All Fiber-Optic Immunosensors Based on Elliptical Core Helical Intermediate-Period Fiber Grating with Low-Sensitivity to Environmental Disturbances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
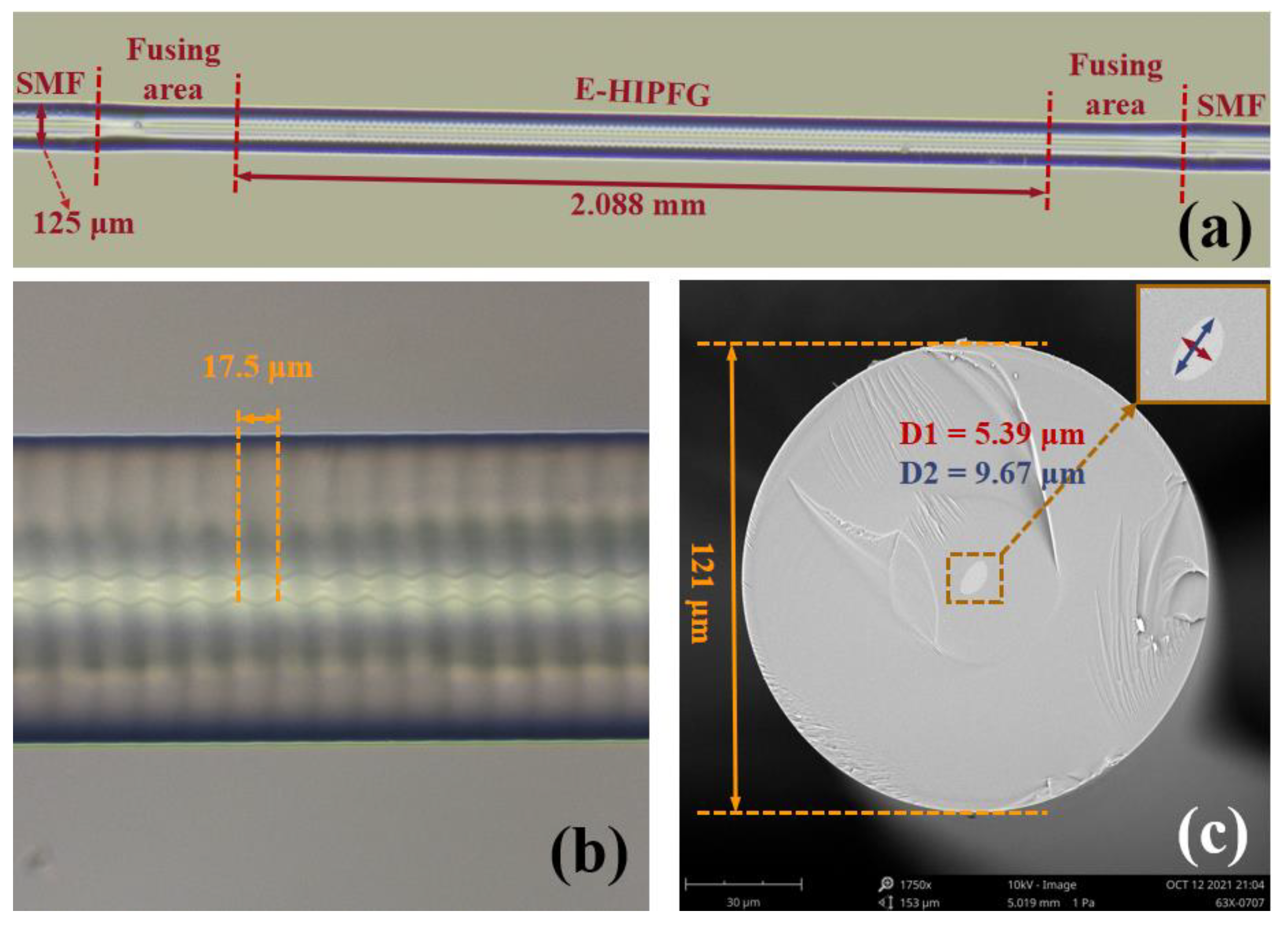
2.2. Fabrication of the E-HIPFG
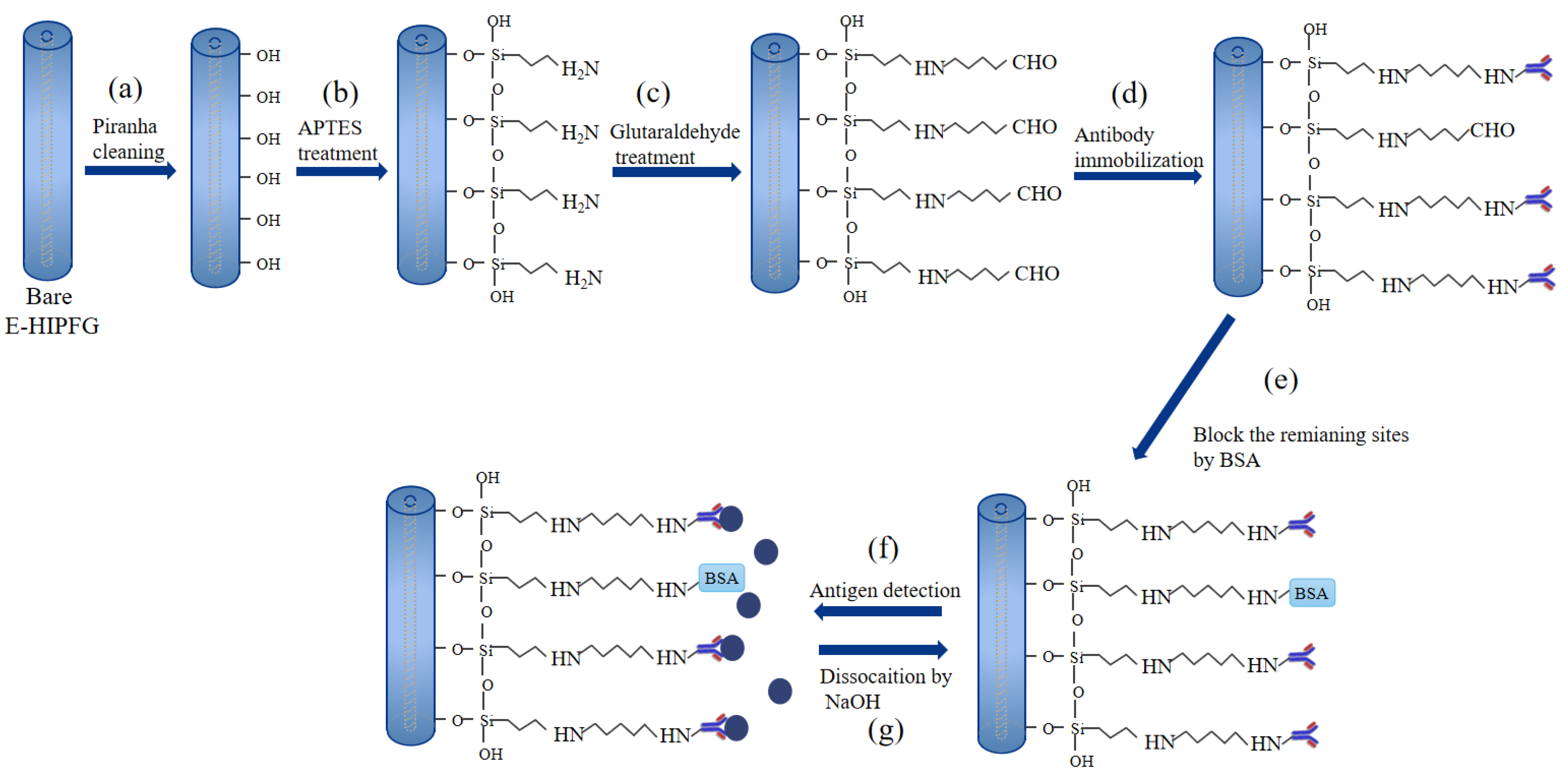
2.3. The Surface Functionalization of E-HIPFG
2.4. Sensing System
3. Results
3.1. The Characteristic of Bare E-HIPFG
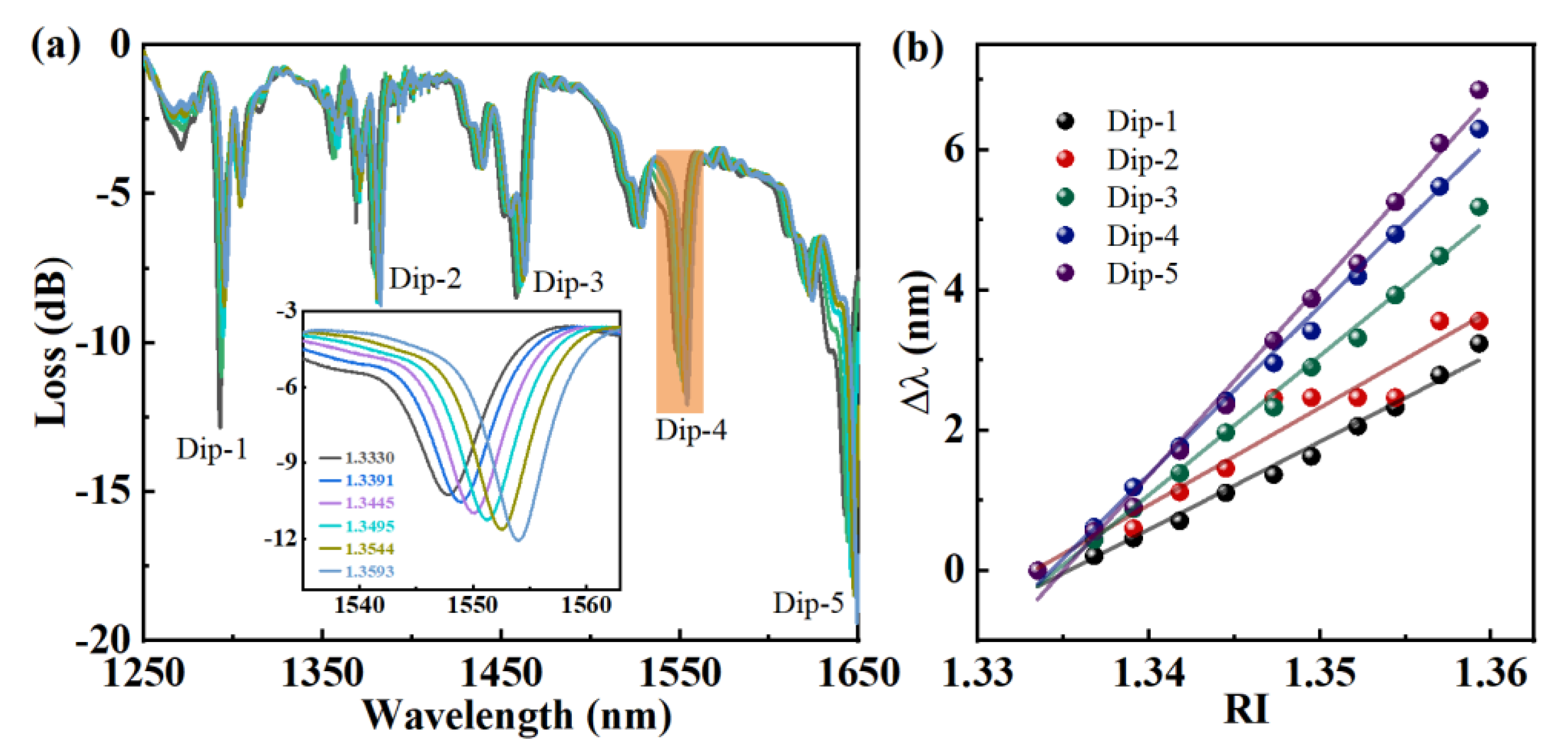
3.2. Immunosensing Properties of Functionalization E-HIPFG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, E.B.; Alter, G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 2020, 30, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, H.Y. Immunoglobulin G and Its Function in the Human Respiratory Tract. Mayo Clin. Proc. 1988, 63, 161–174. [Google Scholar] [CrossRef]
- Biburger, M.; Aschermann, S.; Schwab, I.; Lux, A.; Albert, H.; Danzer, H.; Woigk, M.; Dudziak, D.; Nimmerjahn, F. Monocyte Subsets Responsible for Immunoglobulin G-Dependent Effector Functions In Vivo. Immunity 2011, 35, 932–944. [Google Scholar] [CrossRef] [Green Version]
- Lekchnov, E.A.; Sedykh, S.E.; Dmitrenok, P.S.; Buneva, V.; Nevinsky, G.A. Human placenta: Relative content of antibodies of different classes and subclasses (IgG1–IgG4) containing lambda- and kappa-light chains and chimeric lambda-kappa-immunoglobulins. Int. Immunol. 2015, 27, 297–306. [Google Scholar] [CrossRef]
