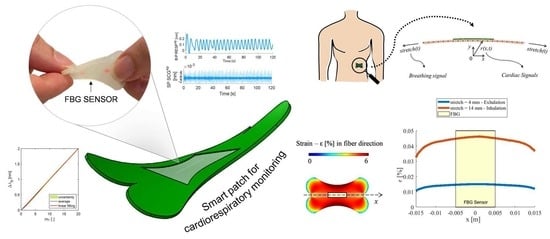
A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring
Abstract
:1. Introduction
2. The Smart Patch Working Principle, Computation Model, and Fabrication
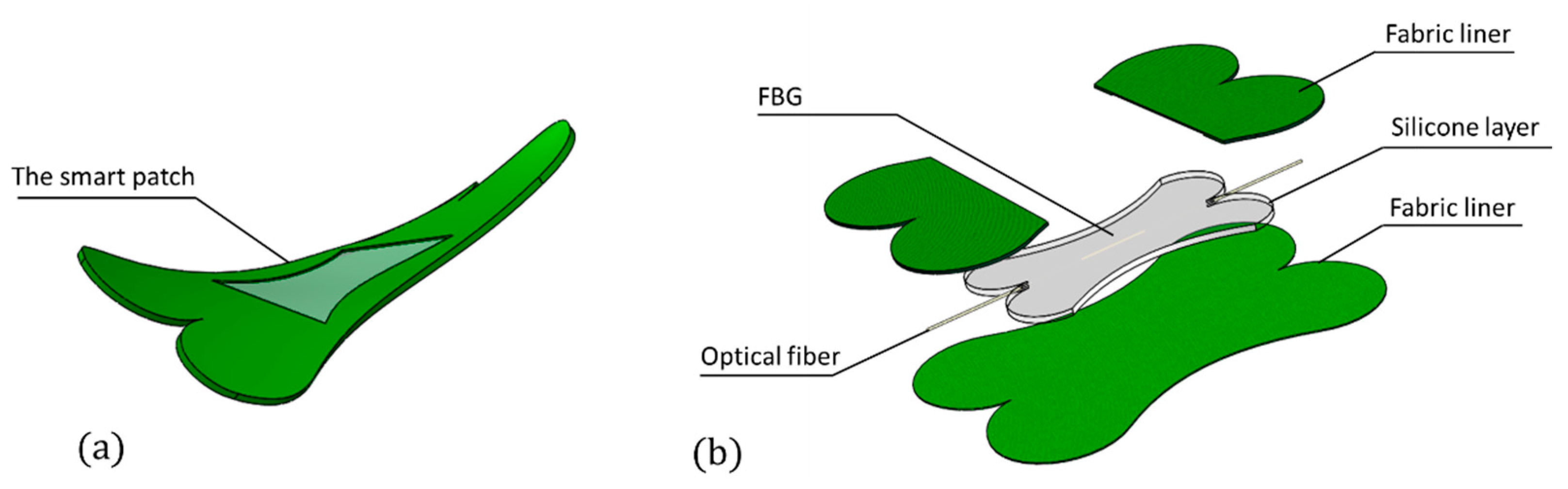
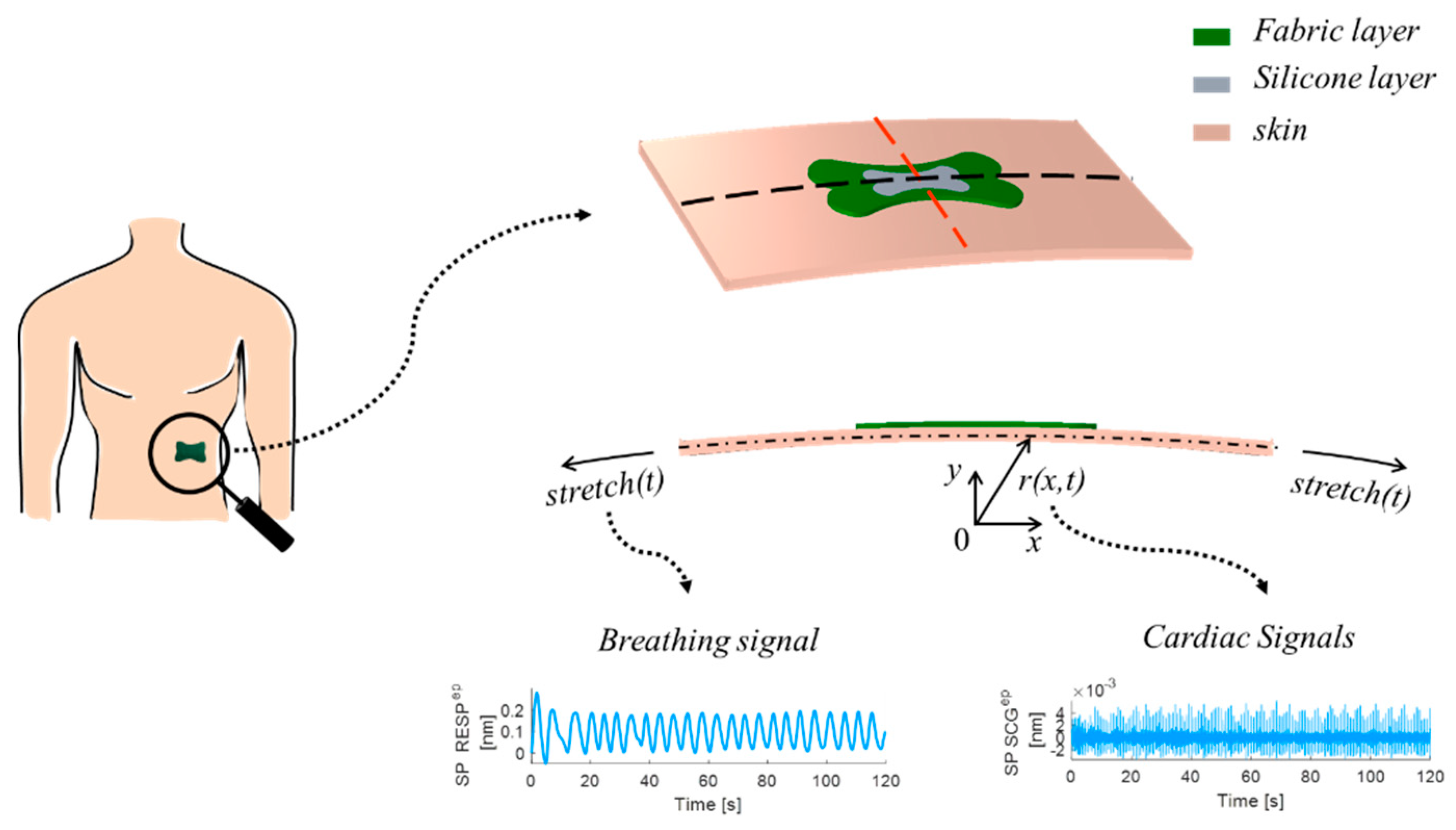
2.1. The Smart Patch Working Principle
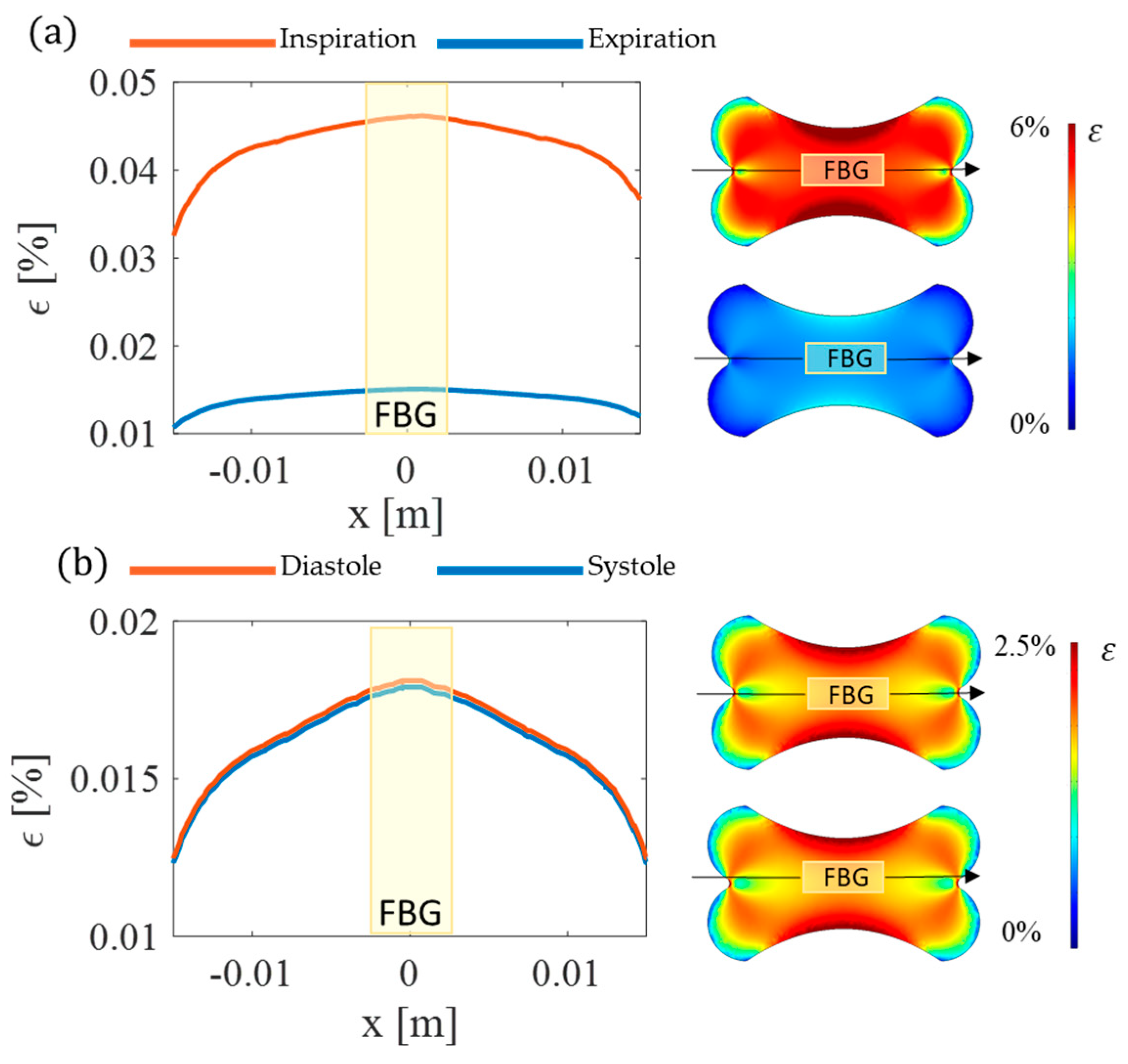
2.2. In Silico Model
2.3. The Smart Patch Fabrication Process
3. The Smart Patch Metrological Assessment
3.1. Strain Response
3.2. Influence of Temperature and Relative Humidity
3.3. Hysteresis Error
4. The Smart Patch Feasibility Assessment of Healthy Volunteers
4.1. Experimental Protocol and Setup
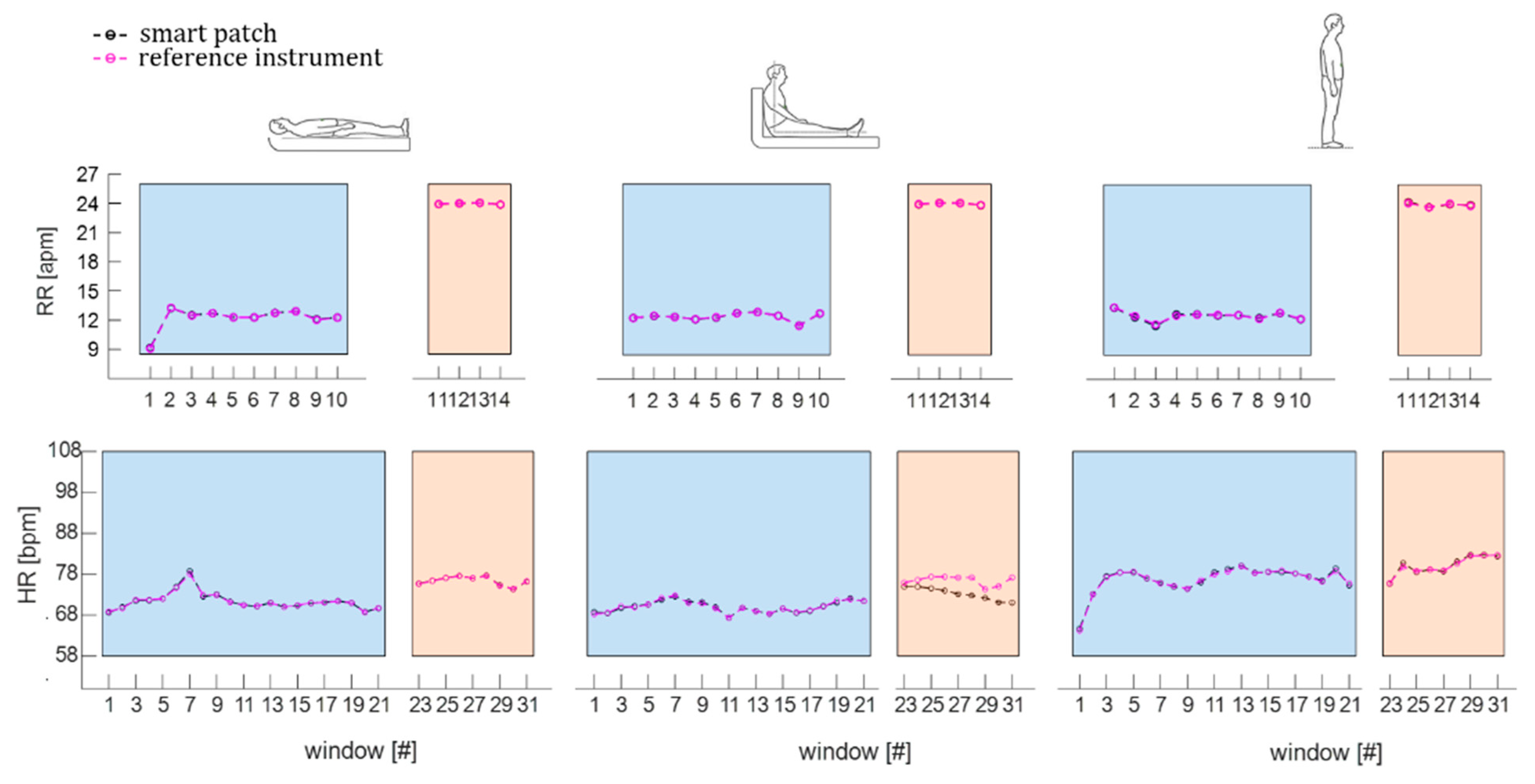
4.2. Data Analysis and Results
- The signal filtering and windowing;
- The power spectral density (PSD) analysis;
- The statistical analysis.
4.2.1. The Signal Filtering and Windowing
4.2.2. The Power Spectral Density Analysis
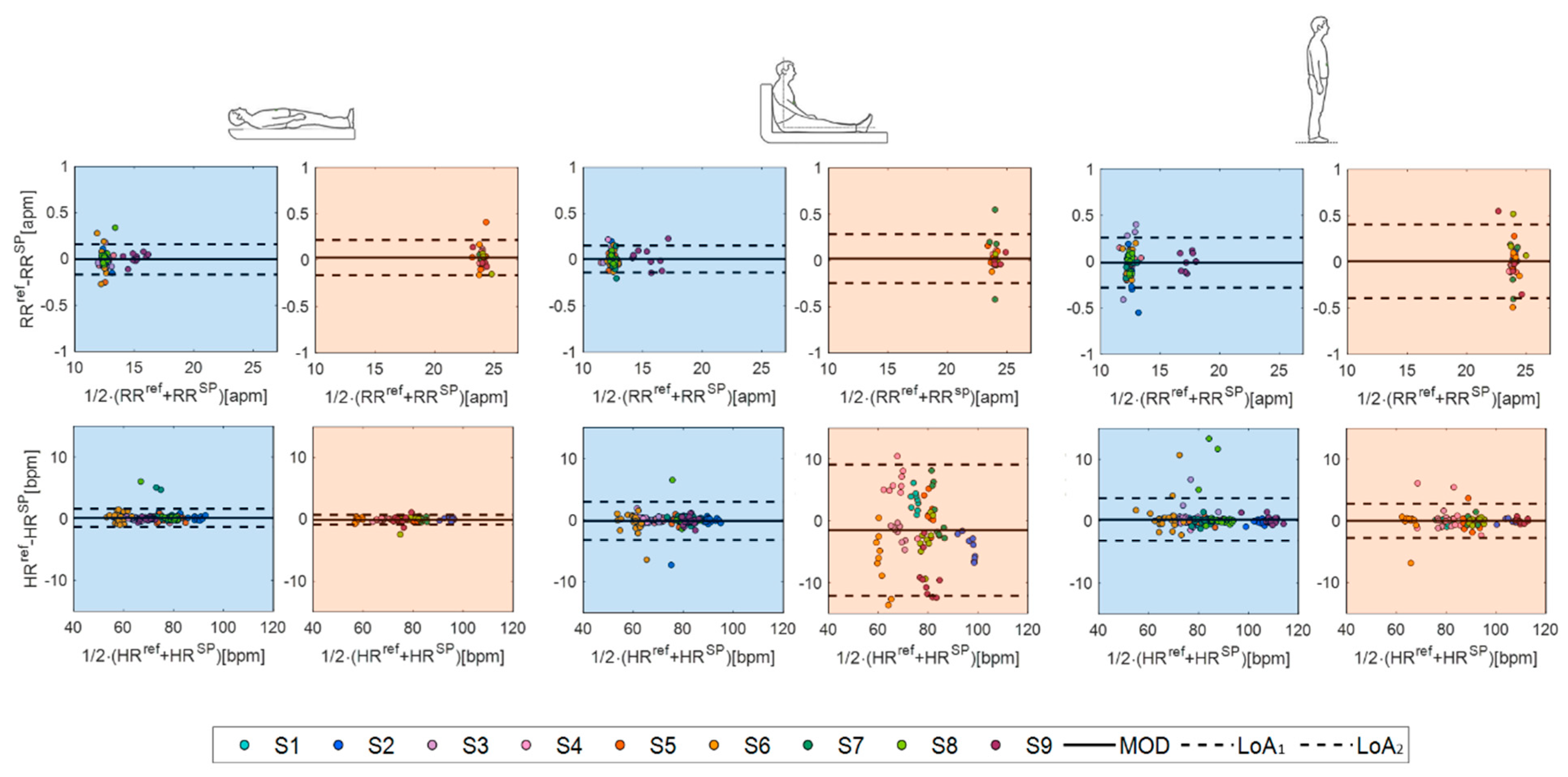
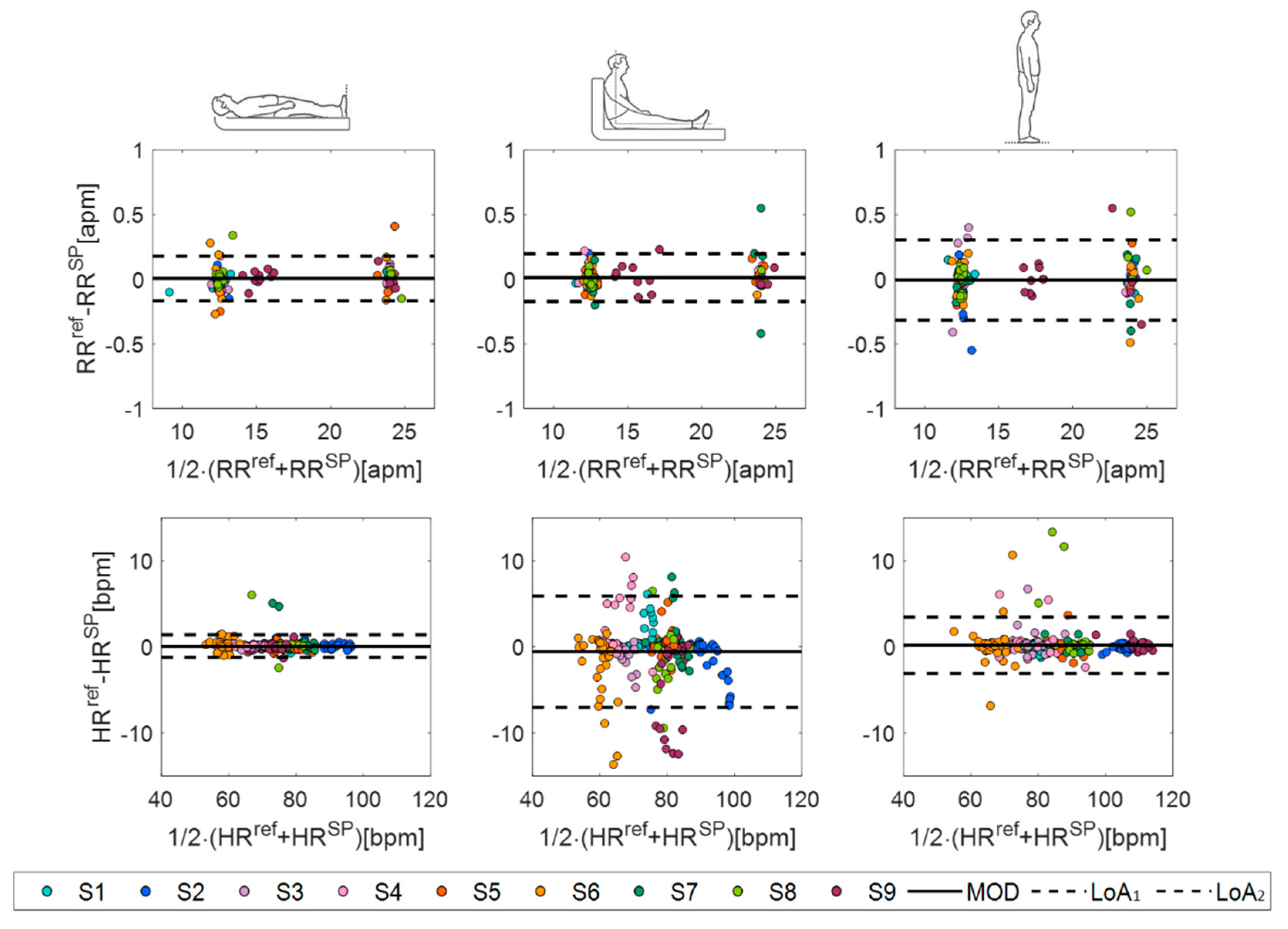
4.2.3. The Statistical Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabhakaran, D.; Anand, S.; Gaziano, T.A.; Mbanya, J.-C.; Wu, Y.; Nugent, R. Disease Control Priorities. In Cardiovascular, Respiratory, and Related Disorders, 3rd ed.; World Bank: Washington, DC, USA, 2017; Volume 5. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Julius, S. Elevated heart rate: A major risk factor for cardiovascular disease. Clin. Exp. Hypertens. 