nikoleli, G.-P.; Tzamtzis, N.; Nikolelis, D.P.; Psaroudakis, N.; Danielsson, B.; Israr, M.Q.; Willander, M. Potentiometric Cholesterol Biosensor Based on ZnO Nanowalls and Stabilized Polymerized Lipid Film. Electroanalysis 2013, 25, 367–372. [Google Scholar] [CrossRef]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. Plasmonics 2014, 9, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, K.; Sun, C. Plasmonics for Biosensing. Materials 2019, 12, 1411. [Google Scholar] [CrossRef]
- Bochenkov, V.; Baumberg, J.; Noginov, M.; Benz, F.; Aldewachi, H.; Schmid, S.; Podolskiy, V.; Aizpurua, J.; Lin, K.; Ebbesen, T.; et al. Applications of Plasmonics: General Discussion. Faraday Discuss. 2015, 178, 435–466. [Google Scholar] [CrossRef][Green Version]
- Robbie, K.; Brett, M.J. Sculptured Thin Films and Glancing Angle Deposition: Growth Mechanics and Applications Sculptured Thin Films and Glancing Angle Deposition: Growth Mechanics and Applications. J. Vac. Sci. Technol. A 1997, 15, 1460. [Google Scholar] [CrossRef]
- Jensen, M.O.; Brett, M.J. Periodically Structured Glancing Angle Deposition Thin Films. IEEE Trans. Nanotechnol. 2005, 4, 269–276. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Taschuk, M.T.; Brett, M.J. Glancing Angle Deposition of Thin Films: Engineering the Nanoscale; John Wiley & Sons: Hoboken, NJ, USA, 2014; Available online: https://www.wiley.com/en-in/Glancing+Angle+Deposition+of+Thin+Films:+Engineering+the+Nanoscale-p-9781118847565 (accessed on 7 July 2020).
- Hawkeye, M.M.; Brett, M.J. Glancing Angle Deposition: Fabrication, Properties, and Applications of Micro- and Nanostructured Thin Films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2007, 25, 1317. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Ye, D.-X.; Wang, G.-C.; Lu, T.-M. Designing Nanostructures by Glancing Angle Deposition. In Nanotubes and Nanowires; SPIE: Bellingham, WA, USA, 2003. [Google Scholar]
- Van Dijken, J.G.; Fleischauer, M.D.; Brett, M.J. Advanced Nanostructuring of Metal Phthalocyanines for Organic Photovoltaic Devices. In Proceedings of the Conference Record of the IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 3469–3471. [Google Scholar]
- Jensen, M.O.; Brett, M.J. Porosity Engineering in Glancing Angle Deposition Thin Films. Appl. Phys. A Mater. Sci. Process. 2005, 80, 763–768. [Google Scholar] [CrossRef]
- Robbie, K.; Sit, J.C.; Brett, M.J. Advanced Techniques for Glancing Angle Deposition. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1998, 16, 1115–1122. [Google Scholar] [CrossRef]
- Taghavi, A.; Rahbarizadeh, F.; Abbasian, S.; Moshaii, A. Label-Free LSPR Prostate-Specific Antigen Immune-Sensor Based on GLAD-Fabricated Silver Nano-Columns. Plasmonics 2020, 15, 753–760. [Google Scholar] [CrossRef]
- González-García, L.; González-Valls, I.; Lira-Cantu, M.; Barranco, A.; González-Elipe, A.R. Aligned TiO 2 Nanocolumnar Layers Prepared by PVD-GLAD for Transparent Dye Sensitized Solar Cells. Energy Environ. Sci. 2011, 4, 3426–3435. [Google Scholar] [CrossRef]
- Sit, J.C.; Broer, D.J.; Brett, M.J. Optical Devices Fabricated from Porous Thin Films Embedded with Liquid Crystals. In Proceedings of the Technical Digest—International Electron Devices Meeting, Washington, DC, USA, 5–8 December 1999; IEEE: Piscataway, NJ, USA, 1999; pp. 123–126. [Google Scholar]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic Concepts and Recent Advances in Nitrophenol Reduction by Gold- and Other Transition Metal Nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications-A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Uptake of Engineered Gold Nanoparticles into Mammalian Cells. Chem. Rev. 2014, 114, 1258–1288. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Chen, T.; Xie, J. Mechanistic Exploration and Controlled Synthesis of Precise Thiolate-Gold Nanoclusters. Coord. Chem. Rev. 2016, 329, 1–15. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Kolwas, K.; Derkachova, A. Plasmonic Abilities of Gold and Silver Spherical Nanoantennas in Terms of Size Dependent Multipolar Resonance Frequencies and Plasmon Damping Rates. Opto-Electron. Rev. 2010, 18, 429–437. [Google Scholar] [CrossRef][Green Version]
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D. Recent Advances in Silver Nanostructured Substrates for Plasmonic Sensors. Biosensors 2022, 12, 713. [Google Scholar] [CrossRef]
- Namura, K.; Hanai, S.; Kondo, S.; Kumar, S.; Suzuki, M. Gold Micropetals Self-Assembled by Shadow-Sphere Lithography for Optofluidic Control. Adv. Mater. Interfaces 2022, 9, 2200200. [Google Scholar] [CrossRef]
- Moores, A.; Goettmann, F. The Plasmon Band in Noble Metal Nanoparticles: An Introduction to Theory and Applications. New J. Chem. 2006, 30, 1121–1132. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticels: Synthesis, properites, toxicology, applications and perspective. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 03300. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, W.; Tan, Y.; Ding, S. A Label-Free Biosensor Based on Gold Nanoshell Monolayers for Monitoring Biomolecular Interactions in Diluted Whole Blood. Biosens. Bioelectron. 2008, 23, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Chilkoti, A. Label-Free Biosensing by Surface Plasmon Resonance of Nanoparticles on Glass: Optimization of Nanoparticle Size. Anal. Chem. 2004, 76, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Song, J.; Li, M.X.; Zhang, T.T.; Zhao, W.; Xu, J.J.; Liu, M.; Chen, H.Y. Ultrasensitive MicroRNA Assay via Surface Plasmon Resonance Responses of Au@Ag Nanorods Etching. Anal. Chem. 2017, 89, 10585–10591. [Google Scholar] [CrossRef] [PubMed]
- Gish, D.A.; Nsiah, F.; McDermott, M.T.; Brett, M.J. Localized Surface Plasmon Resonance Biosensor Using Silver Nanostructures Fabricated by Glancing Angle Deposition. Anal. Chem. 2007, 79, 4228–4232. [Google Scholar] [CrossRef]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.; Dluhy, R.; Tripp, R.A. Rapid and Sensitive Detection of Respiratory Virus Molecular Signatures Using a Silver Nanorod Array SERS Substrate. Nano Lett. 2006, 6, 2630–2636. [Google Scholar] [CrossRef]
- Deng, J.; Jin, Y.; Wang, L.; Chen, G.; Zhang, C. Sensitive Detection of Endonuclease Activity and Inhibition Using Gold Nanorods. Biosens. Bioelectron. 2012, 34, 144–150. [Google Scholar] [CrossRef]