- Pitcher-Wilmott, R.W.; Hindocha, P.; Wood, C.B. The placental transfer of IgG subclasses in human pregnancy. Clin. Exp. Immunol. 1980, 41, 303–308. [Google Scholar]
- Chenoweth, A.M.; Wines, B.D.; Anania, J.C.; Hogarth, P.M. Harnessing the immune system via FcγR function in immune therapy: A pathway to next-gen mAbs. Immunol. Cell Biol. 2020, 98, 287–304. [Google Scholar] [CrossRef] [Green Version]
- Quinn, C.P.; Semenova, V.A.; Elie, C.M.; Romero-Steiner, S.; Greene, C.; Li, H.; Stamey, K.; Steward-Clark, E.; Schmidt, D.S.; Mothershed, E.; et al. Specific, Sensitive, and Quantitative Enzyme-Linked Immunosorbent Assay for Human Immunoglobulin G Antibodies to Anthrax Toxin Protective Antigen. Emerg. Infect. Dis. 2002, 8, 1103–1110. [Google Scholar] [CrossRef]
- Medhi, A.; Baruah, S.; Singh, J.; Betty, C.; Mohanta, D. Au nanoparticle modified GO/PEDOT-PSS based immunosensor probes for sensitive and selective detection of serum immunoglobulin g (IgG). Appl. Surf. Sci. 2021, 575, 151775. [Google Scholar] [CrossRef]
- Jaruwongrungsee, K.; Waiwijit, U.; Wisitsoraat, A.; Sangworasil, M.; Pintavirooj, C.; Tuantranont, A. Real-time multianalyte biosensors based on interference-free multichannel monolithic quartz crystal microbalance. Biosens. Bioelectron. 2015, 67, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Wang, W.; Wang, F.; Zhao, Y.; Cai, Q.W.; Xie, R.; Ju, X.-J.; Liu, Z.; Faraj, Y.; Chu, L.Y. Smart hydrogel grating immunosensors for highly selective and sensitive detection of human-IgG. Ind. Eng. Chem. Res. 2020, 59, 10469–10475. [Google Scholar] [CrossRef]