2004, 26, 637–644. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.O.; Abate, K.H.; Abd-Allah, F.; Ahmed, M.; Alam, K.; Alam, T.; Alvis-Guzman, N.; Ansari, H.; Ärnlöv, J.; et al. The burden of cardiovascular diseases among US states, 1990–2016. JAMA Cardiol. 2018, 3, 375–389. [Google Scholar] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 22 February 2022).
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The importance of respiratory rate monitoring: From healthcare to sport and exercise. Sensors 2020, 20, 6393. [Google Scholar] [CrossRef]
- Palatini, P.; Julius, S. Heart rate and the cardiovascular risk. J. Hypertens. 1997, 15, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, M.V.; Mastropasqua, F.; Massari, F.; Passantino, A.; Colombo, R.; Mannarini, A.; Forleo, C.; Rizzon, P. Effect of respiratory rate on the relationships between RR interval and systolic blood pressure fluctuations: A frequency-dependent phenomenon. Cardiovasc. Res. 1998, 38, 332–339. [Google Scholar] [CrossRef]
- Schein, R.M.H.; Hazday, N.; Pena, M.; Ruben, B.H.; Sprung, C.L. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest 1990, 98, 1388–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.; Borer, J.S.; Camm, A.J.; Danchin, N.; Ferrari, R.; Lopez Sendon, J.L.; Steg, P.G.; Tardif, J.C.; Tavazzi, L.; Tendera, M. Resting Heart Rate in Cardiovascular Disease. J. Am. Coll. Cardiol. 