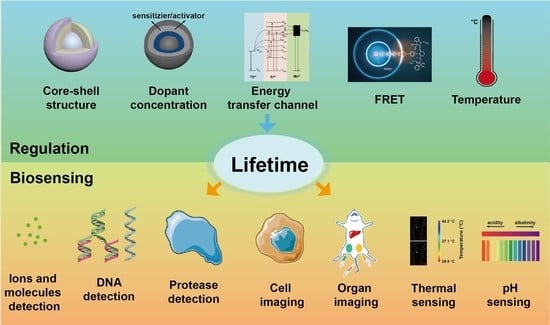
Luminescent Lifetime Regulation of Lanthanide-Doped Nanoparticles for Biosensing
Abstract
:1. Introduction
2. Lifetime Regulation
2.1. Variation of Core–Shell Structures
2.1.1. Core Size
2.1.2. Inert Shell Passivation
2.1.3. Active Shell

2.2. Changing Concentration of Sensitizer and Emitter

2.3. Adjusting the Energy Transfer Channel

2.4. Fluorescence Resonance Energy Transfer

2.5. Changing Temperature

3. Bioapplications
3.1. Ions and Molecules Detection

3.2. DNA Detection

3.3. Protein Detection

3.4. Cell Labeling

3.5. Organ Imaging

3.6. Thermal Sensing

3.7. pH Sensing

4. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Braun, G.B.; Qin, M.; Ruoslahti, E.; Sugahara, K.N. In Vivo Cation Exchange in Quantum Dots for Tumor-specific Imaging. Nat. Commun. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Peng, C.S.; Yang, T.; Joubert, L.-M.; Chu, S. Single Upconversion Nanoparticle Imaging at Sub-10 W cm−2 Irradiance. Nat. Photon. 2018, 12, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xiong, C.; Liu, H.; Wan, Q.; Hou, J.; He, Q.; Badu-Tawiah, A.; Nie, Z. Mass Spectrometry Imaging Reveals the Sub-organ Distribution of Carbon Nanomaterials. Nat. Nanotechnol. 2015, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Swallow, J.G.; Kim, J.J.; Maloney, J.M.; Chen, D.; Smith, J.F.; Bishop, S.R.; Tuller, H.L.; Van Vliet, K.J. Dynamic Chemical Expansion of Thin-film Non-stoichiometric Oxides at Extreme Temperatures. Nat. Mater. 2017, 16, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, G.C.; Vargas, P.A.; Zhu, H.; Grigoriev, A.; Zhu, P. Tetradic Phosphor White Light with Variable CCT and Superlative CRI through Organolead Halide Perovskite Nanocrystals. Nanoscale Adv. 2019, 1, 1791–1798. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, Y.; Song, S.; Zhang, H. Tumor Diagnosis and Therapy Mediated by Metal Phosphorus-Based Nanomaterials. Adv. Mater. 2021, 33, 2103936. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, Y.-J.; Yang, P.-P.; Qi, G.-B.; Zhang, J.-P.; Wang, L.; Wang, H. Self-Assembled Fluorescent Organic Nanomaterials for Biomedical Imaging. Adv. Healthc. Mater. 2018, 7, 1800344. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Qiu, X.; Liu, Y.; Feng, W.; Li, F. Upconversion Nanocomposite for Programming Combination Cancer Therapy by Precise Control of Microscopic Temperature. Nat. Commun. 2018, 9, 2176. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, X.; Su, X.; Xu, M.; Yuan, W.; Liu, Q.; Xue, M.; Liu, Y.; Feng, W.; Li, F. Near-Infrared Upconversion Luminescence and Bioimaging In Vivo Based on Quantum Dots. Adv. Sci. 2019, 6, 1801834. [Google Scholar] [CrossRef]
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-Stokes Shift Luminescent Materials for Bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Li, J.; Su, Q.; Yuan, W.; Dai, Y.; Han, C.; Wang, Q.; Feng, W.; Li, F. Ultrasensitive Near-Infrared Fluorescence-Enhanced Probe for In Vivo Nitroreductase Imaging. J. Am. Chem. Soc. 2015, 137, 6407–6416. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.S.; Lichtman, J.W. Clarifying Tissue Clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebibula, A.; Alifu, N.; Xia, L.; Sun, C.; Yu, X.; Xue, D.; Liu, L.; Li, G.; Qian, J. Ultrastable and Biocompatible NIR-II Quantum Dots for Functional Bioimaging. Adv. Funct. Mater. 2018, 28, 1703451. [Google Scholar] [CrossRef]