- Wang, B.T.; Wang, Q. An interferometric optical fiber biosensor with high sensitivity for IgG/anti-IgG immunosensing. Opt. Commun. 2018, 42, 388–394. [Google Scholar] [CrossRef]
- Sridevi, S.; Vasu, K.; Asokan, S.; Sood, A. Sensitive detection of C-reactive protein using optical fiber Bragg gratings. Biosens. Bioelectron. 2015, 65, 251–256. [Google Scholar] [CrossRef]
- Juste-Dolz, A.; Delgado-Pinar, M.; Avella-Oliver, M.; Fernández, E.; Pastor, D.; Andrés, M.V. Maquieira, Ángel BIO bragg gratings on microfibers for label-free biosensing. Biosens. Bioelectron. 2021, 176, 112916. [Google Scholar] [CrossRef]
- Ran, Y.; Long, J.; Xu, Z.; Yin, Y.; Hu, D.; Long, X.; Zhang, Y.; Liang, L.; Liang, H.; Guan, B.O. Harmonic optical microfiber Bragg grating immunosensor for the accelerative test of cardiac biomarker (cTn-I). Biosens. Bioelectron. 2021, 179, 113081. [Google Scholar] [CrossRef]
- Xiao, P.; Xu, Z.; Hu, D.; Liang, L.; Sun, L.; Li, J.; Ran, Y.; Guan, B.-O. Efficiently Writing Bragg Grating in High-Birefringence Elliptical Microfiber for Label-Free Immunosensing with Temperature Compensation. Adv. Fiber Mater. 2021, 3, 321–330. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2018, 68, 3350–3357. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Su, R.; Liu, B.; Huang, R.; Qi, W.; He, Z. A polydopamine-modified optical fiber SPR biosensor using electroless-plated gold films for immunoassays. Biosens. Bioelectron. 2015, 74, 454–460. [Google Scholar] [CrossRef]
