An Eco-Friendly Synthetic Approach for Copper Nanoclusters and Their Potential in Lead Ions Sensing and Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Instrumentation
2.3. Synthesis of Pep-CuNCs
2.4. Detection of Metal Ions
2.5. Biological Application—In-Vitro Methods
2.5.1. Human Tumor Cell Lines Utilized
2.5.2. Monolayer Cytotoxicity Bioassay
2.5.3. IC50 Determination
3. Results and Discussion
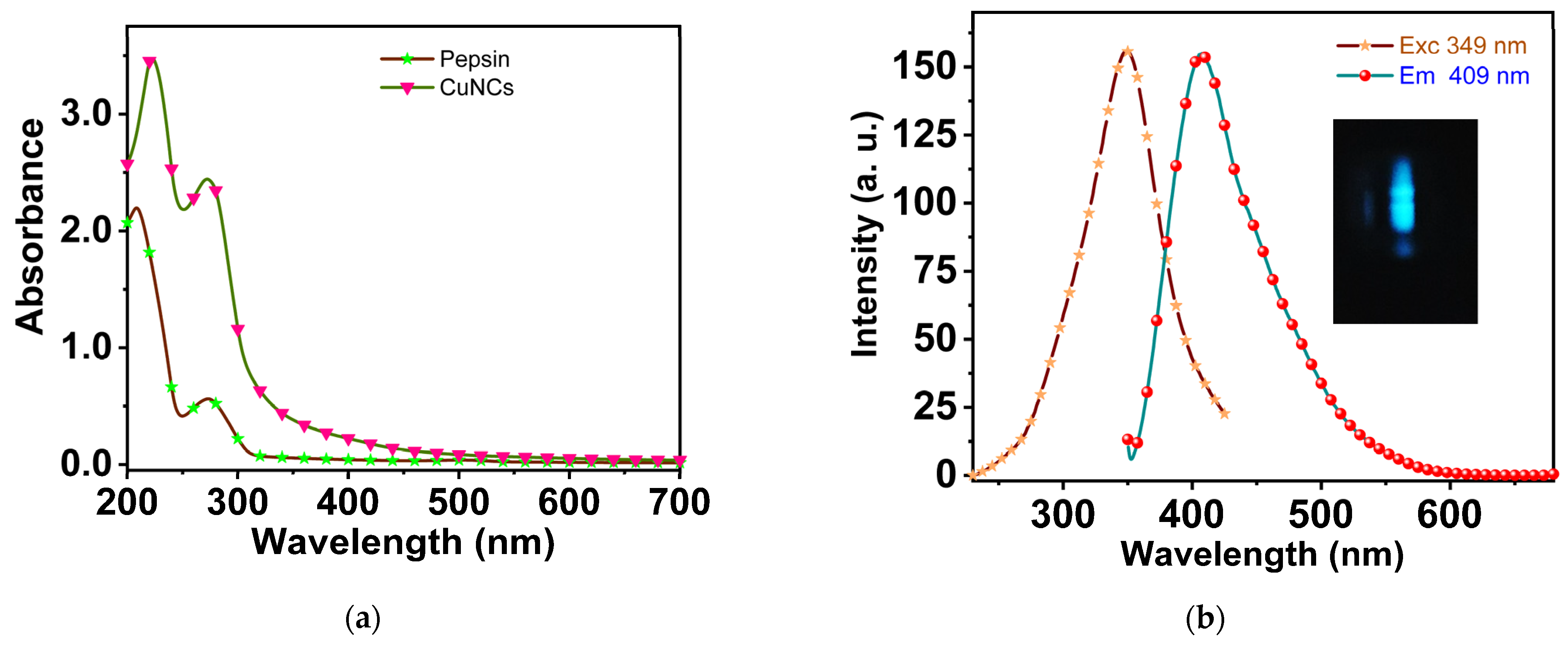
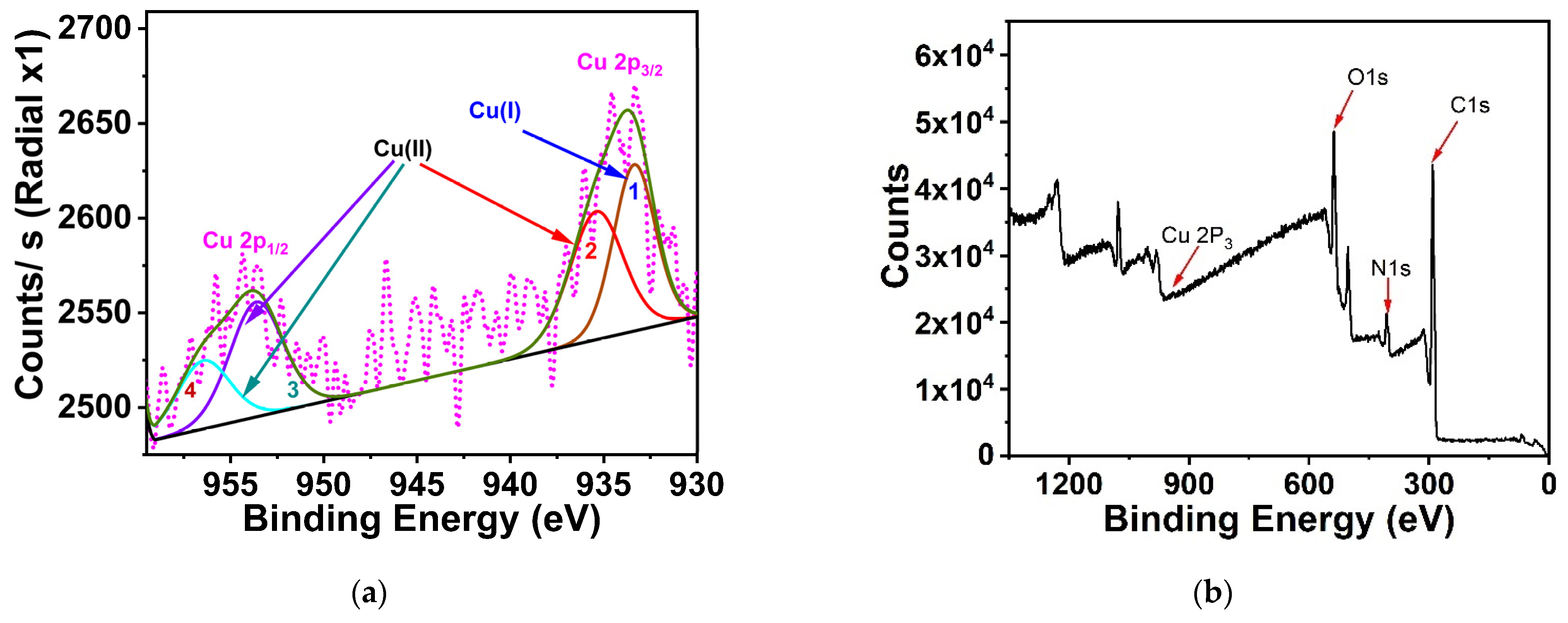
3.1. Optical Characteristics
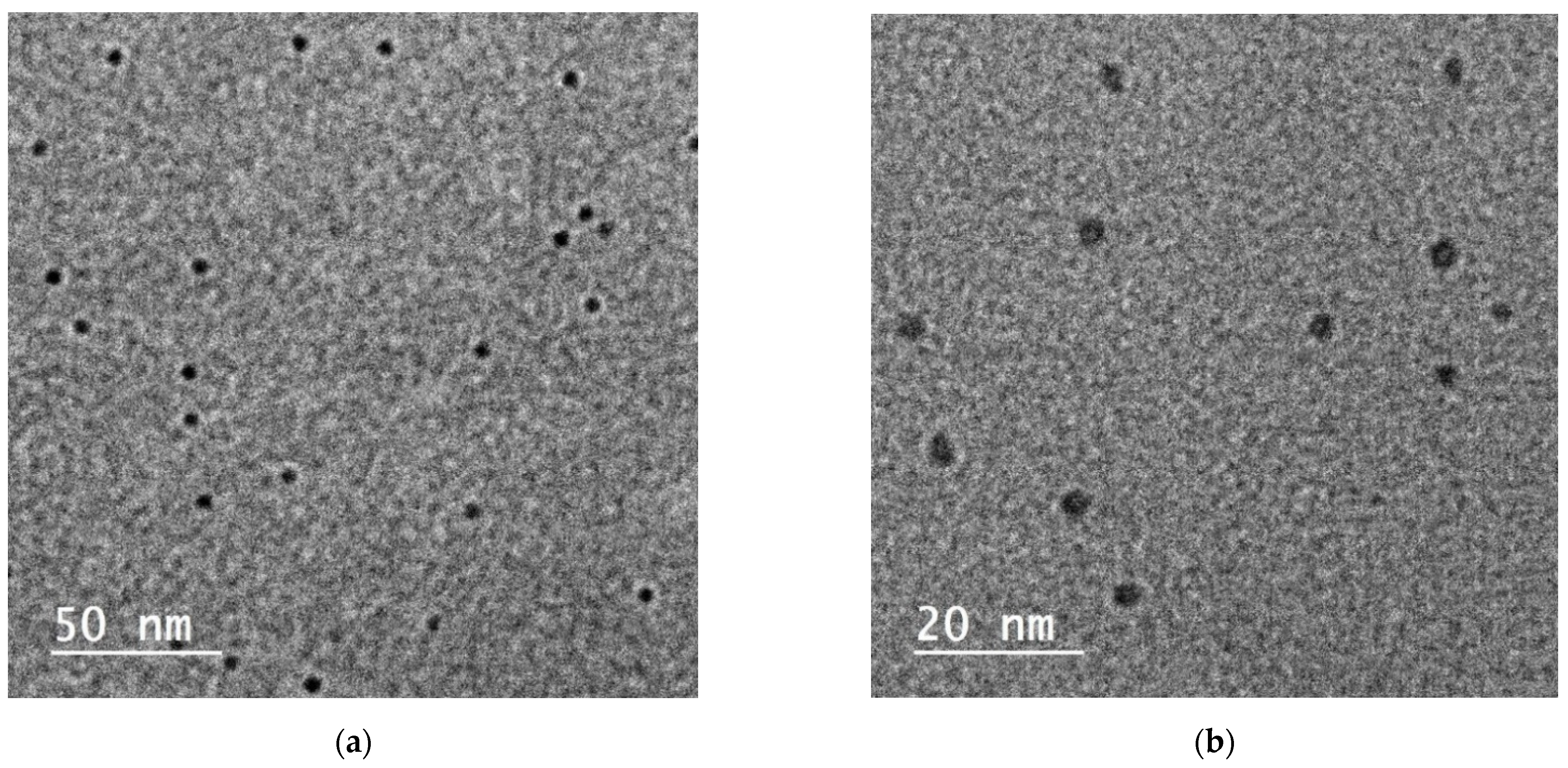
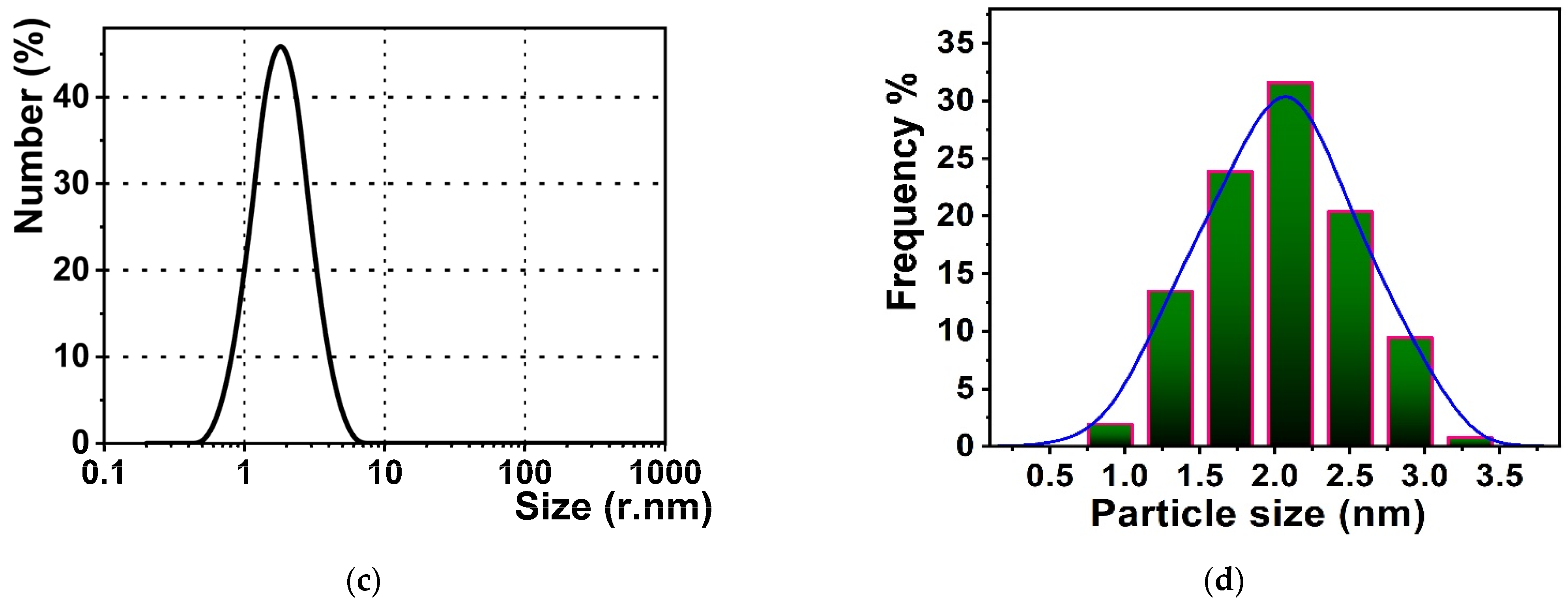
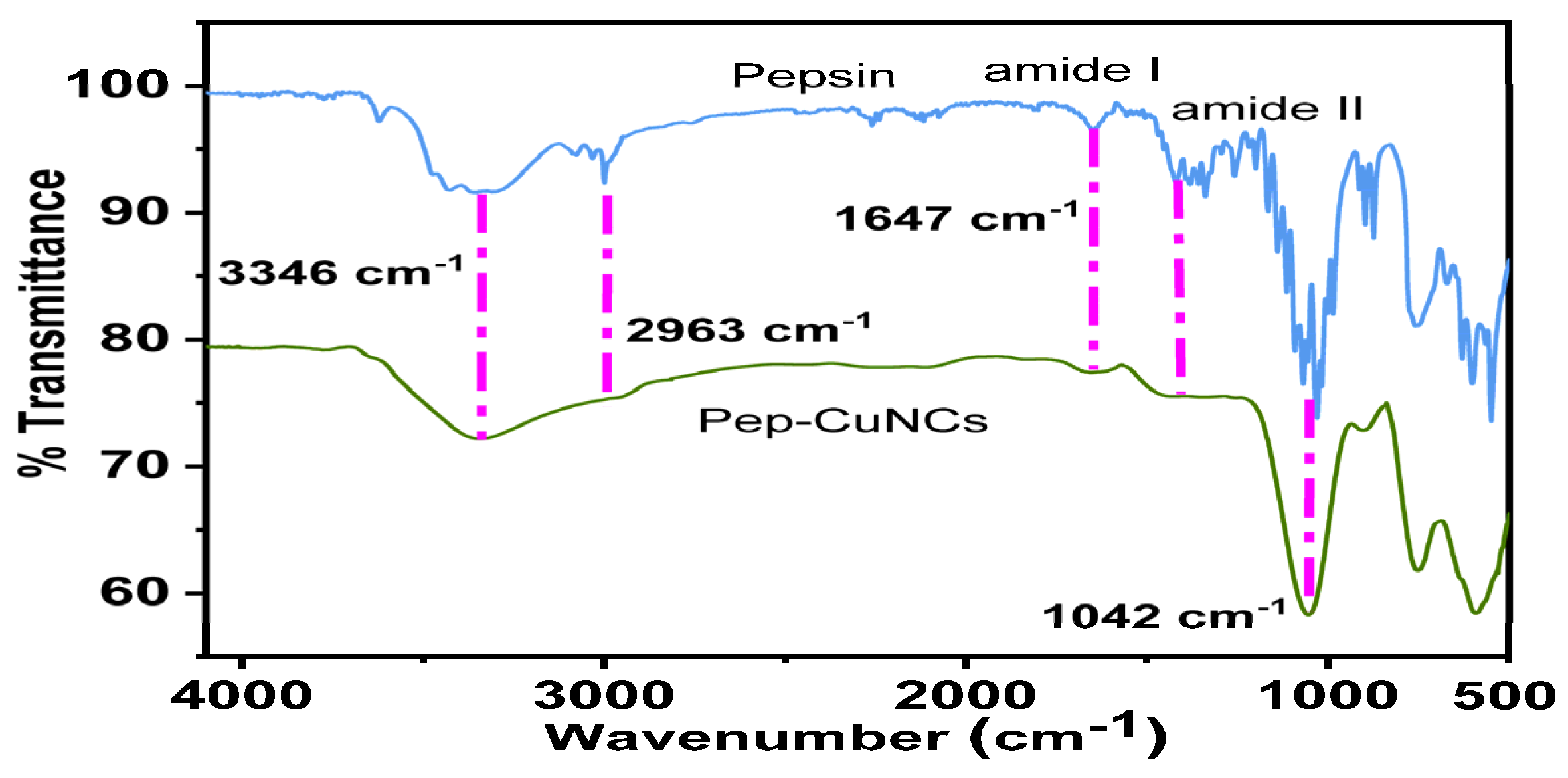
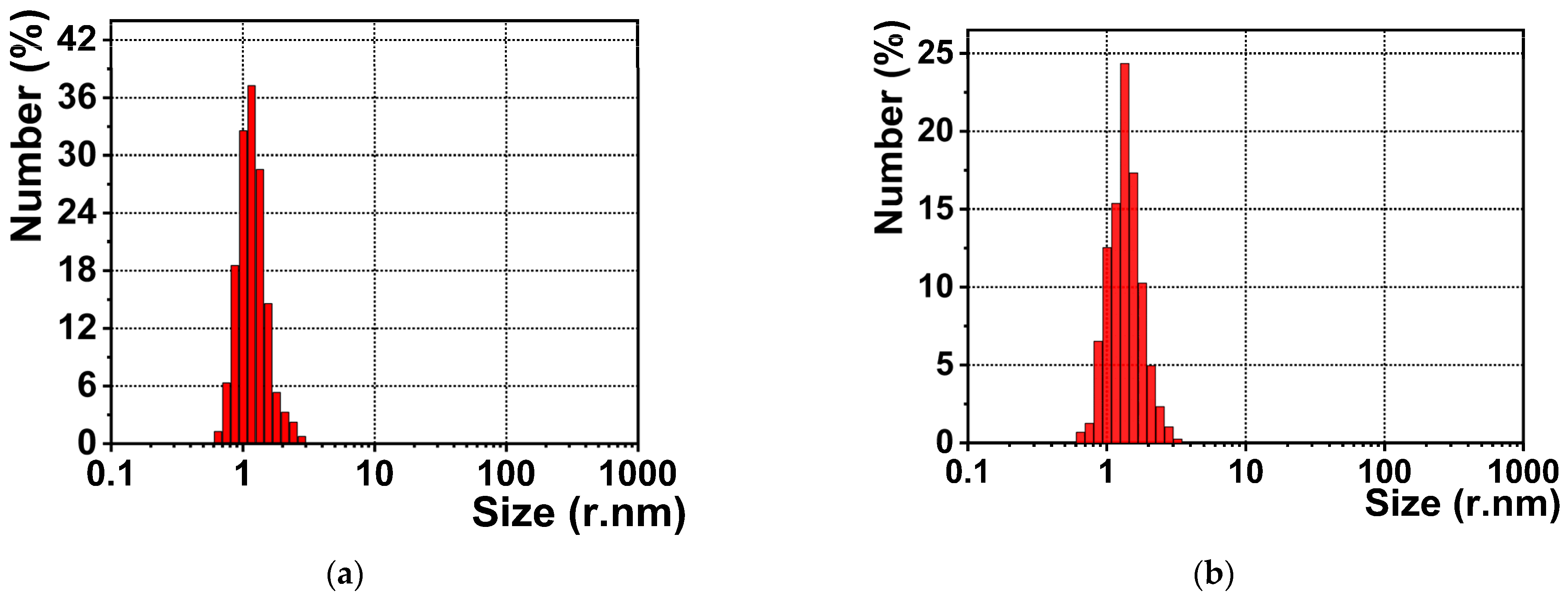
3.2. Particles’ Characterizations
3.3. Sensing Process
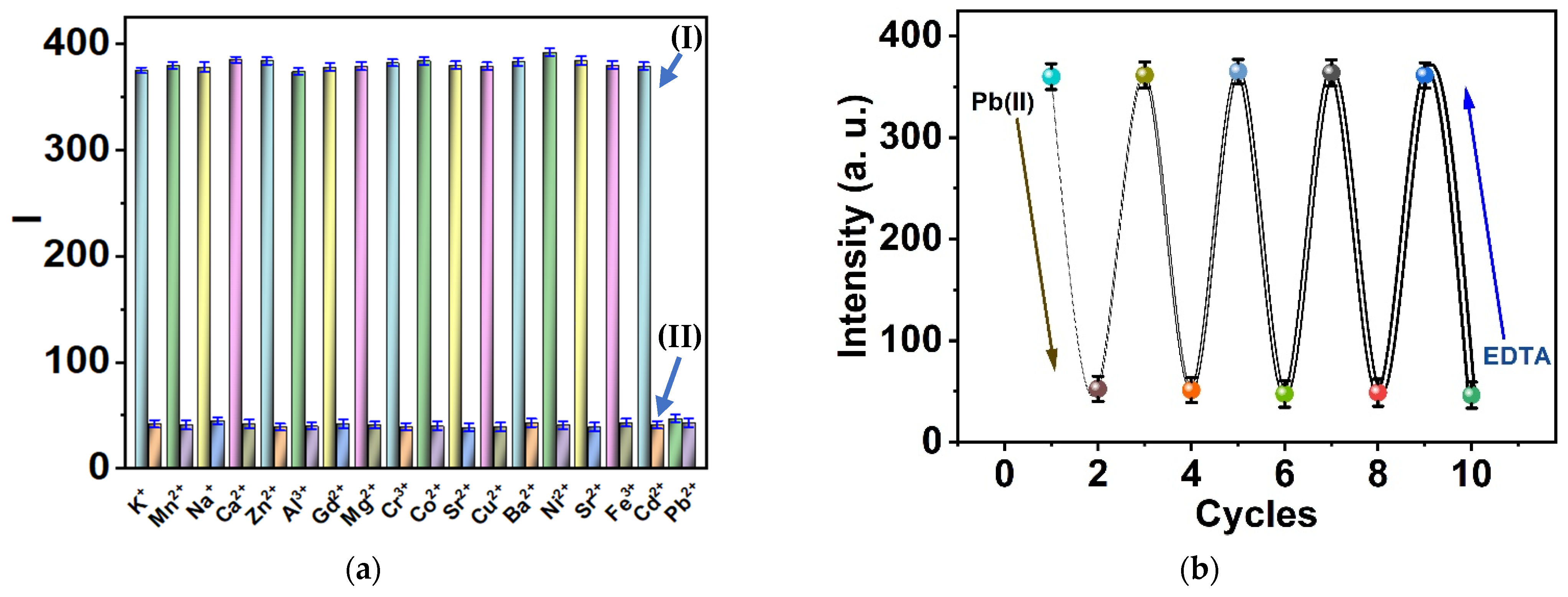
3.4. Selectivity and Reversibility
3.5. Detection of Pb(II) Ions in Environmental Samples
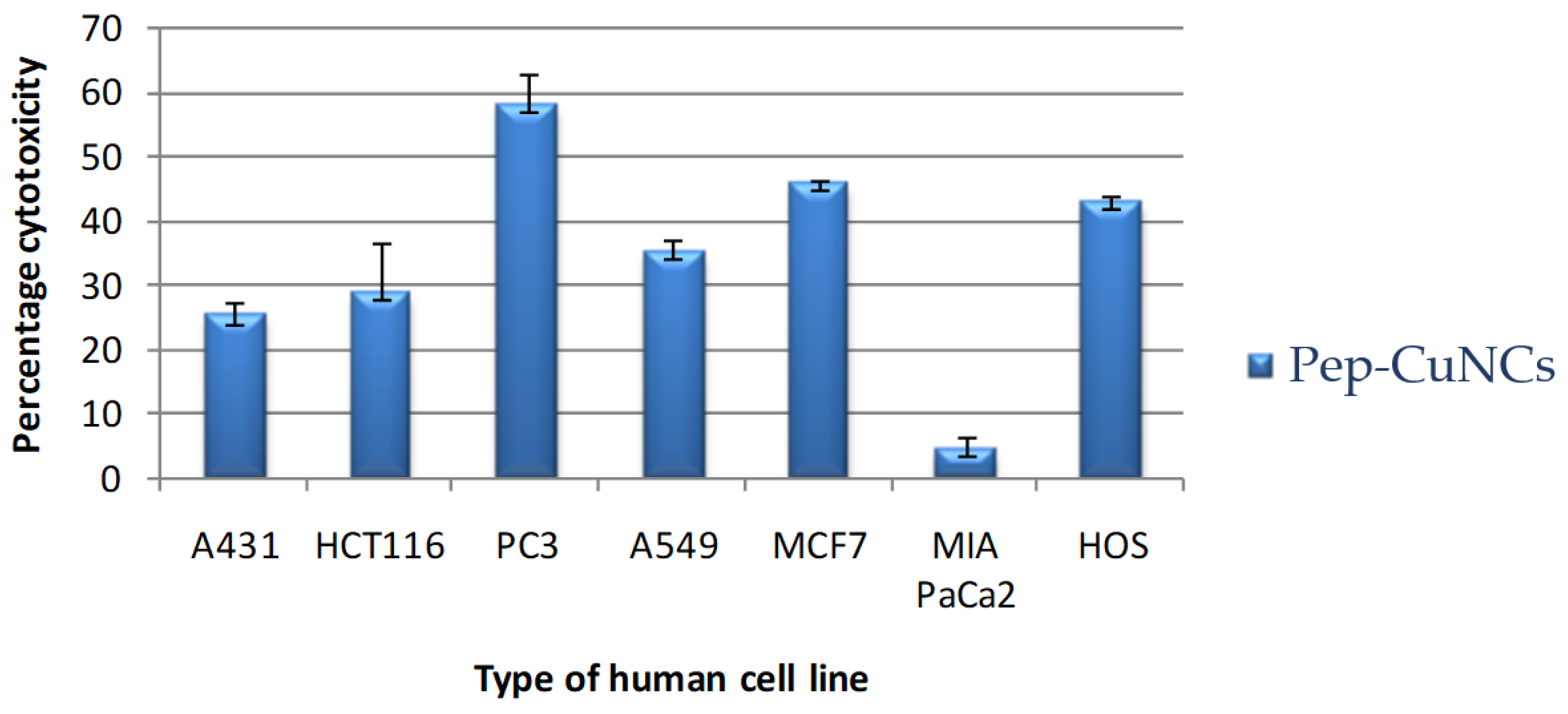
3.6. Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohmand, J.; Eqani, S.A.M.A.S.; Fasola, M.; Alamdar, A.; Mustafa, I.; Ali, N.; Liu, L.; Peng, S.; Shen, H. Human exposure to toxic metals via contaminated dust: Bio-accumulation trends and their potential risk estimation. Chemosphere 2015, 132, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K. An aptasensor for selective, sensitive and fast detection of lead (II) based on polyethyleneimine and gold nanoparticles. Environ. Toxicol. Pharmacol. 2015, 39, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, X.; Cheng, T.; Li, S.; An, G. Graphene enhanced optical fiber SPR sensor for liquid concentration measurement. Opt. Fiber Technol. 2018, 43, 62–66. [Google Scholar] [CrossRef]
- Hotzer, B.; Medintz, I.L.; Hildebrandt, N. Fluorescence in nanobiotechnology: Sophisticated fluorophores for novel applications. Small 2012, 8, 2297–2326. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Alminderej, F.; Ali, I.A. Ultrasensitive optical chemosensor for Cu (II) detection. Int. J. Anal. Chem. 2019, 2019, 7381046. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.M.; Ali, R.; Wolfbeis, O.S. New silica and polystyrene nanoparticles labeled with longwave absorbing and fluorescent chameleon dyes. Microchim. Acta 2011, 174, 429–434. [Google Scholar] [CrossRef]
- Feldmann, C. Luminescent nanomaterials. Nanoscale 2011, 3, 1947–1948. [Google Scholar] [CrossRef]
- Cheng, Z.; Kuru, E.; Sachdeva, A.; Vendrell, M. Fluorescent amino acids as versatile building blocks for chemical biology. Nat. Rev. Chem. 2020, 4, 275–290. [Google Scholar] [CrossRef]
- Ali, R.; Alminderej, F.M.; Messaoudi, S.; Saleh, S.M. Ratiometric ultrasensitive optical chemisensor film based antibiotic drug for Al (III) and Cu (II) detection. Talanta 2021, 221, 121412. [Google Scholar] [CrossRef]
- Saleh, S.M.; Elkady, E.M.; Ali, R.; Alminderej, F.; Mohamed, T.A. Novel chemical sensor for detection Ca (II) ions based on ferutinin. Spectrochim. Acta A 2018, 205, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Ali, R.; Hegazy, M.E.F.; Alminderej, F.M.; Mohamed, T.A. The natural compound chrysosplenol-D is a novel, ultrasensitive optical sensor for detection of Cu (II). J. Mol. Liq. 2020, 302, 112558. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z.; et al. Carbon-quantum-dots-loaded ruthenium nanoparticles as an efficient electrocatalyst for hydrogen production in alkaline