- Qaddare, S.H.; Salimi, A. Amplified Fluorescent Sensing of DNA Using Luminescent Carbon Dots and AuNPs/GO as a Sensing Platform: A Novel Coupling of FRET and DNA Hybridization for Homogeneous HIV-1 Gene Detection at Femtomolar Level. Biosens. Bioelectron. 2017, 89, 773–780. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Savargaonkar, D.; Sharan, C.; Yadav, S.; Mishra, P.; Singh, J.P. SERS Platform for Dengue Diagnosis from Clinical Samples Employing a Hand Held Raman Spectrometer. Anal. Chem. 2020, 92, 2527–2534. [Google Scholar] [CrossRef]
- Goel, P.; Kumar, S.; Sarkar, J.; Singh, J.P. Mechanical Strain Induced Tunable Anisotropic Wetting on Buckled PDMS Silver Nanorods Arrays. ACS Appl. Mater. Interfaces 2015, 7, 8419–8426. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Kalyani, N.; Sharan, C.; Mishra, P.; Singh, J.P. Smartphone Based Dual Mode in Situ Detection of Viability of Bacteria Using Ag Nanorods Array. Biosens. Bioelectron. 2019, 126, 478–484. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Brett, M.J. Optimized Colorimetric Photonic-Crystal Humidity Sensor Fabricated Using Glancing Angle Deposition. Adv. Funct. Mater. 2011, 21, 3652–3658. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.A.; Bell, G.R.; Rourke, J.P.; Sanchez, A.M.; Elkin, M.D.; Hickey, B.J.; Wilson, N.R. Physical Vapor Deposition of Metal Nanoparticles on Chemically Modified Graphene: Observations on Metal-Graphene Interactions. Small 2011, 7, 3202–3210. [Google Scholar] [CrossRef]
- Mattox, D.M. Physical Vapor Deposition (PVD) Processes. Met. Finish. 2000, 98, 410–423. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Ibrahim, A.A.; Riad, A.S. Physical Properties of Thermal Coating CdS Thin Films Using a Modified Evaporation Source. Thin Solid Film. 2000, 372, 144–148. [Google Scholar] [CrossRef]
- Messier, R.; Venugopal, V.C.; Sunal, P.D. Origin and Evolution of Sculptured Thin Films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 1538–1545. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Jing, K. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Kumar, S.; Goel, P.; Singh, D.P.; Singh, J.P. Fabrication of Superhydrophobic Silver Nanorods Array Substrate Using Glancing Angle Deposition. AIP Conf. Proc. 2015, 1591, 872. [Google Scholar] [CrossRef]
- Maithani, Y.; Choudhuri, B.; Mehta, B.R.; Singh, J.P. Self-Adhesive, Stretchable, and Dry Silver Nanorods Embedded Polydimethylsiloxane Biopotential Electrodes for Electrocardiography. Sens. Actuators A Phys. 2021, 332, 113068. [Google Scholar] [CrossRef]
- Suzuki, M.; Taga, Y. Integrated Sculptured Thin Films. Jpn. J. Appl. Phys. 2001, 40, L358. [Google Scholar] [CrossRef]
- Ratsch, C.; Venables, J.A. Nucleation Theory and the Early Stages of Thin Film Growth. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, S96–S109. [Google Scholar] [CrossRef]
- Dirks, A.G.; Leamy, H.J. Columnar Microstructure in Vapor-Deposited Thin Films. Thin Solid Film. 1977, 47, 219–233. [Google Scholar] [CrossRef]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and Characterization of Columnar Growth in Evaporated Films. Thin Solid Film. 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Nakhodkin, N.G.; Shaldervan, A.I. Effect of Vapour Incidence Angles on Profile and Properties of Condensed Films. Thin Solid Film. 1972, 10, 109–122. [Google Scholar] [CrossRef]
- Salazar, P.; Rico, V.; Rodríguez-Amaro, R.; Espinós, J.P.; González-Elipe, A.R. New Copper Wide Range Nanosensor Electrode Prepared by Physical Vapor Deposition at Oblique Angles for the Non-Enzimatic Determination of Glucose. Electrochim. Acta 2015, 169, 195–201. [Google Scholar] [CrossRef]
- Yadav, S.; Senapati, S.; Desai, D.; Gahlaut, S.; Kulkarni, S.; Singh, J.P. Portable and Sensitive Ag Nanorods Based SERS Platform for Rapid HIV-1 Detection and Tropism Determination. Colloids Surf. B Biointerfaces 2021, 198, 111477. [Google Scholar] [CrossRef]
- Zhang, N.; Su, X.; Free, P.; Zhou, X.; Neoh, K.G.; Teng, J.; Knoll, W. Plasmonic Metal Nanostructure Array by Glancing Angle Deposition for Biosensing Application. Sens. Actuators B Chem. 2013, 183, 310–318. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Member, S.; Yadav, S.; Dhawan, A. Enhanced Sensitivity of SPR Biological Sensor Based on Nanohole Arrays in Gold Films. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology, Cork, Ireland, 23–26 July 2018; pp. 1–4. [Google Scholar]
- Almaguer-Flores, A.; Ximénez-Fyvie, L.A.; Rodil, S.E. Oral Bacterial Adhesion on Amorphous Carbon and Titanium Films: Effect of Surface Roughness and Culture Media. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92B, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, E.; Fasching, R.; Varahram, M.; Jobst, G.; Urban, G.; Nicolussi, G.; Husinsky, W.; Friedbacher, G.; Grasserbauer, M. Surface Modification of Platinum Thin Film Electrodes towards a Defined Roughness and Microporosity. J. Electroanal. Chem. 1997, 426, 157–165. [Google Scholar] [CrossRef]
- Steele, J.J.; Brett, M.J. Nanostructure Engineering in Porous Columnar Thin Films: Recent Advances. J. Mater. Sci. Mater. Electron. 2007, 18, 367–379. [Google Scholar] [CrossRef]
- Abdulhalim, I. Plasmonic Sensing Using Metallic Nano-Sculptured Thin Films. Small 2014, 10, 3499–3514. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Park, B.; Siragusa, G.; Jones, L.; Tripp, R.; Zhao, Y.; Cho, Y.J. An Au/Si Hetero-Nanorod-Based Biosensor for Salmonella Detection. Nanotechnology 2008, 19, 155502. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Tomar, M.; Gupta, V. Glad Assisted Synthesis of NiO Nanorods for Realization of Enzymatic Reagentless Urea Biosensor. Biosens. Bioelectron. 2014, 52, 196–201. [Google Scholar] [CrossRef]
- Yadav, S.; Khanam, R.; Singh, J.P. A Purview into Highly Sensitive Magnetic SERS Detection of Hemozoin Biomarker for Rapid Malaria Diagnosis. Sens. Actuators B Chem. 2022, 355, 131303. [Google Scholar] [CrossRef]
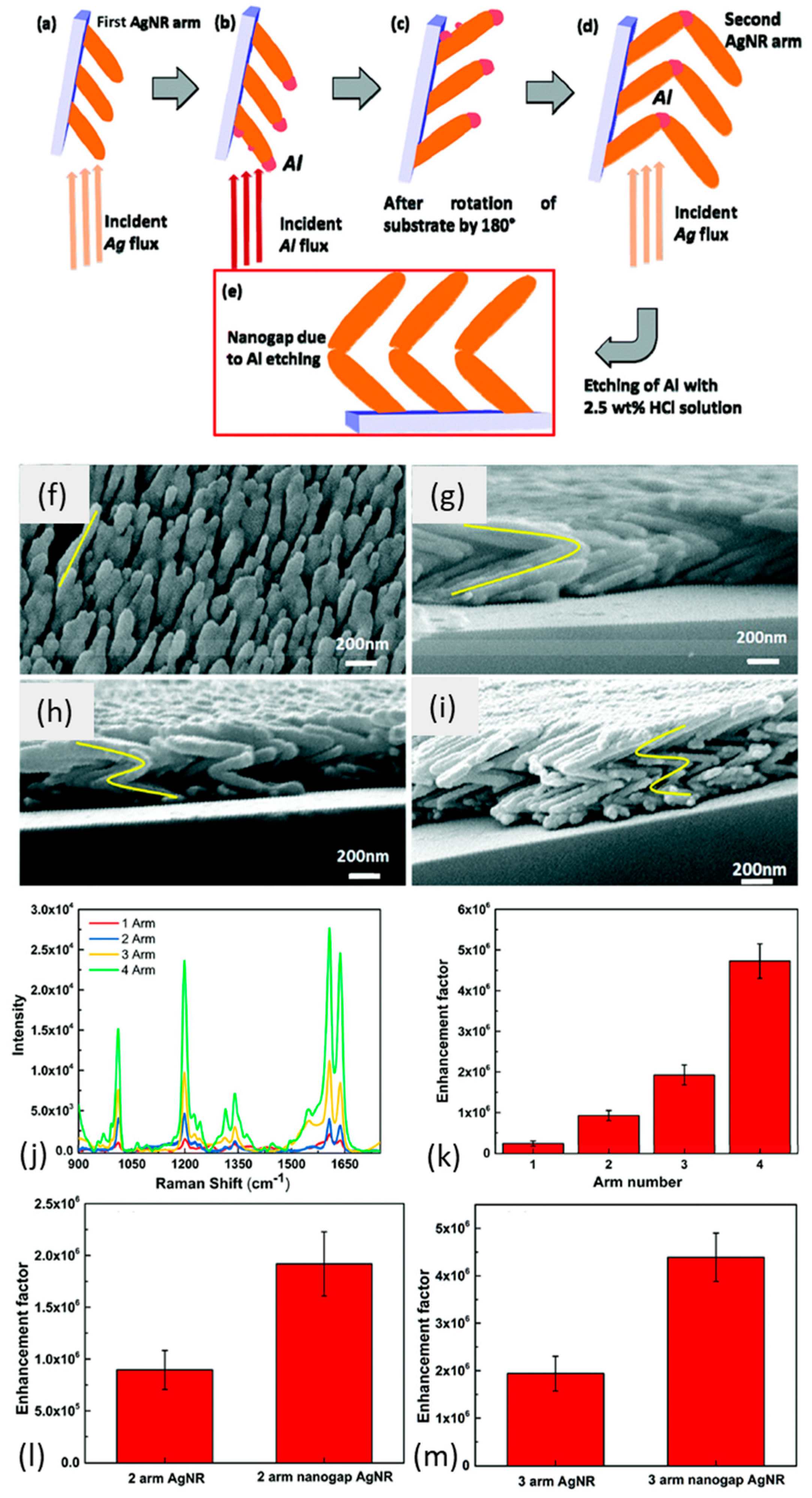
- Rajput, A.; Kumar, S.; Singh, J.P. Vertically Standing Nanoporous Al-Ag Zig-Zag Silver Nanorod Arrays for Highly Active SERS Substrates. Analyst 2017, 142, 3959–3966. [Google Scholar] [CrossRef]
- Yarbakht, M.; Nikkhah, M.; Moshaii, A.; Abbasian, S.; Dellith, A. Fabrication of Silver Chevron Arrays as an Efficient and Stable SERS Substrate: Implications in Biological Sensing. Plasmonics 2018, 13, 715–726. [Google Scholar] [CrossRef]
- Dick, B.; Brett, M.J.; Smy, T. Investigation of Substrate Rotation at Glancing Incidence on Thin-Film Morphology. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2003, 21, 2569–2575. [Google Scholar] [CrossRef]
- Kumar, S.; Gahlaut, S.K.; Singh, J.P. Sculptured Thin Films: Overcoming the Limitations of Surface-Enhanced Raman Scattering Substrates. Appl. Surf. Sci. Adv. 2022, 12, 100322. [Google Scholar] [CrossRef]