2007, 50, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Cooney, M.T.; Vartiainen, E.; Laakitainen, T.; Juolevi, A.; Dudina, A.; Graham, I.M. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am. Heart J. 2010, 159, 612–619.e3. [Google Scholar] [CrossRef]
- Carter, P.; Lagan, J.; Fortune, C.; Bhatt, D.L.; Vestbo, J.; Niven, R.; Chaudhuri, N.; Schelbert, E.B.; Potluri, R.; Miller, C.A. Association of Cardiovascular Disease with Respiratory Disease. J. Am. Coll. Cardiol. 2019, 73, 2166–2177. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.; Redline, S.; Lee, E.; Newman, A.; O’Connor, G.; Boland, L.; Schwartz, J.; Samet, J. Sleep-disordered Breathing and Cardiovascular Disease: Cross-sectional Results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-based methods for measuring respiratory rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMarco, J.P.; Philbrick, J.T. Use of ambulatory electrocardiographic (Holter) monitoring. Ann. Intern. Med. 1990, 113, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, R.; De Vittorio, M.; Visconti, P. Innovative iot solutions and wearable sensing systems for monitoring human biophysical parameters: A review. Electron 2021, 10, 1660. [Google Scholar] [CrossRef]
- Aliverti, A. Wearable technology: Role in respiratory health and disease. Breathe 2017, 13, e27–e36. [Google Scholar] [CrossRef] [Green Version]
- Sana, F.; Isselbacher, E.M.; Singh, J.P.; Heist, E.K.; Pathik, B.; Armoundas, A.A. Wearable Devices for Ambulatory Cardiac Monitoring: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1582–1592. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Brüser, C.; Antink, C.H.; Wartzek, T.; Walter, M.; Leonhardt, S. Ambient and unobtrusive cardiorespiratory monitoring techniques. IEEE Rev. Biomed. Eng. 2015, 8, 30–43. [Google Scholar] [CrossRef]
- Huang, C.T.; Shen, C.L.; Tang, C.F.; Chang, S.H. A wearable yarn-based piezo-resistive sensor. Sens. Actuators A Phys. 2008, 141, 396–403. [Google Scholar] [CrossRef]
- Atalay, O. Textile-based, interdigital, capacitive, soft-strain sensor for wearable applications. Materials 2018, 11, 768. [Google Scholar] [CrossRef] [Green Version]
- Lo Presti, D.; Massaroni, C.; Jorge Leitao, C.S.; De Fatima Domingues, M.; Sypabekova, M.; Barrera, D.; Floris, I.; Massari, L.; Oddo, C.M.; Sales, S.; et al. Fiber bragg gratings for medical applications and future challenges: A review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Cosoli, G.; Spinsante, S.; Scalise, L. Wrist-worn and chest-strap wearable devices: Systematic review on accuracy and metrological characteristics. Meas. J. Int. Meas. Confed. 2020, 159, 107789. [Google Scholar] [CrossRef]