media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, J.; Tang, Z.; Yang, B.; Lu, S. Near-infrared emissive carbon dots with 33.96% emission in aqueous solution for cellular sensing and light-emitting diodes. Sci. Bull. 2019, 64, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Guo, Q.; Xu, L. Study on the chemotherapeutic effect and mechanism of doxorubicin hydrochloride on drug-resistant gastric cancer cell lines using metal-organic framework fluorescent nanoparticles as carriers. J. Nanomater. 2020, 2020, 6681749. [Google Scholar] [CrossRef]
- Neumeier, B.L.; Khorenko, M.; Alves, F.; Goldmann, O.; Napp, J.; Schepers, U.; Reichardt, H.M.; Feldmann, C. Fluorescent inorganic-organic hybrid nanoparticles. Chem. Nano. Mat. 2019, 5, 24–45. [Google Scholar] [CrossRef]
- Saleh, S.M.; Almotiri, M.K.; Ali, R. Green synthesis of highly luminescent gold nanoclusters and their application in sensing Cu(II) and Hg(II). J. Photochem. Photobiol. A Chem. 2022, 426, 113719. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Qing, T.; Zhang, K.; Qing, Z.; Wang, X.; Long, C.; Zhang, P.; Hu, H.; Feng, B. Recent progress in copper nanocluster-based flurescent probing: A review. Microchim. Acta 2019, 186, 670. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W.; Guo, X. Facile one-pot synthesis of near-infrared luminescent gold nanoparticles for sensing copper (II). Nanotechnology 2011, 22, 095701. [Google Scholar] [CrossRef]
- Aires, A.; Lopez-Martinez, E.; Cortajarena, A.L. Sensors based on metal nanoclusters stabilized on designed proteins. Biosensors 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kumari, B.; Makabenta, J.M.; Gupta, A.; Rotello, V. Effective detection of bacteria using metal nanoclusters. Nanoscale 2019, 11, 22172–22181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, F.; Lu, C.; Fauconnier, M.L.; Zheng, J. Bio-Specific Au/Fe3+ Porous Spongy Nanoclusters for Sensitive SERS Detection of Escherichia coli O157: H7. Biosensors 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Moßhammer, M.; Scholz, V.V.; Borisov, S.M.; Holst, G.; Kühl, M. Luminescence lifetime imaging of chemical sensors-A comparison between time-domain and frequency-domain based camera systems. Anal. Chem. 2019, 91, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Obliosca, J.M.; Liu, C.; Batson, R.A.; Babin, M.C.; Werner, J.H.; Yeh, H.C. DNA/RNA detection using DNA-templated few-atom silver nanoclusters. Biosensors 2013, 3, 185–200. [Google Scholar] [CrossRef] [Green Version]
- Farzin, L.; Shamsipur, M.; Samandari, L.; Sadjadi, S.; Sheibani, S. Biosensing strategies based on organic-scaffolded metal nanoclusters for ultrasensitive detection of tumor markers. Talanta 2020, 214, 120886. [Google Scholar] [CrossRef]
- Ali, R.; Saleh, S.M.; Aly, S.M. Fluorescent gold nanoclusters as pH sensors for the pH 5 to 9 range and for imaging of blood cell pH values. Microchim. Acta 2017, 184, 3309–3315. [Google Scholar] [CrossRef]
- Ali, R.; Alfeneekh, B.; Chigurupati, S.; Saleh, S.M. Green synthesis of pregabalin-stabilized gold nanoclusters and their applications in sensing and drug release. Arch. Pharm. 2022, e2100426. [Google Scholar] [CrossRef]
- Osman, M.E.; Eid, M.M.; Khattab, O.H.; El-Hallouty, S.M.; El-Manawaty, M.; Mahmoud, D.A. In vitro cytotoxicity of biosynthesized Ag/CS NP against MCf7, PC3 and A549 cancer cell lines. Int. J. Pharm. Tech. Res. 2015, 8, 1011–1017. [Google Scholar]