- Kim, K.; Lee, K.J.; Jo, N.R.; Jo, E.J.; Shin, Y.B.; Kim, M.G. Wafer-Scale LSPR Substrate: Oblique Deposition of Gold on a Patterned Sapphire Substrate. Biosensors 2022, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Jen, Y.-J.; Huang, J.-W.; Liu, W.-C.; Chan, S.; Tseng, C.-H. Glancing Angle Deposited Gold Nanohelix Arrays on Smooth Glass as Three-Dimensional SERS Substrates. Opt. Mater. Express 2016, 6, 697. [Google Scholar] [CrossRef]
- Martínez, J.L.; Gao, Y.; Lopez-Ríos, T.; Wirgin, A. Anisotropic Surface-Enhanced Raman Scattering at Obliquely Evaporated Ag Films. Phys. Rev. B 1987, 35, 9481–9488. [Google Scholar] [CrossRef]
- Martínez, J.L.; Gao, Y.; Lãpez-Ríos, T. Surface-Enhanced Raman Scattering of Obliquely Evaporated Ag Films. Phys. Rev. B 1986, 33, 5917–5919. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Chaney, S.B.; Zhang, Z.Y. Absorbance Spectra of Aligned Ag Nanorod Arrays Prepared by Oblique Angle Deposition. J. Appl. Phys. 2006, 100, 063527. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhao, Y.P. Extinction Spectra and Electrical Field Enhancement of Ag Nanorods with Different Topologic Shapes. J. Appl. Phys. 2007, 102, 113308. [Google Scholar] [CrossRef]
- Oh, M.K.; Shin, Y.S.; Lee, C.L.; De, R.; Kang, H.; Yu, N.E.; Kim, B.H.; Kim, J.H.; Yang, J.K. Morphological and SERS Properties of Silver Nanorod Array Films Fabricated by Oblique Thermal Evaporation at Various Substrate Temperatures. Nanoscale Res. Lett. 2015, 10, 259. [Google Scholar] [CrossRef]
- Singh, J.P.; Lanier, T.E.; Zhu, H.; Dennis, W.M.; Tripp, R.A.; Zhao, Y. Highly Sensitive and Transparent Surface Enhanced Raman Scattering Substrates Made by Active Coldly Condensed Ag Nanorod Arrays. J. Phys. Chem. C 2012, 116, 20550–20557. [Google Scholar] [CrossRef]
- Singh, J.P.; Chu, H.; Abell, J.; Tripp, R.A.; Zhao, Y. Flexible and Mechanical Strain Resistant Large Area SERS Active Substrates. Nanoscale 2012, 4, 3410–3414. [Google Scholar] [CrossRef]
- Kumar, S.; Goel, P.; Singh, J.P. Flexible and Robust SERS Active Substrates for Conformal Rapid Detection of Pesticide Residues from Fruits. Sens. Actuators B Chem. 2017, 241, 577–583. [Google Scholar] [CrossRef]
- Kumar, S.; Lodhi, D.K.; Goel, P.; Neeti, N.; Mishra, P.; Singh, J.P. A Facile Method for Fabrication of Buckled PDMS Silver Nanorod Arrays as Active 3D SERS Cages for Bacterial Sensing. Chem. Commun. 2015, 51, 12411–12414. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Z.; Wang, Y.; Wang, B.; Zhan, Q.; Zhang, Y.; Ao, X. Broadband SERS Substrates by Oblique Angle Deposition Method. Opt. Mater. Express 2016, 6, 2644. [Google Scholar] [CrossRef]
- Suzuki, M.; Maekita, W.; Wada, Y.; Nakajima, K.; Kimura, K.; Fukuoka, T.; Mori, Y. Surface Enhanced Raman Scattering on Physically Self-Assembled Ag Nanorod Arrays. MRS Online Proc. Libr. 2005, 900, 217–222. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakajima, K.; Kimura, K.; Fukuoka, T.; Mori, Y. Au Nanorod Arrays Tailored for Surface-Enhanced Raman Spectroscopy. Anal. Sci. 2007, 23, 829–833. [Google Scholar] [CrossRef]
- Bužavaitė-Vertelienė, E.; Maciulis, V.; Anulytė, J.; Tolenis, T.; Baskys, A.; Plikusiene, I.; Balevičius, Z. Total Internal Reflection Ellipsometry Approach for Bloch Surface Waves Biosensing Applications. Biosensors 2022, 12, 584. [Google Scholar] [CrossRef]
- Kumar, S.; Kanagawa, M.; Namura, K.; Fukuoka, T.; Suzuki, M. Multilayer Thin-Film Flake Dispersion Gel for Surface-Enhanced Raman Spectroscopy. Appl. Nanosci. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Sharma, Y.; Dhawan, A. Plasmonic “Nano-Fingers on Nanowires” as SERS Substrates. Opt. Lett. 2016, 41, 2085. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Sathish, S.; Galvin, C.J.; Campbell, R.A.; Sinha, A.; Shen, A.Q. Plasma-Assisted Large-Scale Nanoassembly of Metal-Insulator Bioplasmonic Mushrooms. ACS Appl. Mater. Interfaces 2018, 10, 219–226. [Google Scholar] [CrossRef]
- Patzig, C.; Zajadacz, J.; Zimmer, K.; Fechner, R.; Khare, C.; Rauschenbach, B. Patterning Concept for Sculptured Nanostructures with Arbitrary Periods. Appl. Phys. Lett. 2009, 95, 103107. [Google Scholar] [CrossRef]
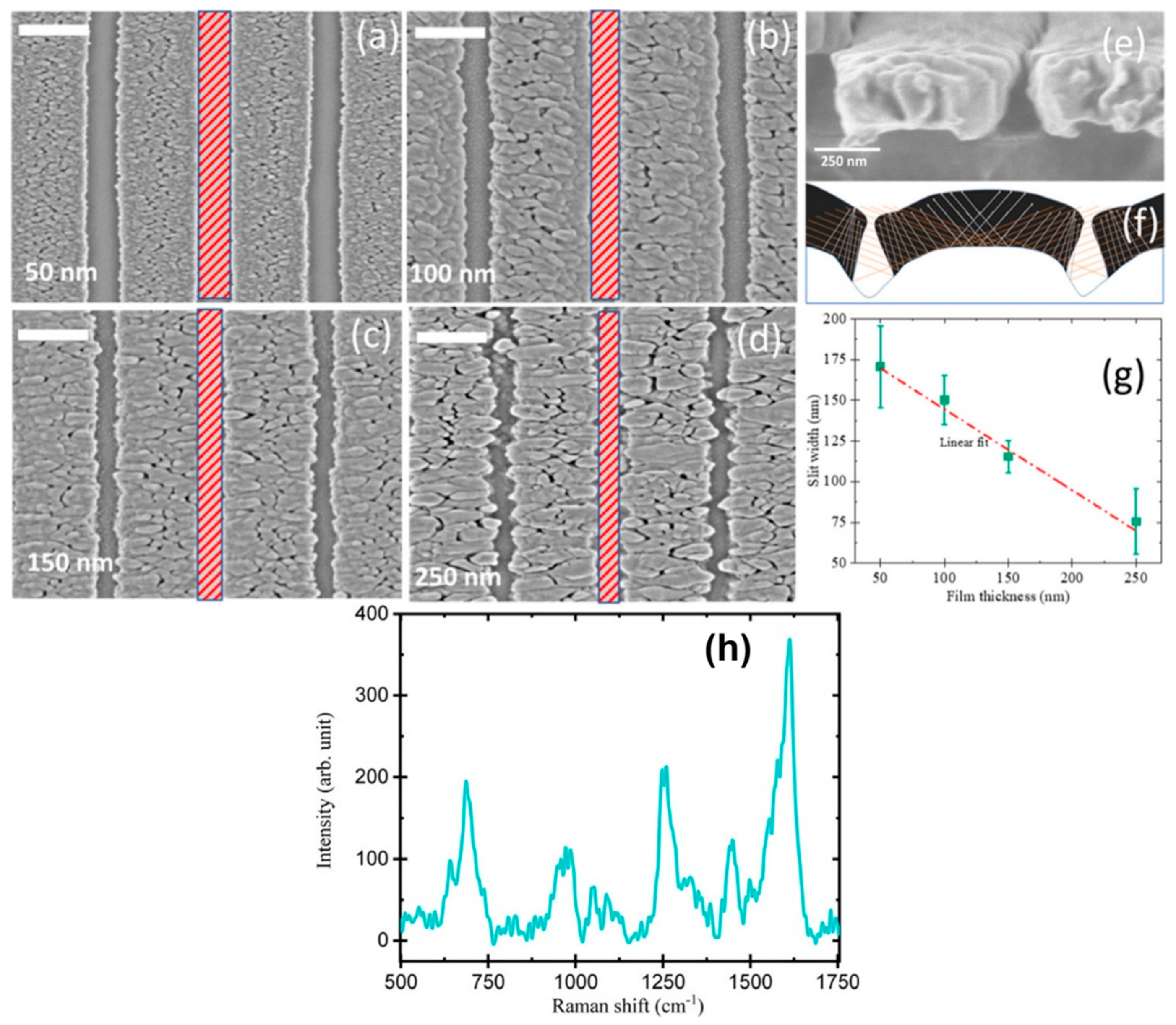
- Kumar, S.; Doi, Y.; Namura, K.; Suzuki, M. Plasmonic Nanoslit Arrays Fabricated by Serial Bideposition: Optical and Surface-Enhanced Raman Scattering Study. ACS Appl. Bio Mater. 2020, 3, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Cao, Z.; Huang, Z.; Xu, Z.; Fu, J.; Yobas, L. Microfluidic Integration of Plasmonic Applications for Highly Sensitive Bioanalysis. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2013), Freiburg, Germany, 27–31 October 2013; Zengerle, R., Ed.; Curran: Freiburg, Germany, 2014; Volume 2. [Google Scholar]
- Hu, X.; Meng, G.; Huang, Q.; Zhu, C.; Chen, B.; Huang, Z.; Li, F.; Wang, Z. Nano-Petri-Dish Array Assisted Glancing Angle Sputtering for Ag-NP Assembled Bi-Nanoring Arrays as Effective SERS Substrates. ACS Appl. Mater. Interfaces 2014, 6, 7991–7995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Q.; Wang, W.; Shen, L.; Li, Z.; Zhang, Z. Latticing Vertically Aligned Ag Nanorods to Enhance Its SERS Sensitivity. Mater. Res. Bull. 2012, 47, 921–924. [Google Scholar] [CrossRef]
- Giordano, M.C.; Foti, A.; Messina, E.; Gucciardi, P.G.; Comoretto, D.; Buatier De Mongeot, F. SERS Amplification from Self-Organized Arrays of Plasmonic Nanocrescents. ACS Appl. Mater. Interfaces 2016, 8, 6629–6638. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhang, W.; Cunningham, B.T. Coupling Discrete Metal Nanoparticles to Photonic Crystal Surface Resonant Modes and Application to Raman Spectroscopy. Opt. Express 2010, 18, 4300. [Google Scholar] [CrossRef]
- Castillo-Seoane, J.; Contreras-Bernal, L.; Obrero-Perez, J.M.; García-Casas, X.; Lorenzo-Lázaro, F.; Aparicio, F.J.; Lopez-Santos, C.; Rojas, T.C.; Anta, J.A.; Borrás, A.; et al. Highly Anisotropic Organometal Halide Perovskite Nanowalls Grown by Glancing-Angle Deposition (Adv. Mater. 18/2022). Adv. Mater. 2022, 34, 2270137. [Google Scholar] [CrossRef]
- Lee, B.S.; Lin, P.C.; Lin, D.Z.; Yen, T.J. Rapid Biochemical Mixture Screening by Three-Dimensional Patterned Multifunctional Substrate with Ultra-Thin Layer Chromatography (UTLC) and Surface Enhanced Raman Scattering (SERS). Sci. Rep. 2018, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Eiamchai, P.; Chananonnawathorn, C.; Horprathum, M.; Patthanasettakul, V.; Limwichean, S.; Nuntawong, N. Spatial Elemental Investigations in Nanostructured Alloyed Ag/Au SERS Substrates by Magnetron Sputtering Oblique-Angle Co-Deposition towards Increased Performance and Shelf Life. Appl. Surf. Sci. 2020, 513, 145748. [Google Scholar] [CrossRef]