- Aray, A.; Chiavaioli, F.; Arjmand, M.; Trono, C.; Tombelli, S.; Giannetti, A.; Cennamo, N.; Soltanolkotabi, M.; Zeni, L.; Baldini, F. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J. Biophotonics 2016, 9, 1077–1084. [Google Scholar] [CrossRef]
- Esposito, F.; Sansone, L.; Srivastava, A.; Baldini, F.; Campopiano, S.; Chiavaioli, F.; Giordano, M.; Giannetti, A.; Iadicicco, A. Long period grating in double cladding fiber coated with graphene oxide as high-performance optical platform for biosensing. Biosens. Bioelectron. 2021, 172, 112747. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Baldini, F.; Tombelli, S.; Trono, C.; Giannetti, A. Biosensing with optical fiber gratings. Nanophotonics 2017, 6, 663–679. [Google Scholar] [CrossRef]
- Dong, J.; Sang, M.; Wang, S.; Xu, T.; Yu, X.; Liu, T. Ultrasensitive Label-Free Biosensor Based on the Graphene-Oxide-Coated-U-Bent Long-Period Fiber Grating Inscribed in a Two-Mode Fiber. J. Light. Technol. 2021, 39, 4013–4019. [Google Scholar] [CrossRef]
- Liu, C.; Cai, Q.; Xu, B.; Zhu, W.; Zhang, L.; Zhao, J.; Chen, X. Graphene oxide functionalized long period grating for ultrasensitive label-free immunosensing. Biosens. Bioelectron. 2017, 94, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; Srivastava, A.; Sansone, L.; Giordano, M.; Campopiano, S.; Iadicicco, A. Label-Free Biosensors Based on Long Period Fiber Gratings: A Review. IEEE Sens. J. 2021, 21, 12692–12705. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, Y.; Han, M.; Gong, J.; Wang, A. Fully Distributed Fiber-Optic Biological Sensing. IEEE Photon- Technol. Lett. 2010, 22, 1553–1555. [Google Scholar] [CrossRef]
- Pilla, P.; Sandomenico, A.; Malachovská, V.; Borriello, A.; Giordano, M.; Cutolo, A.; Ruvo, M.; Cusano, A. A protein-based biointerfacing route toward label-free immunoassays with long period gratings in transition mode. Biosens. Bioelectron. 2012, 31, 486–491. [Google Scholar] [CrossRef]
- Biswas, P.; Dey, T.K.; Basumallick, N.; Bandyopadhyay, S.; Ghosh, A.; Bandyopadhyay, S. Realization of high sensitive refractive index sensor with enhanced contrast using an over coupled long period fiber grating operating at mode transition. In Proceedings of the 13th International Conference on Fiber Optics and Photonics, Kanpur, India, 4–8 December 2016. [Google Scholar]
- Zou, T.; Zhong, J.; Liu, S.; Zhu, G.; Zhao, Y.; Luo, J.; Lu, S.; Zhang, Q.; He, J.; Bai, Z.; et al. Helical Intermediate-Period Fiber Grating for Refractive Index Measurements with Low-Sensitive Temperature and Torsion Response. J. Light. Technol. 2021, 39, 6678–6685. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Luo, J.; Chen, Y.; Fu, C.; Xiong, C.; Wang, Y.; Jing, S.; Bai, Z.; Liao, C. Torsion, Refractive Index, and Temperature Sensors Based on An Improved Helical Long Period Fiber Grating. J. Light. Technol. 