- Ismail, N.S.; George, R.F.; Serya, R.A.; Baselious, F.N.; El-Manawaty, M.; Shalaby, E.M.; Girgis, A.S. Rational design, synthesis and 2D-QSAR studies of antiproliferative tropane-based compounds. RSC Adv. 2016, 6, 101911–101923. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Dolma, K.; Kaur, N.; Malhotra, A.; Kumar, N.; Dixit, P.; Sharma, D.; Mayilraj, S.; Choudhury, A.R. Marine microbe as nano-factories for copper biomineralization. Biotechnol. Bioprocess Eng. 2015, 20, 51–57. [Google Scholar] [CrossRef]
- Luo, J.J.; Liu, Y.F.; Niu, Y.; Jiang, Q.; Huang, R.; Zhang, B.S.; Su, D.S. Insight into the chemical adsorption properties of CO molecules supported on Au or Cu and hybridized Au-CuO nanoparticles. Nanoscale 2017, 9, 15033–15043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, R.; Lang, T.; Saleh, S.M.; Meier, R.J.; Wolfbeis, O.S. Optical sensing scheme for carbon dioxide using a solvatochromic probe. Anal. Chem. 2011, 83, 2846–2851. [Google Scholar] [CrossRef]
- Available online: http://water.epa.gov/drink/contaminants/index.cfm (accessed on 25 February 2022).