- Kim, S.M.; Zhang, W.; Cunningham, B.T. Photonic Crystals with SiO2-Ag “Post-Cap” Nanostructure Coatings for Surface Enhanced Raman Spectroscopy. Appl. Phys. Lett. 2008, 93, 143112. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Hou, M.; Xie, Z.; Zhang, Z. Silver Nanorods Wrapped with Ultrathin Al2O3layers Exhibiting Excellent Sers Sensitivity and Outstanding Sers Stability. Sci. Rep. 2015, 5, 12890. [Google Scholar] [CrossRef]
- Savaloni, H.; Babaei, R. Surface Enhanced Raman Spectroscopy and Structural Characterization of Ag/Cu Chiral Nano-Flower Sculptured Thin Films. Appl. Surf. Sci. 2013, 280, 439–445. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Ma, L.; Zou, S.; Ling, Y.; Zhang, Z. Highly Stable and Active SERS Substrates with Ag-Ti Alloy Nanorods. Nanoscale 2018, 10, 19863–19870. [Google Scholar] [CrossRef] [PubMed]
- Savaloni, H.; Babaei, R.; Goli-Haghighi, S. Application of Mn–Cu Helical Star-Shaped (Pine-Tree-Like) Sculpted Thin Films with Different Symmetries Using Surface-Enhanced Raman Spectroscopy (SERS). Appl. Spectrosc. 2019, 73, 879–892. [Google Scholar] [PubMed]
- Kumar, S.; Lodhi, D.K.; Singh, J.P. Highly Sensitive Multifunctional Recyclable Ag-TiO2 Nanorod SERS Substrates for Photocatalytic Degradation and Detection of Dye Molecules. RSC Adv. 2016, 6, 45120–45126. [Google Scholar] [CrossRef]
- Hu, L.; Huo, K.; Chen, R.; Gao, B.; Fu, J.; Chu, P.K. Recyclable and High-Sensitivity Electrochemical Biosensing Platform Composed of Carbon-Doped TiO2 Nanotube Arrays. Anal. Chem. 2011, 83, 8138–8144. [Google Scholar] [CrossRef]
- Hunt, H.K.; Armani, A.M. Recycling Microcavity Optical Biosensors. Opt. Lett. 2011, 36, 1092. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Park, S.-G.; Kang, M.-H.; Choi, J.-H.; Nam, Y.; Jeong, K.-H. Beyond the SERS: Raman Enhancement of Small Molecules Using Nanofluidic Channels with Localized Surface Plasmon Resonance. Small 2011, 7, 184–188. [Google Scholar] [CrossRef]
- Abbas, A.; Linman, M.J.; Cheng, Q. New Trends in Instrumental Design for Surface Plasmon Resonance-Based Biosensors. Biosens. Bioelectron. 2011, 26, 1815–1824. [Google Scholar] [CrossRef]
- Homola, J. Present and Future of Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Liao, H.; Nehl, C.L.; Hafner, J.H. Biomedical Applications of Plasmon Resonant Metal Nanoparticles. Nanomedicine 2006, 1, 201–208. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P. Use of Surface Plasmon Resonance to Probe The Equilibrium and Dynamic Aspects of Interactions between Biological Macromolecules. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Guedon, P.; Livache, T.; Martin, F.; Lesbre, F.; Roget, A.; Bidan, G.; Levy, Y. Characterization and Optimization of a Real-Time, Parallel, Label-Free, Polypyrrole-Based DNA Sensor by Surface Plasmon Resonance Imaging. Anal. Chem. 2000, 72, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.E.; Frutos, A.G.; Thiel, A.J.; Corn, R.M.; Peterlinz, K.A.; Georgiadis, R.M.; He, L.; Musick, M.D.; Nicewarner, S.R.; Salinas, F.G.; et al. Surface Plasmon Resonance Imaging Measurements of DNA and RNA Hybridization Adsorption onto DNA Microarrays. Gen. Anal. Biomol. Eng 1997, 69, 1–7. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Bhalla, N.; Yu, Z.; Pauly, S.; Kumar, A.; Maddi, C.; Mariotti, D.; Zhao, P.; Payam, A.F.; Soin, N. Total Electrification of Large-Scale Nanophotonic Arrays by Frictional Charges. Nanoscale Horiz. 2022, 7, 1513–1522. [Google Scholar] [CrossRef]
- Kumar Agrawal, A.; Das, A.; Dhawan, A. Enhanced Sensitivity of SPR Sensing and Imaging Using Plasmonic Nanopillar Arrays. In Proceedings of the IEEE Conference on Nanotechnology, Cork, Ireland, 23–26 July 2018; IEEE Computer Society: Washington, DC, USA, 2019; Volume 2018, pp. 1–4. [Google Scholar]
- Agrawal, A.; Gupta, N.; Das, A.; Ahmed, K.; Dhawan, A. Nanostructured Plasmonic Gold Films for Enhanced Sensitivity of SPR Biological Sensing and Imaging. In Proceedings of the Plasmonics in Biology and Medicine XVI, San Francisco, CA, USA, 7 March 2019; Vo-Dinh, T., Ho, H.-P.A., Ray, K., Eds.; SPIE: Bellingham, WA, USA, 2019; Volume 10894, p. 21. [Google Scholar]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly Sensitive Graphene Biosensors Based on Surface Plasmon Resonance. Opt. Express 2010, 18, 14395. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.L.; Byun, K.M. Graphene-on-Silver Substrates for Sensitive Surface Plasmon Resonance Imaging Biosensors. Opt. Express 2011, 19, 458. [Google Scholar] [CrossRef]
- McGaughey, G.B.; Gagné, M.; Rappé, A.K. π-Stacking Interactions. Alive and Well in Proteins. J. Biol. Chem. 1998, 273, 15458–15463. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, H.; Cort, J.R.; Buchko, G.W.; Zhang, Y.; Shao, Y.; Aksay, I.A.; Liu, J.; Lin, Y. Constraint of DNA on Functionalized Graphene Improves Its Biostability and Specificity. Small 2010, 6, 1205–1209. [Google Scholar] [CrossRef]
- Leong, H.S.; Guo, J.; Lindquist, R.G.; Liu, Q.H. Surface Plasmon Resonance in Nanostructured Metal Films under the Kretschmann Configuration. J. Appl. Phys. 2009, 106, 124314. [Google Scholar] [CrossRef]
- Kaur, G.; Tomar, M.; Gupta, V. Nanostructured Zinc Oxide Thin Film for Application to Surface Plasmon Resonance Based Cholesterol Biosensor. In Proceedings of the International Workshop on Thin Films for Electronics, Electro-Optics, Energy, and Sensors, Dayton, OH, USA, 4–6 July 2015; Subramanyam, G., Ed.; SPIE: Bellingham, WA, USA, 2015; Volume 9667, p. 966706. [Google Scholar]
- Shalabney, A.; Khare, C.; Rauschenbach, B.; Abdulhalim, I. Sensitivity of Surface Plasmon Resonance Sensors Based on Metallic Columnar Thin Films in the Spectral and Angular Interrogations. Sens. Actuators B Chem. 2011, 159, 201–212. [Google Scholar] [CrossRef]
- Byun, K.M.; Yoon, S.J.; Kim, D.; Kim, S.J. Experimental Study of Sensitivity Enhancement in Surface Plasmon Resonance Biosensors by Use of Periodic Metallic Nanowires. Opt. Lett. 2007, 32, 1902. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, E.; Lausted, C.; Lin, T.; Yang, C.W.T.; Hood, L.; Lagally, E.T. Parallel Microfluidic Surface Plasmon Resonance Imaging Arrays. Lab Chip 2010, 10, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Carlen, E.T.; Deboer, H.L.; Van Den Berg, A.; Schasfoort, R.B.M. Electrokinetic Lab-on-a-BioChip for Multi-Ligand/Multi-Analyte Biosensing. Anal. Chem. 2010, 82, 4145–4150. [Google Scholar] [CrossRef]