- Thor, W.; Wu, Y.; Wang, L.; Zhang, Y.; Tanner, P.A.; Wong, K.-L. Charging and Ultralong Phosphorescence of Lanthanide Facilitated Organic Complex. Nat. Commun. 2021, 12, 6532. [Google Scholar] [CrossRef] [PubMed]
- Suhling, K.; French, P.M.W.; Phillips, D. Time-resolved Fluorescence Microscopy. Photoch. Photobio. Sci. 2005, 4, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Xie, Y.; Arno, M.C.; Husband, J.T.; Torrent-Sucarrat, M.; O’Reilly, R.K. Manipulating the Fluorescence Lifetime at the Sub-cellular Scale via Photo-switchable Barcoding. Nat. Commun. 2020, 11, 2460. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, J.; Zhang, R.; Liu, Y.; Liu, D.; Goldys, E.M.; Yang, X.; Xi, P.; Sunna, A.; Lu, J.; et al. Tunable Lifetime Multiplexing Using Luminescent Nanocrystals. Nat. Photon. 2014, 8, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Li, R.; Tu, D.; Shang, X.; Liu, Y.; You, W.; Sun, C.; Zhang, F.; Chen, X. Intense Near-infrared-II Luminescence from NaCeF4: Er/Yb Nanoprobes for in vitro Bioassay and In Vivo Bioimaging. Chem. Sci. 2018, 9, 4682–4688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gu, Y.; Yuan, W.; Zhou, X.; Qiu, X.; Kong, M.; Wang, Q.; Feng, W.; Li, F. Quantitative Mapping of Liver Hypoxia in Living Mice Using Time-Resolved Wide-Field Phosphorescence Lifetime Imaging. Adv. Sci. 2020, 7, 1902929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Zhu, X.; Lu, Y.; Zhao, J.; Feng, W.; Jia, G.; Wang, F.; Li, F.; Jin, D. High-Contrast Visualization of Upconversion Luminescence in Mice Using Time-Gating Approach. Anal. Chem. 2016, 88, 3449–3454. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Wang, J.; Li, Z.; Lv, X.; Liang, L.; Yuan, Q. Recent Progress in Time-Resolved Biosensing and Bioimaging Based on Lanthanide-Doped Nanoparticles. Small 2019, 15, 1804969. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tu, D.; Huang, P.; Zhou, S.; Chen, Z.; Chen, X. Time-resolved Luminescent Biosensing Based on Inorganic Lanthanide-doped Nanoprobes. Chem. Commun. 2015, 51, 4129–4143. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, J.; Liu, Q.; Huang, B.; Peng, X.; Würth, C.; Resch-Genger, U.; Zhan, Q.; Widengren, J.; Ågren, H.; Liu, H. On the Decay Time of Upconversion Luminescence. Nanoscale 2019, 11, 4959–4969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinet, P.; Palmeri, P.; Biémont, E.; Li, Z.S.; Zhang, Z.G.; Svanberg, S. Radiative Lifetime Measurements and Transition Probability Calculations in Lanthanide Ions. J. Alloys Compd. 2002, 344, 255–259. [Google Scholar] [CrossRef]
- Kong, J.; Shang, X.; Zheng, W.; Chen, X.; Tu, D.; Wang, M.; Song, J.; Qu, J. Revisiting the Luminescence Decay Kinetics of Energy Transfer Upconversion. J. Phys. Chem. Lett. 2020, 11, 3672–3680. [Google Scholar] [CrossRef]
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial Core–shell and Core–multishell Nanowire Heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef]