- Shafiq, G.; Veluvolu, K.C. Surface chest motion decomposition for cardiovascular monitoring. Sci. Rep. 2014, 4, 5093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Tocco, J.; Lo Presti, D.; Zaltieri, M.; D’Alesio, G.; Filosa, M.; Massari, L.; Aliperta, A.; Di Rienzo, M.; Carrozza, M.C.; Ferrarin, M.; et al. A Wearable System Based on Flexible Sensors for Unobtrusive Respiratory Monitoring in Occupational Settings. IEEE Sens. J. 2021, 21, 14369–14378. [Google Scholar] [CrossRef]
- Di Tocco, J.; Raiano, L.; Sabbadini, R.; Massaroni, C.; Formica, D.; Schena, E. A wearable system with embedded conductive textiles and an imu for unobtrusive cardio-respiratory monitoring. Sensors 2021, 21, 3018. [Google Scholar] [CrossRef]
- Hung, K.; Zhang, Y.T.; Tai, B. Wearable medical devices for tele-home healthcare. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Online, 1–5 September 2004; pp. 5384–5387. [Google Scholar]
- Lo Presti, D.; Massaroni, C.; Caponero, M.; Formica, D.; Schena, E. Cardiorespiratory monitoring using a mechanical and an optical system. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications, MeMeA, Lausanne, Switzerland, 23–25 June 2021. [Google Scholar]
- Massaroni, C.; Zaltieri, M.; Lo Presti, D.; Nicolò, A.; Tosi, D.; Schena, E. Fiber Bragg grating sensors for cardiorespiratory monitoring: A review. IEEE Sens. J. 2021, 21, 14069–14080. [Google Scholar] [CrossRef]