- Zordan, M.D.; Grafton, M.M.G.; Park, K.; Leary, J.F. The Design of a Microfluidic Biochip for the Rapid, Multiplexed Detection of Foodborne Pathogens by Surface Plasmon Resonance Imaging. In Proceedings of the Frontiers in Pathogen Detection: From Nanosensors to Systems, San Francisco, CA, USA, 23–25 January 2010; Fauchet, P.M., Ed.; SPIE: Bellingham, WA, USA, 2010; Volume 7553, p. 755307. [Google Scholar]
- Malic, L.; Veres, T.; Tabrizian, M. Two-Dimensional Droplet-Based Surface Plasmon Resonance Imaging Using Electrowetting-on-Dielectric Microfluidics. Lab Chip 2009, 9, 473–475. [Google Scholar] [CrossRef]
- Natarajan, S.; Katsamba, P.S.; Miles, A.; Eckman, J.; Papalia, G.A.; Rich, R.L.; Gale, B.K.; Myszka, D.G. Continuous-Flow Microfluidic Printing of Proteins for Array-Based Applications Including Surface Plasmon Resonance Imaging. Anal. Biochem. 2008, 373, 141–146. [Google Scholar] [CrossRef]
- Liu, C.; Cui, D.; Li, H. A Hard-Soft Microfluidic-Based Biosensor Flow Cell for SPR Imaging Application. Biosens. Bioelectron. 2010, 26, 255–261. [Google Scholar] [CrossRef]
- Galopin, E.; Beaugeois, M.; Pinchemel, B.; Camart, J.-C.; Bouazaoui, M.; Thomy, V. SPR Biosensing Coupled to a Digital Microfluidic Microstreaming System. Biosens. Bioelectron. 2007, 23, 746–750. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Kumar, S.; Taneichi, T.; Fukuoka, T.; Namura, K.; Suzuki, M. Study on Transport of Molecules in Gel by Surface-Enhanced Raman Spectroscopy. Cellulose 2021, 28, 10803–10813. [Google Scholar] [CrossRef]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-Enhanced Raman Spectroscopy Applied to Food Safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.H.; Schlotter, N.E.; Crawford, A.C.; Porter, M.D. Prospects for Point-of-Care Pathogen Diagnostics Using Surface-Enhanced Raman Scattering (SERS). Chem. Soc. Rev. 2016, 45, 3865–3882. [Google Scholar] [CrossRef]
- Hughes, J.; Izake, E.L.; Lott, W.B.; Ayoko, G.A.; Sillence, M. Ultra Sensitive Label Free Surface Enhanced Raman Spectroscopy Method for the Detection of Biomolecules. Talanta 2014, 130, 20–25. [Google Scholar] [CrossRef][Green Version]
- Kong, K.V.; Leong, W.K.; Lam, Z.; Gong, T.; Goh, D.; Lau, W.K.O.; Olivo, M. A Rapid and Label-Free SERS Detection Method for Biomarkers in Clinical Biofluids. Small 2014, 10, 5030–5034. [Google Scholar] [CrossRef]
- Radziuk, D.; Moehwald, H. Prospects for Plasmonic Hot Spots in Single Molecule SERS towards the Chemical Imaging of Live Cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef]
- Hakonen, A.; Andersson, P.O.; Stenbæk Schmidt, M.; Rindzevicius, T.; Käll, M. Explosive and Chemical Threat Detection by Surface-Enhanced Raman Scattering: A Review. Anal. Chim. Acta 2015, 893, 1–13. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S.; Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S. Surface-Enhanced Raman Scattering: Introduction and Applications. Recent Adv. Nanophotonics-Fundam. Appl. 2020. [Google Scholar] [CrossRef]
- Sharma, B.; Fernanda Cardinal, M.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-Performance SERS Substrates: Advances and Challenges. MRS Bull. 2013, 38, 615–624. [Google Scholar] [CrossRef]
- Kumar, S.; Tokunaga, K.; Namura, K.; Fukuoka, T.; Suzuki, M. Experimental Evidence of a Twofold Electromagnetic Enhancement Mechanism of Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2020, 124, 21215–21222. [Google Scholar] [CrossRef]
- Ko, H.; Singamaneni, S.; Tsukruk, V.V. Nanostructured Surfaces and Assemblies as SERS Media. Small 2008, 4, 1576–1599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Fukuoka, T.; Takahashi, R.; Yoshida, M.; Utsumi, Y.; Yamaguchi, A.; Namura, K.; Suzuki, M. Highly Stable and Reproducible Au Nanorod Arrays for Near-Infrared Optofluidic SERS Sensor. Mater. Lett. 2021, 286, 129106. [Google Scholar] [CrossRef]
- Song, C.; Chen, J.; Zhao, Y.; Wang, L. Gold-Modified Silver Nanorod Arrays for SERS-Based Immunoassays with Improved Sensitivity. J. Mater. Chem. B 2014, 2, 7488–7494. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Shalabney, A.; Khalaila, I.; Grüner, C.; Rauschenbach, B.; Abdulhalim, I. SERS Biosensor Using Metallic Nano-Sculptured Thin Films for the Detection of Endocrine Disrupting Compound Biomarker Vitellogenin. Small 2014, 10, 3579–3587. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Driskell, J.D.; Seto, A.G.; Jones, L.P.; Jokela, S.; Dluhy, R.A.; Zhao, Y.P.; Tripp, R.A. Rapid MicroRNA (MiRNA) Detection and Classification via Surface-Enhanced Raman Spectroscopy (SERS). Biosens. Bioelectron. 2008, 24, 917–922. [Google Scholar] [CrossRef]
- Driskell, J.D.; Tripp, R.A. Label-Free SERS Detection of MicroRNA Based on Affinity for an Unmodified Silver Nanorod Array Substrate. Chem. Commun. 2010, 46, 3298–3300. [Google Scholar] [CrossRef]
- Hou, M.; Huang, Y.; Ma, L.; Zhang, Z. Sensitivity and Reusability of SiO2 NRs@ Au NPs SERS Substrate in Trace Monochlorobiphenyl Detection. Nanoscale Res. Lett. 2015, 10, 444. [Google Scholar] [CrossRef]
- Nuntawong, N.; Eiamchai, P.; Somrang, W.; Denchitcharoen, S.; Limwichean, S.; Horprathum, M.; Patthanasettakul, V.; Chaiya, S.; Leelapojanaporn, A.; Saiseng, S.; et al. Detection of Methamphetamine/Amphetamine in Human Urine Based on Surface-Enhanced Raman Spectroscopy and Acidulation Treatments. Sens. Actuators B Chem. 2017, 239, 139–146. [Google Scholar] [CrossRef]
- Li, B.; Wang, T.; Su, Q.; Wu, X.; Dong, P. Fabrication of Au Nanorods by the Oblique Angle Deposition Process for Trace Detection of Methamphetamine with Surface-Enhanced Raman Scattering. Sensors 2019, 19, 3742. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Huang, Y.; Ma, L.; Zhang, Z. Quantitative Analysis of Single and Mix Food Antiseptics Basing on SERS Spectra with PLSR Method. Nanoscale Res. Lett. 2016, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yao, Y.; Wang, W.; Qu, L.; Bradley, L.; Sun, S.; Zhao, Y. Rapid and Sensitive Detection of Sodium Saccharin in Soft Drinks by Silver Nanorod Array SERS Substrates. Sens. Actuators B Chem. 2017, 251, 272–279. [Google Scholar] [CrossRef]
- Devor, E.J.; Behlke, M.A.; Huang, L.; Bogh, L.; Rose, S. Fluorescence and Fluorescence Applications; Integrated DNA Technologies: Coralville, IA, USA, 2005. [Google Scholar]
- Nanobiosensors and Fluorescence Based Biosensors: An Overview. Available online: http://www.ijnd.ir/article_660965.html (accessed on 19 July 2020).