- Hudry, D.; Howard, I.A.; Popescu, R.; Gerthsen, D.; Richards, B.S. Structure–Property Relationships in Lanthanide-Doped Upconverting Nanocrystals: Recent Advances in Understanding Core–Shell Structures. Adv. Mater. 2019, 31, 1900623. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Yin, Y.; McRae, C.; Piper, J.A.; Dawes, J.M.; Jin, D.; Goldys, E.M. Upconversion Luminescence with Tunable Lifetime in NaYF4: Yb,Er Nanocrystals: Role of Nanocrystal Size. Nanoscale 2013, 5, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, R.; Xie, X.; Huang, L.; Liu, X. Nonlinear Spectral and Lifetime Management in Upconversion Nanoparticles by Controlling Energy Distribution. Nanoscale 2016, 8, 6666–6673. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Han, S.; Xie, X.; Zhu, H.; Chen, H.; Chen, C.-K.; Liu, R.-S.; Chen, X.; Wang, F.; Liu, X. The Effect of Surface Coating on Energy Migration-Mediated Upconversion. J. Am. Chem. Soc. 2012, 134, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, H.; Mao, Y. Understanding the Effect of Mn2+ on Yb3+/Er3+ Upconversion and Obtaining a Maximum Upconversion Fluorescence Enhancement in Inert-core/active-shell/inert-shell Structures. RSC Adv. 2016, 6, 83321–83327. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Wang, R.; Ji, C.; Wei, Y.; Shi, M.; Wang, Y.; Du, Y.; Zhang, Y.; Yuan, Q.; et al. Bioactive Core–Shell CaF2 Upconversion Nanostructure for Promotion and Visualization of Engineered Bone Reconstruction. ACS Nano 2020, 14, 16085–16095. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, L.-D.; Li, L.-D.; Si, R.; Liu, R.; Yan, C.-H. Selective Cation Exchange Enabled Growth of Lanthanide Core/Shell Nanoparticles with Dissimilar Structure. J. Am. Chem. Soc. 2017, 139, 18492–18495. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; del Rosal, B.; Zhang, Y.; Martín Rodríguez, E.; Hu, J.; Zhou, Z.; Fan, R.; Ortgies, D.H.; Fernández, N.; Chaves-Coira, I.; et al. Rare-earth-doped Fluoride Nanoparticles with Engineered Long Luminescence Lifetime for Time-gated In Vivo Optical Imaging in the Second Biological Window. Nanoscale 2018, 10, 17771–17780. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Wei, H.-L.; Liu, Y.; Chen, C.; Guan, M.; Wang, S.; Su, Y.; Wang, H.; Chen, Z.; Jin, D. Six-photon Upconverted Excitation Energy Lock-in for Ultraviolet-C Enhancement. Nat. Commun. 2021, 12, 4367. [Google Scholar] [CrossRef]
- Huang, Q.; Ye, W.; Jiao, X.; Yu, L.; Liu, Y.; Liu, X. Efficient Upconversion Fluorescence in NaYF4: Yb3+, Er3+/mNaYF4 Multilayer core–shell Nanoparticles. J. Alloys Compd. 2018, 763, 216–222. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Z.; Yuan, W.; Kong, M.; Liu, Y.; Liu, Y.; Gao, Y.; Feng, W.; Wang, F.; Zhou, J.; et al. High-sensitivity Imaging of Time-domain Near-infrared Light Transducer. Nat. Photon. 2019, 13, 525–531. [Google Scholar] [CrossRef]
- Li, H.; Tan, M.; Wang, X.; Li, F.; Zhang, Y.; Zhao, L.; Yang, C.; Chen, G. Temporal Multiplexed In Vivo Upconversion Imaging. J. Am. Chem. Soc. 2020, 142, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, Y.; Wang, R.; Li, X.; Fan, L.; Zhang, F. High-capacity Upconversion Wavelength and Lifetime Binary Encoding for Multiplexed Biodetection. Angew. Chem. 2018, 130, 13006–13011. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, P.; Lu, Y.; Wang, R.; Zhou, L.; Zheng, X.; Li, X.; Piper, J.A.; Zhang, F. Lifetime-engineered NIR-II Nanoparticles Unlock Multiplexed In Vivo Imaging. Nature Nanotechnol. 2018, 13, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, A.; Runowski, M.; Shyichuk, A.; Majewska, M.; Grzyb, T. Tunable Yellow-green Up-conversion Emission and Luminescence Lifetimes in Yb3+-Er3+-Ho3+ Multi-doped β-NaLuF4 Crystals. J. Alloys Compd. 2019, 793, 96–106. [Google Scholar] [CrossRef]