2020, 38, 2504–2510. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Xiong, C.; Wang, Y.; Li, Z.; Sun, Z.; Li, J. Magnetic field sensor based on helical long-period fiber grating with a three-core optical fiber. Opt. Express 2021, 29, 20649–20656. [Google Scholar] [CrossRef]
- Sun, B.; Wei, W.; Liao, C.; Zhang, L.; Zhang, Z.; Chen, M.-Y.; Wang, Y. Automatic Arc Discharge-Induced Helical Long Period Fiber Gratings and Its Sensing Applications. IEEE Photon-Technol. Lett. 2017, 29, 873–876. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhang, Z.; Shao, L.; Liu, S.; Liu, Y.; Pang, Y.; Bai, Z.; Fu, C.; Cui, W.; Qi, L.; et al. Broadband tunable orbital angular momentum mode converter based on a conventional single-mode all-fiber configuration. Opt. Express 2021, 29, 15595–15603. [Google Scholar] [CrossRef]
- Fu, C.; Liu, S.; Bai, Z.; He, J.; Liao, C.; Wang, Y.; Li, Z.; Zhang, Y.; Yang, K.; Bin, Y.; et al. Orbital Angular Momentum Mode Converter Based on Helical Long Period Fiber Grating Inscribed by Hydrogen–Oxygen Flame. J. Light. Technol. 2018, 36, 1683–1688. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Wang, C.; Sun, Z.; Yin, G.; Zhou, K.; Wang, Y.; Zhao, W.; Zhang, L. Theoretical and experimental analysis of excessively tilted fiber gratings. Opt. Express 2016, 24, 12107–12115. [Google Scholar] [CrossRef] [Green Version]