- Hirshberg, M.; Henrick, K.; Lloyd Haire, L.; Vasisht, N.; Brune, M.; Corrie, J.E.T.; Webb, M.R. Crystal Structure of Phosphate Binding Protein Labeled with a Coumarin Fluorophore, a Probe for Inorganic Phosphate. Biochemistry 1998, 37, 10381–10385. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, M.S.; Tibbles, K.L.; Hunter, J.L.; Bell, J.C.; Kowalczykowski, S.C.; Webb, M.R. Fluorescent Single-Stranded DNA Binding Protein as a Probe for Sensitive, Real-Time Assays of Helicase Activity. Biophys. J. 2008, 95, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-Enhanced Fluorescence: An Emerging Tool in Biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef]
- Matveeva, E.G.; Gryczynski, Z.; Lakowicz, J.R. Myoglobin Immunoassay Based on Metal Particle-Enhanced Fluorescence. J. Immunol. Methods 2005, 302, 26–35. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar, S.; Singh, J.P. Morphology Dependent Surface Enhanced Fluorescence Study on Silver Nanorod Arrays Fabricated by Glancing Angle Deposition. RSC Adv. 2015, 5, 31341–31346. [Google Scholar] [CrossRef]
- Zhong, W. Nanomaterials in Fluorescence-Based Biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59. [Google Scholar] [CrossRef]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Metal-Enhanced Fluorescence Using Anisotropic Silver Nanostructures: Critical Progress to Date. Anal. Bioanal. Chem. 2005, 382, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Badshah, M.A.; Ju, J.; Lu, X.; Abbas, N.; Kim, S. min Enhancing the Sensitivity of DNA Microarrays by Metal-Enhanced Fluorescence Using Vertical Nanorod Structures. Sens. Actuators B Chem. 2018, 274, 451–457. [Google Scholar] [CrossRef]
- Li, H.; Chen, C.Y.; Wei, X.; Qiang, W.; Li, Z.; Cheng, Q.; Xu, D. Highly Sensitive Detection of Proteins Based on Metal-Enhanced Fluorescence with Novel Silver Nanostructures. Anal. Chem. 2012, 84, 8656–8662. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Bu, W.; Zhang, S.; Zheng, X.; Li, M.; Chen, F.; He, Q.; Zhou, L.; Peng, W.; Hua, Y.; et al. Multifunctional Nanoprobes for Upconversion Fluorescence, MR and CT Trimodal Imaging. Biomaterials 2012, 33, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, Y.; Li, G.; Zhao, R.Y.; Lakowicz, J.R. Direct Observation of Chemokine Receptors 5 on T-Lymphocyte Cell Surfaces Using Fluorescent Metal Nanoprobes 2: Approximation of CCR5 Populations. Biochem. Biophys. Res. Commun. 2011, 407, 63–67. [Google Scholar] [CrossRef]
- Yang, J.; Moraillon, A.; Siriwardena, A.; Boukherroub, R.; Ozanam, F.; Gouget-Laemmel, A.C.; Szunerits, S. Carbohydrate Microarray for the Detection of Glycan-Protein Interactions Using Metal-Enhanced Fluorescence. Anal. Chem. 2015, 87, 3721–3728. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on Oblique Angle Deposition of Thin Films: From Fundamentals to Devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Geddes, C.D.; Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Metal enhanced fluorescence: Potential applications in HTS. Comb. Chem. High Throughput Screen. 2003, 6, 109–117. [Google Scholar] [CrossRef]
- Park, B.; Fu, J.; Zhao, Y.; Siragusa, G.R.; Cho, Y.-J.; Lawrence, K.C.; Windham, W.R. Bio-Functional Au/Si Nanorods for Pathogen Detection. In Proceedings of the Nanosensing: Materials, Devices, and Systems III, Boston, MA, USA, 11–12 September 2007; Islam, M.S., Dutta, A.K., Eds.; SPIE: Bellingham, WA, USA, 2007; Volume 6769, pp. 159–167. [Google Scholar]
- Haustein, E.; Schwille, P. Single-Molecule Spectroscopic Methods. Curr. Opin. Struct. Biol. 2004, 14, 531–540. [Google Scholar] [CrossRef]
- Zhang, J. Target Molecule Imaging on Tissue Specimens by Fluorescent Metal Nanoprobes. J. Biomed. Opt. 2011, 16, 116004. [Google Scholar] [CrossRef]
- Punj, D.; Regmi, R.; Devilez, A.; Plauchu, R.; Moparthi, S.B.; Stout, B.; Bonod, N.; Rigneault, H.; Wenger, J. Self-Assembled Nanoparticle Dimer Antennas for Plasmonic-Enhanced Single-Molecule Fluorescence Detection at Micromolar Concentrations. ACS Photonics 2015, 2, 1099–1107. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.X.; Collins, A.; Zhao, Y.P. Optical Properties and Biosensor Application of Ultrathin Silver Films Prepared by Oblique Angle Deposition. J. Phys. Chem. C 2008, 112, 16784–16791. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, W.; Yuan, Z.; Lu, C. Colorimetric Detection of Biological Hydrogen Sulfide Using Fluorosurfactant Functionalized Gold Nanorods. Analyst 2015, 140, 7443–7450. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.P.; Yep, T.; Mutus, B. Microplate-Based Colorimetric Detection of Free Hydrogen Sulfide. Anal. Chem. 2013, 85, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, S.; White, C.E. A Study of the Reaction between Hydrogen Sulfide and Silver. J. Am. Chem. Soc. 1930, 52, 885–892. [Google Scholar] [CrossRef]
- Graedel, T.E.; Franey, J.P.; Gualtieri, G.J.; Kammloty, G.W.; Malm, D.L. On the mechanism of silver and copper sulfidation by atmospheric h2s and ocs. Corros. Sci. 1985, 25, 1163–1180. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion Mechanisms for Silver Exposed to the Atmosphere. J. Electrochem. Soc. 1992, 139, 1963. [Google Scholar] [CrossRef]
- Kim, H. Corrosion Process of Silver in Environments Containing 0.1 Ppm H2S and 1.2 Ppm NO2. Mater. Corros. 2003, 54, 243–250. [Google Scholar] [CrossRef]
- Chen, R.; Morris, H.R.; Whitmore, P.M. Fast Detection of Hydrogen Sulfide Gas in the Ppmv Range with Silver Nanoparticle Films at Ambient Conditions. Sens. Actuators B Chem. 2013, 186, 431–438. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Yadav, K.; Sharan, C.; Singh, J.P. Quick and Selective Dual Mode Detection of H2S Gas by Mobile App Employing Silver Nanorods Array. Anal. Chem. 2017, 89, 13582–13588. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Lee, Y.J.; Lee, J.; Lee, D.; Park, H.K.; Lee, G.J. Colorimetric Detection of Endogenous Hydrogen Sulfide Production in Living Cells. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2017, 177, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Xiong, B.; Cheng, X.; He, Y.; Yeung, E.S. High-Throughput Sulfide Sensing with Colorimetric Analysis of Single Au-Ag Core-Shell Nanoparticles. Anal. Chem. 2014, 86, 4663–4667. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Ghasemian, A.; Mostafavi, S.K.S.; Teimouri, M.; Vardanjani, H.R.; Mirforughi, S.A. Gold Nanoparticle-Based Colorimetric Platform Technology as Rapid and Efficient Bacterial Pathogens Detection Method from Various Sources. Rev. Med. Microbiol. 2019, 30, 128–132. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold Nanoparticle-Based Colorimetric Biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Peng, H.; Chen, I.A. Rapid Colorimetric Detection of Bacterial Species through the Capture of Gold Nanoparticles by Chimeric Phages. ACS Nano 2019, 13, 1244–1252. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Chen, Y.; Kang, X.; Yang, C.; Wang, F.; Liu, Y.; Xiong, X. Self-Stabilized and Strongly Adhesive Supramolecular Polymer Protective Layer Enables Ultrahigh-Rate and Large-Capacity Lithium-Metal Anode. Angew. Chemie Int. Ed. 2020, 59, 2055–2060. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Fiber-Optic Plasmonic Sensor Utilizing CTAB-Functionalized ZnO Nanoparticle-Decorated Carbon Nanotubes on Silver Films for the Detection of Catechol in Wastewater. ACS Appl. Nano Mater. 2020, 3, 2582–2593. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Zhang, Y.; Ma, X.; Iqbal, M.Z.; Miao, L.; Zhou, Z.; Shen, Z.; Wu, A. High-Performance Colorimetric Detection of Thiosulfate by Using Silver Nanoparticles for Smartphone-Based Analysis. ACS Sensors 2017, 2, 1152–1159. [Google Scholar] [CrossRef]