- Siefe, C.; Mehlenbacher, R.D.; Peng, C.S.; Zhang, Y.; Fischer, S.; Lay, A.; McLellan, C.A.; Alivisatos, A.P.; Chu, S.; Dionne, J.A. Sub-20 nm Core–Shell–Shell Nanoparticles for Bright Upconversion and Enhanced Förster Resonant Energy Transfer. J. Am. Chem. Soc. 2019, 141, 16997–17005. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Wang, Y.-F.; Xiao, J.-W.; Tu, D.; Chen, X.; Yan, C.-H. Photon Upconversion in Yb3+–Tb3+ and Yb3+–Eu3+ Activated Core/shell Nanoparticles with Dual-band Excitation. J. Mater. Chem. C 2016, 4, 4186–4192. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.H.; Zhang, H.; Fan, Y.; Zhang, F. Independent Luminescent Lifetime and Intensity Tuning of Upconversion Nanoparticles by Gradient Doping for Multiplexed Encoding. Angew. Chem. 2021, 133, 7117–7121. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, Y.; Pei, P.; Sun, C.; Lu, L.; Zhang, F. Tm3+-Sensitized NIR-II Fluorescent Nanocrystals for In Vivo Information Storage and Decoding. Angew. Chem. Int. Ed. 2019, 58, 10153–10157. [Google Scholar] [CrossRef]
- Liu, X.; Yi, Z.; Qin, X.; Liu, H.; Huang, W.; Liu, X. Tuning Long-Lived Mn(II) Upconversion Luminescence through Alkaline-Earth Metal Doping and Energy-Level Tailoring. Adv. Opt. Mater. 2019, 7, 1900519. [Google Scholar] [CrossRef]
- Han, X.; Song, E.; Chen, W.; Zhou, Y.; Zhang, Q. Color-tunable Upconversion Luminescence and Prolonged Eu3+ Fluorescence Lifetime in Fluoride KCdF3: Yb3+,Mn2+,Eu3+ via Controllable and Efficient Energy Transfer. J. Mater. Chem. C 2020. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Li, X.; Yi, Z.; Deng, R.; Liang, L.; Xie, X.; Loong, D.T.B.; Song, S.; Fan, D.; et al. Binary Temporal Upconversion Codes of Mn2+-activated Nanoparticles for Multilevel Anti-counterfeiting. Nat. Commun. 2017, 8, 899. [Google Scholar] [CrossRef]
- Qin, H.; Wu, D.; Sathian, J.; Xie, X.; Ryan, M.; Xie, F. Tuning the Upconversion Photoluminescence Lifetimes of NaYF4: Yb3+, Er3+ through Lanthanide Gd3+ Doping. Sci. Rep. 2018, 8, 12683. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.C.; Kalyakina, A.S.; Singh, K.; Tye, M.A.; Mazitschek, R. Bright and Stable Luminescent Probes for Target Engagement Profiling in Live Cells. Nat. Chem. Biol. 2021, 17, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Muhr, V.; Würth, C.; Kraft, M.; Buchner, M.; Baeumner, A.J.; Resch-Genger, U.; Hirsch, T. Particle-Size-Dependent Förster Resonance Energy Transfer from Upconversion Nanoparticles to Organic Dyes. Anal. Chem. 2017, 89, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Gu, Y.; Liu, Y.; Shi, Y.; Wu, N.; Feng, W.; Li, F. Luminescence Lifetime–Based In Vivo Detection with Responsive Rare Earth–Dye Nanocomposite. Small 2019, 15, 1904487. [Google Scholar] [CrossRef]
- Wang, M.; Wei, H.; Wang, S.; Hu, C.; Su, Q. Dye Sensitization for Ultraviolet Upconversion Enhancement. Nanomaterials 2021, 11, 3114. [Google Scholar] [CrossRef]
- Xue, M.; Cao, C.; Zhou, X.; Xu, M.; Feng, W.; Li, F. Tuning the Upconversion Efficiency and Spectrum of Upconversion Nanoparticles through Surface Decorating of an Organic Dye. Inorg. Chem. 2019, 58, 14490–14497. [Google Scholar] [CrossRef]
- Lyu, C.; Li, H.; Wyatt, P.B.; Gillin, W.P.; Ye, H. Prolonged and Efficient Near-infrared Photoluminescence of a Sensitized Organic Ytterbium-containing Molecular Composite. J. Mater. Chem. C 2020, 8, 9502–9505. [Google Scholar] [CrossRef]