- Lo Presti, D.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.; Longo, U.G.; Formica, D.; Oddo, C.M.; Schena, E. Wearable system based on flexible fbg for respiratory and cardiac monitoring. IEEE Sens. J. 2019, 19, 7391–7398. [Google Scholar] [CrossRef]
- Liu, Y.; Norton, J.J.S.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016, 2, e1601185. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Feng, X.; Chen, X.; Dai, L.L.; Xu, Y. A wearable mechano-acoustic sensor based on electrochemical redox reaction for continuous cardiorespiratory monitoring. Appl. Phys. Lett. 2021, 118, 023703. [Google Scholar] [CrossRef]
- Yang, C.; Tavassolian, N. Combined Seismo-and Gyro-Cardiography: A More Comprehensive Evaluation of Heart-Induced Chest Vibrations. IEEE J. Biomed. Health Inform. 2018, 22, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Thakkar, H.K.; Rajput, S.S.; Santamaria, J.; Bhatt, C.; Roca, F. A comprehensive review on seismocardiogram: Current advancements on acquisition, annotation, and applications. Mathematics 2021, 9, 2243. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Y.; Gao, W. Skin-Interfaced Sensors in Digital Medicine: From Materials to Applications. Matter 2020, 2, 1414–1445. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-integrated wearable systems and implantable biosensors: A comprehensive review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Phan, D.T.; Nguyen, C.H.; Nguyen, T.D.P.; Tran, L.H.; Park, S.; Choi, J.; Lee, B.-I.; Oh, J. A Flexible, Wearable, and Wireless Biosensor Patch with Internet of Medical Things Applications. Biosensors 2022, 12, 139. [Google Scholar] [CrossRef]
- Ismar, E.; Kurşun Bahadir, S.; Kalaoglu, F.; Koncar, V. Futuristic Clothes: Electronic Textiles and Wearable Technologies. Glob. Chall. 2020, 4, 1900092. [Google Scholar] [CrossRef] [Green Version]
- Tavares, C.; Leitão, C.; Lo Presti, D.; Domingues, M.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and heart rate monitoring using an FBG 3D-printed wearable system. Biomed. Opt. Express 2022, 13, 2299. [Google Scholar] [CrossRef]
- Perezcampos Mayoral, C.; Gutiérrez Gutiérrez, J.; Cano Pérez, J.L.; Vargas Treviño, M.; Gallegos Velasco, I.B.; Hernández Cruz, P.A.; Torres Rosas, R.; Tepech Carrillo, L.; Arnaud Ríos, J.; Apreza, E.L.; et al. Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field. Biosensors 2021, 11, 58. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Martinek, R.; Nazeran, H. Vital sign monitoring and cardiac triggering at 1.5 tesla: A practical solution by an mr-ballistocardiography fiber-optic sensor. Sensors 2019, 19, 470. [Google Scholar] [CrossRef] [Green Version]