- Yang, X.H.; Ling, J.; Peng, J.; Cao, Q.E.; Ding, Z.T.; Bian, L.C. A Colorimetric Method for Highly Sensitive and Accurate Detection of Iodide by Finding the Critical Color in a Color Change Process Using Silver Triangular Nanoplates. Anal. Chim. Acta 2013, 798, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Harris, K.D.; Elias, A.L. Peroxidase-Like Behavior of Ni Thin Films Deposited by Glancing Angle Deposition for Enzyme-Free Uric Acid Sensing. ACS Omega 2020, 5, 9123–9130. [Google Scholar] [CrossRef]
- Kruse, C.; Anderson, T.; Wilson, C.; Zuhlke, C.; Alexander, D.; Gogos, G.; Ndao, S. Extraordinary Shifts of the Leidenfrost Temperature from Multiscale Micro/Nanostructured Surfaces. Langmuir 2013, 29, 9798–9806. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, J.P. Delayed Freezing of Water Droplet on Silver Nanocolumnar Thin Film. Appl. Phys. Lett. 2013, 102, 243112. [Google Scholar] [CrossRef]
- Tyrrell, J.W.G.; Attard, P. Images of Nanobubbles on Hydrophobic Surfaces and Their Interactions. Phys. Rev. Lett. 2001, 87, 176104. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Wang, P.I.; Peles, Y.; Koratkar, N.; Peterson, G.P. Nanostructured Copper Interfaces for Enhanced Boiling. Small 2008, 4, 1084–1088. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Devi, P.; Singh, J.P. Self-Sustainable and Recyclable Ag Nanorods for Developing Ag-Ag2S Nano Heterostructures Using Sewage Gas: Applications in Photocatalytic Water Purification, Hydrogen Evolution, SERS and Antibacterial Activity. Appl. Surf. Sci. 2020, 528, 147037. [Google Scholar] [CrossRef]
- Cassidy, P.J.; Radda, G.K. Molecular Imaging Perspectives. J. R. Soc. Interface 2005, 2, 133–144. [Google Scholar] [CrossRef]
- Minchin, R.F.; Martin, D.J. Minireview: Nanoparticles for Molecular Imaging—An Overview. Endocrinology 2010, 151, 474–481. [Google Scholar] [CrossRef]
- Sheng, Y.; De Liao, L.; Thakor, N.V.; Tan, M.C. Nanoparticles for Molecular Imaging. J. Biomed. Nanotechnol. 2014, 10, 2641–2676. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyás, B. Nanoparticles in Practice for Molecular-Imaging Applications: An Overview. Acta Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ma, X.; Ma, H.; Zheng, K.; Liu, J.; Hou, S.; Meng, J.; Wang, P.C.; Wu, X.; Liang, X.J. Surface Chemistry-Mediated Penetration and Gold Nanorod Thermotherapy in Multicellular Tumor Spheroids. Nanoscale 2013, 5, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Selvan, S.T.; Nogami, M. Field Enhancement Effect of Small Ag Particles on the Fluorescence from Eu3+-Doped SiO2 Glass. Appl. Phys. Lett. 1999, 74, 1513–1515. [Google Scholar] [CrossRef]
- Fulmore, J.S.; Geiger, B.F.; Werner, K.A.; Talbott, L.L.; Jones, D.C. Feature Article. Child. Educ. 2009, 85, 293–299. [Google Scholar] [CrossRef]
- Ju, J.; Byeon, E.; Han, Y.A.; Kim, S.M. Fabrication of a Substrate for Ag-nanorod Metal-enhanced Fluorescence Using the Oblique. Micro Nano Lett. 2013, 8, 370–373. [Google Scholar] [CrossRef]
- Ji, X.; Xiao, C.; Lau, W.F.; Li, J.; Fu, J. Metal Enhanced Fluorescence Improved Protein and DNA Detection by Zigzag Ag Nanorod Arrays. Biosens. Bioelectron. 2016, 82, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Hecht, B. Principles of Nano-Optics; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780511813535. [Google Scholar]
- Schermelleh, L.; Heintzmann, R.; Leonhardt, H. A Guide to Super-Resolution Fluorescence Microscopy. J. Cell Biol. 2010, 190, 165–175. [Google Scholar] [CrossRef]
- Neuman, T.; Alonso-González, P.; Garcia-Etxarri, A.; Schnell, M.; Hillenbrand, R.; Aizpurua, J. Mapping the near Fields of Plasmonic Nanoantennas by Scattering-Type Scanning near-Field Optical Microscopy. Laser Photonics Rev. 2015, 9, 637–649. [Google Scholar] [CrossRef]
- Villarrubia, J.S.; Vladár, A.E.; Ming, B.; Kline, R.J.; Sunday, D.F.; Chawla, J.S.; List, S. Scanning Electron Microscope Measurement of Width and Shape of 10nm Patterned Lines Using a JMONSEL-Modeled Library. Ultramicroscopy 2015, 154, 15–28. [Google Scholar] [CrossRef]
- Hess, S.T.; Girirajan, T.P.K.; Mason, M.D. Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef]
- Kawata, S.; Inouye, Y.; Verma, P. Plasmonics for Near-Field Nano-Imaging and Superlensing. Nat. Photonics 2009, 3, 388–394. [Google Scholar] [CrossRef]
- Wang, Y.; Srituravanich, W.; Sun, C.; Zhang, X. Plasmonic Nearfield Scanning Probe with High Transmission. Nano Lett. 2008, 8, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Holzmeister, P.; Acuna, G.P.; Grohmann, D.; Tinnefeld, P. Breaking the Concentration Limit of Optical Single-Molecule Detection. Chem. Soc. Rev. 2014, 43, 1014–1028. [Google Scholar] [CrossRef]
- Stranahan, S.M.; Willets, K.A. Super-Resolution Optical Imaging of Single-Molecule SERS Hot Spots. Nano Lett. 2010, 10, 3777–3784. [Google Scholar] [CrossRef]
- Willets, K.A. Super-Resolution Imaging of Interactions between Molecules and Plasmonic Nanostructures. Phys. Chem. Chem. Phys. 2013, 15, 5345–5354. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wood, A.; Pathak, A.; Mathai, J.; Bok, S.; Zheng, H.; Hamm, S.; Basuray, S.; Grant, S.; Gangopadhyay, K.; et al. Plasmonic Gratings with Nano-Protrusions Made by Glancing Angle Deposition for Single-Molecule Super-Resolution Imaging. Nanoscale 2016, 8, 12189–12201. [Google Scholar] [CrossRef]
- Qi, H.; Xiao, S.; Shi, R.; Ward, M.O.; Chen, Y.; Tu, W.; Su, Q.; Wang, W.; Wang, X.; Zhang, Z. Enhanced Reader.Pdf. Nature 2018, 388, 539–547. [Google Scholar]
- Liu, C.Y.; Lo, W.C. Large-Area Super-Resolution Optical Imaging by Using Core-Shell Microfibers. Opt. Commun. 2017, 399, 104–111. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Su, J.L.; Wang, B.; Wilson, K.E.; Bayer, C.L.; Chen, Y.S.; Kim, S.; Homan, K.A.; Emelianov, S.Y. Advances in Clinical and Biomedical Applications of Photoacoustic Imaging. Expert Opin. Med. Diagn. 2010, 4, 497–510. [Google Scholar] [CrossRef]
- Laufer, J.; Delpy, D.; Elwell, C.; Beard, P. Quantitative Spatially Resolved Measurement of Tissue Chromophore Concentrations Using Photoacoustic Spectroscopy: Application to the Measurement of Blood Oxygenation and Haemoglobin Concentration. Phys. Med. Biol. 2007, 52, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive Laser-Induced Photoacoustic Tomography for Structural and Functional in Vivo Imaging of the Brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Laufer, J.; Beard, P. Backward-Mode Multiwavelength Photoacoustic Scanner Using a Planar Fabry-Perot Polymer Film Ultrasound Sensor for High-Resolution Three-Dimensional Imaging of Biological Tissues. Appl. Opt. 2008, 47, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Roberts, W.; Carson, P.; Liu, X.; Tao, C.; Wang, X. Evaluation of Bladder Microvasculature with High-Resolution Photoacoustic Imaging. Opt. Lett. 2011, 36, 4815. [Google Scholar] [CrossRef]