- Xu, H.; Han, S.; Deng, R.; Su, Q.; Wei, Y.; Tang, Y.; Qin, X.; Liu, X. Anomalous Upconversion Amplification Induced by Surface Reconstruction in Lanthanide Sublattices. Nat. Photon. 2021, 15, 732–737. [Google Scholar] [CrossRef]
- Singh, N.S.; Ningthoujam, R.S.; Yaiphaba, N.; Singh, S.D.; Vatsa, R.K. Lifetime and Quantum Yield Studies of Dy3+ Doped GdVO4 Nanoparticles: Concentration and Annealing Effect. J. Appl. Phys. 2009, 105, 064303. [Google Scholar] [CrossRef]
- Singh, N.S.; Ningthoujam, R.S.; Luwang, M.N.; Singh, S.D.; Vatsa, R.K. Luminescence, Lifetime and Quantum Yield Studies of YVO4: Ln3+ (Ln3+=Dy3+, Eu3+) Nanoparticles: Concentration and Annealing Effects. Chem. Phys. Lett. 2009, 480, 237–242. [Google Scholar] [CrossRef]
- Yu, W.; Xu, W.; Song, H.; Zhang, S. Temperature-dependent Upconversion Luminescence and Dynamics of NaYF4: Yb3+/Er3+ Nanocrystals: Influence of Particle Size and Crystalline Phase. Dalton Trans. 2014, 43, 6139–6147. [Google Scholar] [CrossRef]
- Chihara, T.; Umezawa, M.; Miyata, K.; Sekiyama, S.; Hosokawa, N.; Okubo, K.; Kamimura, M.; Soga, K. Biological Deep Temperature Imaging with Fluorescence Lifetime of Rare-Earth-Doped Ceramics Particles in the Second NIR Biological Window. Sci. Rep. 2019, 9, 12806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhao, L.; Guo, Y.; Yu, H. Up-conversion Luminescence Lifetime Thermometry Based on the 1G4 State of Tm3+ Modulated by Cross Relaxation Processes. Dalton Trans. 2019, 48, 16034–16040. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, O.A.; Haro-González, P.; Carvajal, J.J.; Jaque, D.; Massons, J.; Aguiló, M.; Díaz, F. Er: Yb: NaY2F5O up-converting nanoparticles for sub-tissue fluorescence lifetime thermal sensing. Nanoscale 2014, 6, 9727–9733. [Google Scholar] [CrossRef]
- Tan, M.; Li, F.; Cao, N.; Li, H.; Wang, X.; Zhang, C.; Jaque, D.; Chen, G. Accurate In Vivo Nanothermometry through NIR-II Lanthanide Luminescence Lifetime. Small 2020, 16, 2004118. [Google Scholar] [CrossRef]
- Lu, K.; Yi, Y.; Xu, L.; Sun, X.; Liu, L.; Li, H. Temperature-Independent Lifetime and Thermometer Operated in a Biological Window of Upconverting NaErF4 Nanocrystals. Nanomaterials 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Feng, W.; Yang, P.; Huang, C.; Li, F. The Biosafety of Lanthanide Upconversion Nanomaterials. Chem. Soc. Rev. 2015, 44, 1509–1525. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.; Wu, Y.; He, H.; Zhu, X.; Zhang, H.; Dou, C.; Feng, L.; Fan, Y.; Zhang, F. A Tumor-Microenvironment-Responsive Lanthanide–Cyanine FRET Sensor for NIR-II Luminescence-Lifetime in Situ Imaging of Hepatocellular Carcinoma. Adv. Mater. 2020, 32, 2001172. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Qu, Z.-b.; Chen, Z.-H.; Han, X.-Y.; Deng, L.-X.; Luo, Q.; Jin, Z.; Shi, G.; Zhang, M. The Marriage of Protein and Lanthanide: Unveiling a Time-Resolved Fluorescence Sensor Array Regulated by pH toward High-Throughput Assay of Metal Ions in Biofluids. Anal. Chem. 2019, 91, 11170–11177. [Google Scholar] [CrossRef]