- Shu, X.; Zhang, L.; Bennion, I. Sensitivity characteristics of long-period fiber gratings. J. Light. Technol. 2002, 20, 255–266. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Gouveia, C.A.J.; Jorge, P.A.S.; Baldini, F. Towards a Uniform Metrological Assessment of Grating-Based Optical Fiber Sensors: From Refractometers to Biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, Y.; Guo, J.; Liu, W.; Liu, L.; Meng, Y.; Yu, X. Temperature-Insensitive Label-Free Sensors for Human IgG Based on S-Tapered Optical Fiber Sensors. IEEE Access 2021, 9, 116286–116293. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.-Z.; Song, H.; Zhao, W.-M.; Jing, J.-Y. A dual channel self-compensation optical fiber biosensor based on coupling of surface plasmon polariton. Opt. Laser Technol. 2020, 124, 106002. [Google Scholar] [CrossRef]
- Shu, X.; Huang, D. Highly sensitive chemical sensor based on the measurement of the separation of dual resonant peaks in a 100-μm-period fiber grating. Opt. Commun. 1999, 171, 65–69. [Google Scholar] [CrossRef]
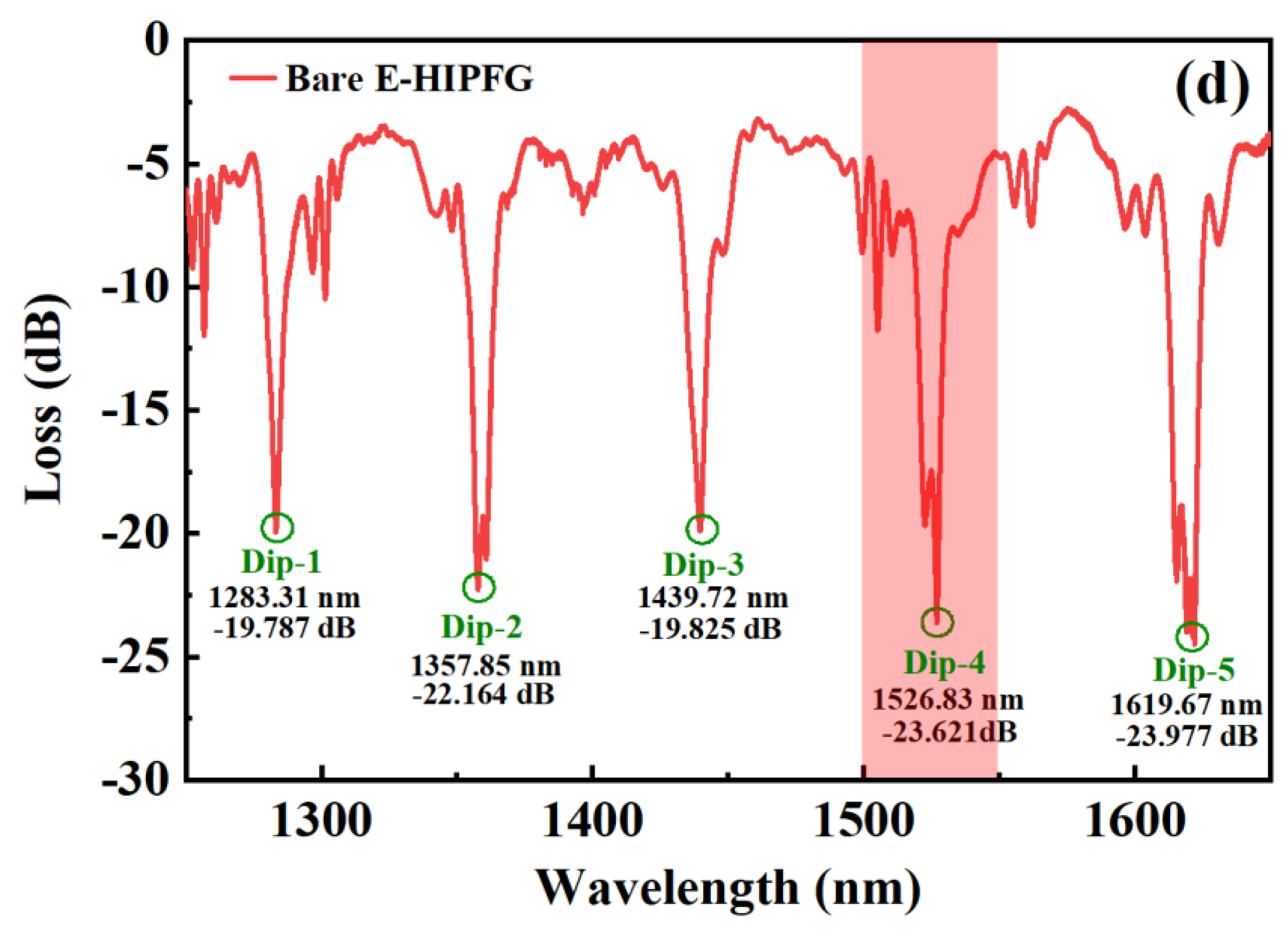
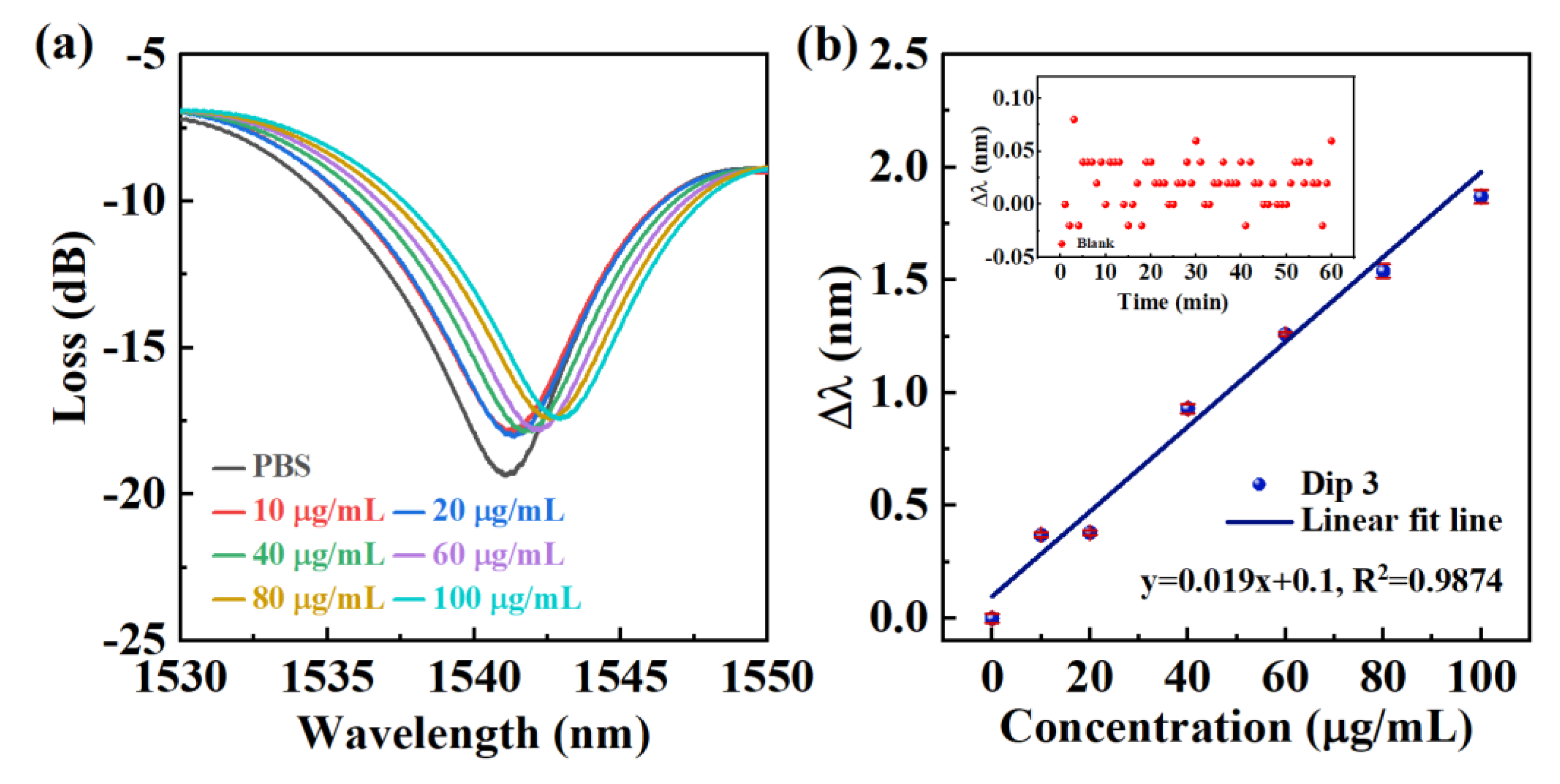

| Dip Features (in Air) | Dip-1 | Dip-2 | Dip-3 | Dip-4 | Dip-5 |
|---|---|---|---|---|---|
| Wavelength (nm) | 1238.31 | 1357.85 | 1439.72 | 1526.83 | 1619.67 |
| Loss (dB) | −19.787 | −22.164 | −19.825 | −23.621 | −23.977 |
| RI sensitivity (nm/RIU)/R2 | 125.16/0.9850 | 138.81/0.9524 | 198.76/0.9929 | 239.78/0.9948 | 270.67/0.9915 |
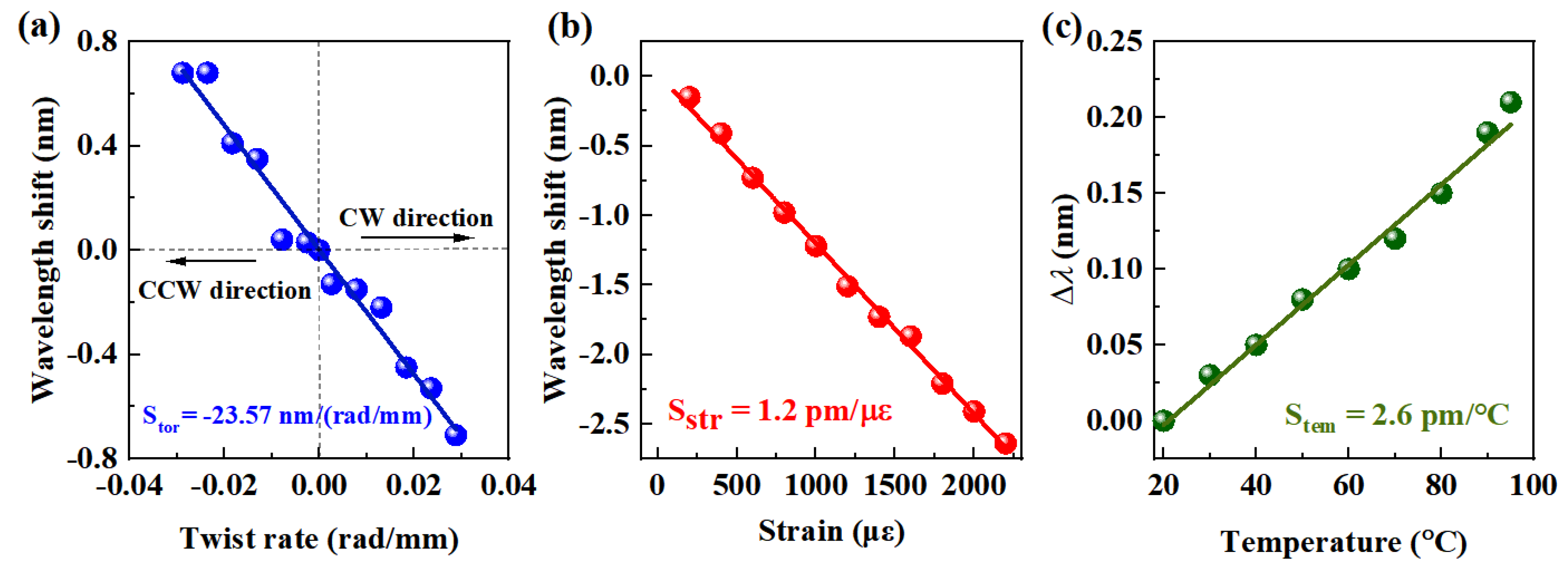