- Hajireza, P.; Sorge, J.; Brett, M.; Zemp, R. In Vivo Optical Resolution Photoacoustic Microscopy Using Glancing Angle-Deposited Nanostructured Fabry–Perot Etalons. Opt. Lett. 2015, 40, 1350. [Google Scholar] [CrossRef] [PubMed]
- Hajireza, P.; Krause, K.; Brett, M.; Zemp, R. Glancing Angle Deposited Nanostructured Film Fabry-Perot Etalons for Optical Detection of Ultrasound. Opt. Express 2013, 21, 6391. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making Polymeric Micro- and Nanoparticles of Complex Shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904. [Google Scholar] [CrossRef]
- Tirrell, D.A.; Langer, R. Materials for Biology and Medicine. Nature 2012, 428, 25. [Google Scholar]
- Love, J.C.; Gates, B.D.; Wolfe, D.B.; Paul, K.E.; Whitesides, G.M. Fabrication and Wetting Properties of Metallic Half-Shells with Submicron Diameters. Nano Lett. 2002, 2, 891–894. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Pawar, A.B.; Kretzschmar, I. Fabrication, Assembly, and Application of Patchy Particles. Macromol. Rapid Commun. 2010, 31, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Grzybowski, B.A.; Granick, S. Janus Particle Synthesis, Assembly, and Application. Langmuir 2017, 33, 6964–6977. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus Particles for Biological Imaging and Sensing. Analyst 2016, 141, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, L.; Liao, T.; Chen, X.; Jiang, W.; Luo, W.; Yang, J.; Sun, Z. Janus Nanoarchitectures: From Structural Design to Catalytic Applications. Nano Today 2018, 22, 62–82. [Google Scholar] [CrossRef]
- Loget, G.; Roche, J.; Gianessi, E.; Bouffier, L.; Kuhn, A. Indirect Bipolar Electrodeposition. J. Am. Chem. Soc. 2012, 134, 20033–20036. [Google Scholar] [CrossRef]
- Nie, Z.; Li, W.; Seo, M.; Xu, S.; Kumacheva, E. Janus and Ternary Particles Generated by Microfluidic Synthesis: Design, Synthesis, and Self-Assembly. J. Am. Chem. Soc. 2006, 128, 9408–9412. [Google Scholar] [CrossRef]
- Roh, K.H.; Martin, D.C.; Lahann, J. Biphasic Janus Particles with Nanoscale Anisotropy. Nat. Mater. 2005, 4, 759–763. [Google Scholar] [CrossRef]
- Pawar, A.B.; Kretzschmar, I. Multifunctional Patchy Particles by Glancing Angle Deposition Technique. In Proceedings of the 2008 Annual Meeting, Washington, DC, USA, 13 October 2008; pp. 355–358. [Google Scholar]
- Imura, Y.; Kato, M.; Kondo, T.; Kawai, T. Strings of Metal Half-Shells Fabricated Using Colloidal Particle Monolayer as a Template. Langmuir 2010, 26, 11314–11318. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Lin, X.; Dai, L.; He, Q. Self-Propelled Janus Mesoporous Silica Nanomotors with Sub-100 Nm Diameters for Drug Encapsulation and Delivery. ChemPhysChem 2014, 15, 2255–2260. [Google Scholar] [CrossRef]
- Peng, F.; Men, Y.; Tu, Y.; Chen, Y.; Wilson, D.A. Nanomotor-Based Strategy for Enhanced Penetration across Vasculature Model. Adv. Funct. Mater. 2018, 28, 1706117. [Google Scholar] [CrossRef]
- Wu, Z.; Troll, J.; Jeong, H.H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A Swarm of Slippery Micropropellers Penetrates the Vitreous Body of the Eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Salazar, P.; Álvarez, R.; Palmero, A.; López-Santos, C.; González-Mora, J.L.; González-Elipe, A.R. Cholesterol Biosensing with a Polydopamine-Modified Nanostructured Platinum Electrode Prepared by Oblique Angle Physical Vacuum Deposition. Sens. Actuators B Chem. 2017, 240, 37–45. [Google Scholar] [CrossRef]
- El Kazzy, M.; Weerakkody, J.S.; Hurot, C.; Mathey, R.; Buhot, A.; Scaramozzino, N.; Hou, Y. An Overview of Artificial Olfaction Systems with a Focus on Surface Plasmon Resonance for the Analysis of Volatile Organic Compounds. Biosensors 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Salazar, P.; Rico, V.; González-Elipe, A.R. Nickel–Copper Bilayer Nanoporous Electrode Prepared by Physical Vapor Deposition at Oblique Angles for the Non-Enzymatic Determination of Glucose. Sens. Actuators B Chem. 2016, 226, 436–443. [Google Scholar] [CrossRef]
- Yadav, J.; Raturi, P.; Yadav, S.; Singh, J.P. Zig-Zag Ag2S Nanostructures for Superior Optical Absorption and Photoelectrochemical Water Splitting Performance. Renew. Energy 2021, 179, 2256–2266. [Google Scholar] [CrossRef]
- Norrod, K.L.; Sudnik, L.M.; Rousell, D.; Rowlen, K.L. Quantitative Comparison of Five SERS Substrates: Sensitivity and Limit of Detection. Appl. Spectrosc. 2016, 51, 994–1001. [Google Scholar] [CrossRef]







| GLAD-Based Optical Biosensors | GLAD-Based Electrochemical Biosensors |
|---|---|
| 1.The aspect ratio and morphology of the nanostructures are tuned and optimized to improve sensitivity to a variety of optical properties (fluorescence, absorption, etc.). | 1. An electrode surface is coated with biological sensing material for potentiometric, amperometric, or conductimetric measurements [249]. |
| 2. Enhanced sensitivity is provided by the plasmonic nature of metals such as Ag, Au, Cu, and Pt. | 2. Involves the modulation of electrical properties such as potential, current, or impedance associated with the interaction of biomolecules with the working electrodes [250]. |
| 3. By forming electromagnetic hotspots, GLAD-based nanostructures significantly improve optical spectroscopy (Raman, fluorescence, and infrared) and plasmon resonance sensing [24]. | 3. High porosity, large exposed areas, and excellent diffusion properties make GLAD-based metallic nanostructures excellent electrochemical sensors [251,252]. |
| 4. LOD ~ 1 fM [253] | 4. LOD ~ 1 µM [251] |
| 5. Nanostructures must be optimized to match optical measurements, must possess high sensitivity, and require trained personnel to operate [54] | 5. Comparatively low sensitivity, costly instruments, trained personnel required for their operation [248]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Senapati, S.; Kumar, S.; Gahlaut, S.K.; Singh, J.P. GLAD Based Advanced Nanostructures for Diversified Biosensing Applications: Recent Progress. Biosensors 2022, 12, 1115. https://doi.org/10.3390/bios12121115
Yadav S, Senapati S, Kumar S, Gahlaut SK, Singh JP. GLAD Based Advanced Nanostructures for Diversified Biosensing Applications: Recent Progress. Biosensors. 2022; 12(12):1115. https://doi.org/10.3390/bios12121115
Chicago/Turabian StyleYadav, Sarjana, Sneha Senapati, Samir Kumar, Shashank K. Gahlaut, and Jitendra P. Singh. 2022. "GLAD Based Advanced Nanostructures for Diversified Biosensing Applications: Recent Progress" Biosensors 12, no. 12: 1115. https://doi.org/10.3390/bios12121115
APA StyleYadav, S., Senapati, S., Kumar, S., Gahlaut, S. K., & Singh, J. P. (2022). GLAD Based Advanced Nanostructures for Diversified Biosensing Applications: Recent Progress. Biosensors, 12(12), 1115. https://doi.org/10.3390/bios12121115