- Hanaoka, K.; Kikuchi, K.; Kobayashi, S.; Nagano, T. Time-Resolved Long-Lived Luminescence Imaging Method Employing Luminescent Lanthanide Probes with a New Microscopy System. J. Am. Chem. Soc. 2007, 129, 13502–13509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Shen, B.; Yuan, W.; Zhou, X.; Liu, Q.; Kong, M.; Shi, Y.; Yang, P.; Feng, W.; Li, F. Time-Gated Ratiometric Detection with the Same Working Wavelength to Minimize the Interferences from Photon Attenuation for Accurate In Vivo Detection. ACS Central Sci. 2019, 5, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Ma, H.; Song, B.; Dai, Z.; Zheng, X.; Zhang, R.; Chen, K.; Yuan, J. Time-gated Luminescence Probe for Ratiometric and Luminescence Lifetime Detection of Hypochorous Acid in Lysosomes of Live Cells. Talanta 2020, 212, 120760. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Ye, Z.; Yang, Y.; Ma, H.; Zheng, X.; Jin, D.; Yuan, J. Background-free in-vivo Imaging of Vitamin C Using Time-gateable Responsive Probe. Sci. Rep. 2015, 5, 14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Lu, J.; Zhao, J.; Cusido, J.; Raymo, F.M.; Yuan, J.; Yang, S.; Leif, R.C.; Huo, Y.; Piper, J.A.; et al. On-the-fly Decoding Luminescence Lifetimes in the Microsecond Region for Lanthanide-encoded Suspension Arrays. Nat. Commun. 2014, 5, 3741. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, S.; Tonai, K.; Kaneko, M.; Kikuchi, K. Lanthanide-Based Protease Activity Sensors for Time-Resolved Fluorescence Measurements. J. Am. Chem. Soc. 2008, 130, 14376–14377. [Google Scholar] [CrossRef]
- Mizukami, S.; Yamamoto, T.; Yoshimura, A.; Watanabe, S.; Kikuchi, K. Covalent Protein Labeling with a Lanthanide Complex and Its Application to Photoluminescence Lifetime-Based Multicolor Bioimaging. Angew. Chem. Int. Ed. 2011, 50, 8750–8752. [Google Scholar] [CrossRef]
- Vuojola, J.; Syrjänpää, M.; Lamminmäki, U.; Soukka, T. Genetically Encoded Protease Substrate Based on Lanthanide-Binding Peptide for Time-Gated Fluorescence Detection. Anal. Chem. 2013, 85, 1367–1373. [Google Scholar] [CrossRef]
- Tu, D.; Liu, L.; Ju, Q.; Liu, Y.; Zhu, H.; Li, R.; Chen, X. Time-Resolved FRET Biosensor Based on Amine-Functionalized Lanthanide-Doped NaYF4 Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 6306–6310. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Tu, D.; Chen, Z.; Huang, M.; Zhu, H.; Ma, E.; Chen, X. Amine-Functionalized Lanthanide-Doped Zirconia Nanoparticles: Optical Spectroscopy, Time-Resolved Fluorescence Resonance Energy Transfer Biodetection, and Targeted Imaging. J. Am. Chem. Soc. 2012, 134, 15083–15090. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, S.; Chen, Z.; Hu, P.; Liu, Y.; Tu, D.; Zhu, H.; Li, R.; Huang, M.; Chen, X. Sub-10 nm Lanthanide-doped CaF2 Nanoprobes for Time-resolved Luminescent Biodetection. Angew. Chem. Int. Ed. 2013, 52, 6671–6676. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zeng, Q.; Zheng, J.; Xing, D.; Zhang, T. Aptamer-Functionalized Upconverting Nanoformulations for Light-Switching Cancer-Specific Recognition and in Situ Photodynamic–Chemo Sequential Theranostics. ACS Appl. Mater. Inter. 2021, 13, 9316–9328. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q. Multiple Fluorescent Labeling of Silica Nanoparticles with Lanthanide Chelates for Highly Sensitive Time-Resolved Immunofluorometric Assays. Clin. Chem. 2007, 53, 1503–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Martin, J.; Lu, Y.; Zhao, J.; Yuan, J.; Ostrowski, M.; Paulsen, I.; Piper, J.A.; Jin, D. Resolving Low-Expression Cell Surface Antigens by Time-Gated Orthogonal Scanning Automated Microscopy. Anal. Chem. 2012, 84, 9674–9678. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, X.; Lei, W.; He, C.; You, W.; Lin, Q.; Zhou, S.; Chen, X.; Chen, Z. Direct Detection of Circulating Tumor Cells in Whole Blood Using Time-Resolved Luminescent Lanthanide Nanoprobes. Angew. Chem. Int. Ed. 2019, 58, 12195–12199. [Google Scholar] [CrossRef]