| Sensor Structure | Extra Materials/Architectures | SIgG (nm/μg/mL) | LOD (μg/mL) | Stor (nm/(rad/mm)) | SStr (pm/με) | Stem (pm/°C) | Ref. |
|---|---|---|---|---|---|---|---|
| U-bent LPFG | 1 GO coating and U-bent structure | - | 0.07 | - | 3.04 | 40.4 | [13] |
| Dual-channel SPR | Au film/GO/Au2 NPs coating and MMF-PCF | - | 0.015 | - | - | 5.1 | [34] |
| S-Tapered Optical Fiber | None | - | 0.028 | - | - | −20 | [33] |
| E-HIPFG | None | 0.018 | 4.7 | −23.566 | 1.2 | 2.6 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Liu, S.; Zou, T.; Yan, W.; Zhou, M.; Liu, B.; Rao, X.; Wang, Y.; Sun, Z.; Wang, Y. All Fiber-Optic Immunosensors Based on Elliptical Core Helical Intermediate-Period Fiber Grating with Low-Sensitivity to Environmental Disturbances. Biosensors 2022, 12, 99. https://doi.org/10.3390/bios12020099
Zhong J, Liu S, Zou T, Yan W, Zhou M, Liu B, Rao X, Wang Y, Sun Z, Wang Y. All Fiber-Optic Immunosensors Based on Elliptical Core Helical Intermediate-Period Fiber Grating with Low-Sensitivity to Environmental Disturbances. Biosensors. 2022; 12(2):99. https://doi.org/10.3390/bios12020099
Chicago/Turabian StyleZhong, Junlan, Shen Liu, Tao Zou, Wenqi Yan, Min Zhou, Bonan Liu, Xing Rao, Ying Wang, Zhongyuan Sun, and Yiping Wang. 2022. "All Fiber-Optic Immunosensors Based on Elliptical Core Helical Intermediate-Period Fiber Grating with Low-Sensitivity to Environmental Disturbances" Biosensors 12, no. 2: 99. https://doi.org/10.3390/bios12020099