- Xiang, Y.; Tong, A.; Lu, Y. Abasic site-containing DNAzyme and aptamer for label-free fluorescent detection of Pb2+ and adenosine with high sensitivity, selectivity, and tunable dynamic range. J. Am. Chem. Soc. 2009, 131, 15352–15357. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Han, S.H.; Chung, B.H. Improving Pb2+ detection using DNAzyme-based fluorescence sensors by pairing fluorescence donors with gold nanoparticles. Biosens. Bioelectron. 2011, 26, 2125–2129. [Google Scholar] [CrossRef]
- Rotaru, A.; Dutta, S.; Jentzsch, E.; Gothelf, K.; Mokhir, A. Selective dsDNA-Templated Formation of Copper Nanoparticles in Solution. Angew. Chem. Int. Ed. 2010, 49, 5665–5667. [Google Scholar] [CrossRef]
- Risto, M.; Takaluoma, T.T.; Bajorek, T.; Oilunkaniemi, R.; Laitinen, R.S.; Chivers, T. Preparation, structure, and metallophilic interactions of dinuclear silver (I) and copper (I) complexes of selenium diimides. Inorg. Chem. 2009, 48, 6271–6279. [Google Scholar] [CrossRef]



| Samples | Added Pb(II) µM | ICP-MS µM | Found µM | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|
| Tap water | 0.50 | 0.513 | 0.481 | 1.35 | 96.20 |
| 1.00 | 1.009 | 0.989 | 0.99 | 98.90 | |
| 2.00 | 2.007 | 1.982 | 0.92 | 99.10 | |
| Mineral water | 0.50 | 0.511 | 0.487 | 1.29 | 97.40 |
| 1.00 | 1.007 | 0.991 | 0.97 | 99.10 | |
| 2.00 | 2.005 | 1.991 | 0.96 | 99.55 |
| Cell | Activity at 100 µM | IC50 (µM) |
|---|---|---|
| A431 | 25.1 ± 2.3 | - |
| HCT116 | 28.9 ± 7.4 | - |
| PC3 | 57.9 ± 4.8 | - |
| A549 | 35.2 ± 1.6 | - |
| MCF7 | 45.9 ± 0.3 | 104.4 ± 16, r2 = 0.94 |
| PaCaII | 4.6 ± 1.9 | - |
| HOS | 42.9 ± 0.9 | - |
| Cell | 100 µM | 50 µM | 25 µM | 12.5 µM |
|---|---|---|---|---|
| HOS | 42.9 ± 0.9 | 50.9 ± 1.3 | 40.8 ± 2.4 | 17.8 ± 1.6 |
| MCF7 | 45.9 ± 0.3 | 29.2 ± 0.5 | 3.6 ± 2.2 | 0.0 ± 0.0 |
| PC3 | 57.9 ± 4.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| A549 | 35.2 ± 1.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| A431 | 25.1 ± 2.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 21.4 ± 2.1 |
| HCT116 | 28.9 ± 7.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PaCaII | 4.6 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, S.M.; El-Sayed, W.A.; El-Manawaty, M.A.; Gassoumi, M.; Ali, R. An Eco-Friendly Synthetic Approach for Copper Nanoclusters and Their Potential in Lead Ions Sensing and Biological Applications. Biosensors 2022, 12, 197. https://doi.org/10.3390/bios12040197
Saleh SM, El-Sayed WA, El-Manawaty MA, Gassoumi M, Ali R. An Eco-Friendly Synthetic Approach for Copper Nanoclusters and Their Potential in Lead Ions Sensing and Biological Applications. Biosensors. 2022; 12(4):197. https://doi.org/10.3390/bios12040197
Chicago/Turabian StyleSaleh, Sayed M., Wael A. El-Sayed, May A. El-Manawaty, Malek Gassoumi, and Reham Ali. 2022. "An Eco-Friendly Synthetic Approach for Copper Nanoclusters and Their Potential in Lead Ions Sensing and Biological Applications" Biosensors 12, no. 4: 197. https://doi.org/10.3390/bios12040197