- Sayyadi, N.; Justiniano, I.; Connally, R.E.; Zhang, R.; Shi, B.; Kautto, L.; Everest-Dass, A.V.; Yuan, J.; Walsh, B.J.; Jin, D.; et al. Sensitive Time-Gated Immunoluminescence Detection of Prostate Cancer Cells Using a TEGylated Europium Ligand. Anal. Chem. 2016, 88, 9564–9571. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Zhang, H.; Zhao, M.; Pei, P.; Chen, Y.; Yang, Y.; Lu, L.; Yu, P.; Sun, C.; et al. High-Fidelity NIR-II Multiplexed Lifetime Bioimaging with Bright Double Interfaced Lanthanide Nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 23545–23551. [Google Scholar] [CrossRef]
- Siaï, A.; Haro-González, P.; Horchani-Naifer, K.; Férid, M. La2O3: Tm, Yb, Er upconverting nano-oxides for sub-tissue lifetime thermal sensing. Sens. Actuat. B-Chem. 2016, 234, 541–548. [Google Scholar] [CrossRef]
- Qiu, X.; Zhou, Q.; Zhu, X.; Wu, Z.; Feng, W.; Li, F. Ratiometric Upconversion Nanothermometry with Dual Emission at the Same Wavelength Decoded via a Time-resolved Technique. Nat. Commun. 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Skripka, A.; Lai, Y.; Jiang, C.; Liu, J.; Vetrone, F.; Liang, J. Fast Wide-field Upconversion Luminescence Lifetime Thermometry Enabled by Single-shot Compressed Ultrahigh-speed Imaging. Nat. Commun. 2021, 12, 6401. [Google Scholar] [CrossRef]
- Kong, M.; Gu, Y.; Chai, Y.; Ke, J.; Liu, Y.; Xu, X.; Li, Z.; Feng, W.; Li, F. Luminescence Interference-free Lifetime Nanothermometry Pinpoints In Vivo Temperature. Sci. China Chem. 2021, 64, 974–984. [Google Scholar] [CrossRef]
- Estebanez, N.; Ferrera-González, J.; Cortez-Cevallos, I.A.; González-Béjar, M.; Pérez-Prieto, J. Lengthening the Lifetime of Common Emissive Probes to Microseconds by a Jigsaw-Like Construction of NIR-Responsive Nanohybrids. Adv. Opt. Mater. 2020, 8, 1902030. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, Q.; Zhou, X.; Gu, Y.; Yuan, W.; Feng, W.; Li, F. Reversible Ratiometric Probe Combined with the Time-Gated Method for Accurate In Vivo Gastrointestinal pH Sensing. ACS Appl. Mater. Inter. 2020, 12, 25557–25564. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Cheng, S.; Wang, J.-X.; Liu, Y.-W.; Feng, W.; Li, F.; Zhang, J.-L. Fluorescence Lifetime Imaging of Upper Gastrointestinal pH In Vivo with a Lanthanide Based Near-infrared τ Probe. Chem. Sci. 2019, 10, 4227–4235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, J.; Yoon, J.; Zhou, M.; Wei, H.-L.; Goh, Y.; Lok, K.; Liu, Y.; Li, Z.; Wang, H.; Su, Q.; et al. Deciphering Nanoparticle Trafficking into Glioblastomas Uncovers an Augmented Antitumor Effect of Metronomic Chemotherapy. Adv. Mater. 2022, 34, 2106194. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Hu, C.; Su, Q. Luminescent Lifetime Regulation of Lanthanide-Doped Nanoparticles for Biosensing. Biosensors 2022, 12, 131. https://doi.org/10.3390/bios12020131
Wang M, Hu C, Su Q. Luminescent Lifetime Regulation of Lanthanide-Doped Nanoparticles for Biosensing. Biosensors. 2022; 12(2):131. https://doi.org/10.3390/bios12020131
Chicago/Turabian StyleWang, Mingkai, Chuanyu Hu, and Qianqian Su. 2022. "Luminescent Lifetime Regulation of Lanthanide-Doped Nanoparticles for Biosensing" Biosensors 12, no. 2: 131. https://doi.org/10.3390/bios12020131