- Nedoma, J.; Fajkus, M.; Novak, M.; Strbikova, N.; Vasinek, V.; Nazeran, H.; Vanus, J.; Perecar, F.; Martinek, R. Validation of a novel fiber-optic sensor system for monitoring cardiorespiratory activities during mri examinations. Adv. Electr. Electron. Eng. 2017, 15, 536–543. [Google Scholar] [CrossRef]
- Ranganathan, N.; Sivaciyan, V.; Saksena, F.B. Precordial Pulsations; Humana Press: Totowa, NJ, USA, 2006; pp. 113–139. [Google Scholar]
- Boonkerd, C.; Limroongreungrat, W. Elastic therapeutic tape: Do they have the same material properties? J. Phys. Ther. Sci. 2016, 28, 1303–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.P.; Ha, G.; Wright, D.E.; Ma, Y.; Sen-Gupta, E.; Haubrich, N.R.; Branche, P.C.; Li, W.; Huppert, G.L.; Johnson, M.; et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. NPJ Digit. Med. 2018, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdogan, T. Fiber grating spectra. J. Light. Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef] [Green Version]
- Welch, P.D. The Use of Fast Fourier Transform for the Estimation of Power Spectra: A Method Based on Time Averaging Over Short, Modified Periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Martin Bland, J.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2019, 40, 07TR01. [Google Scholar] [CrossRef] [Green Version]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef]


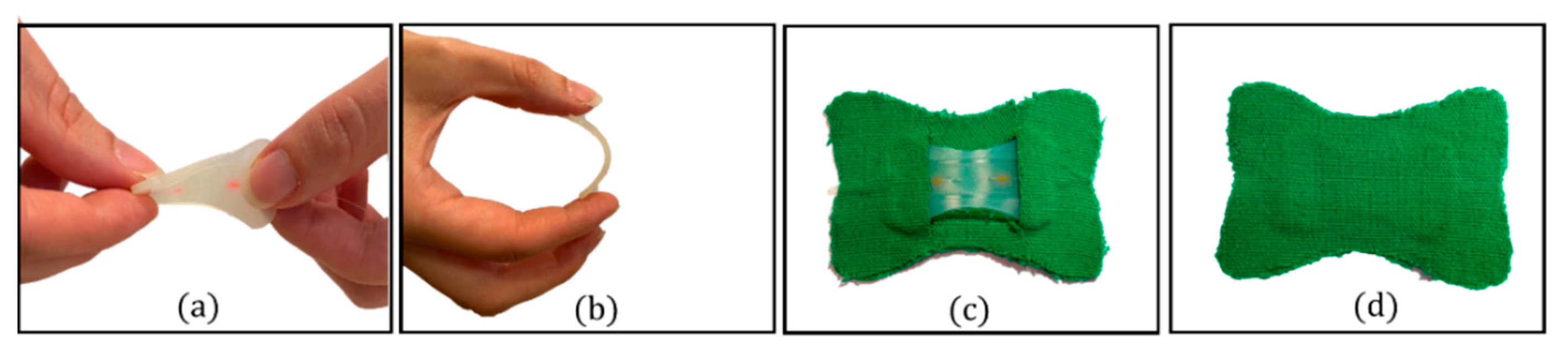
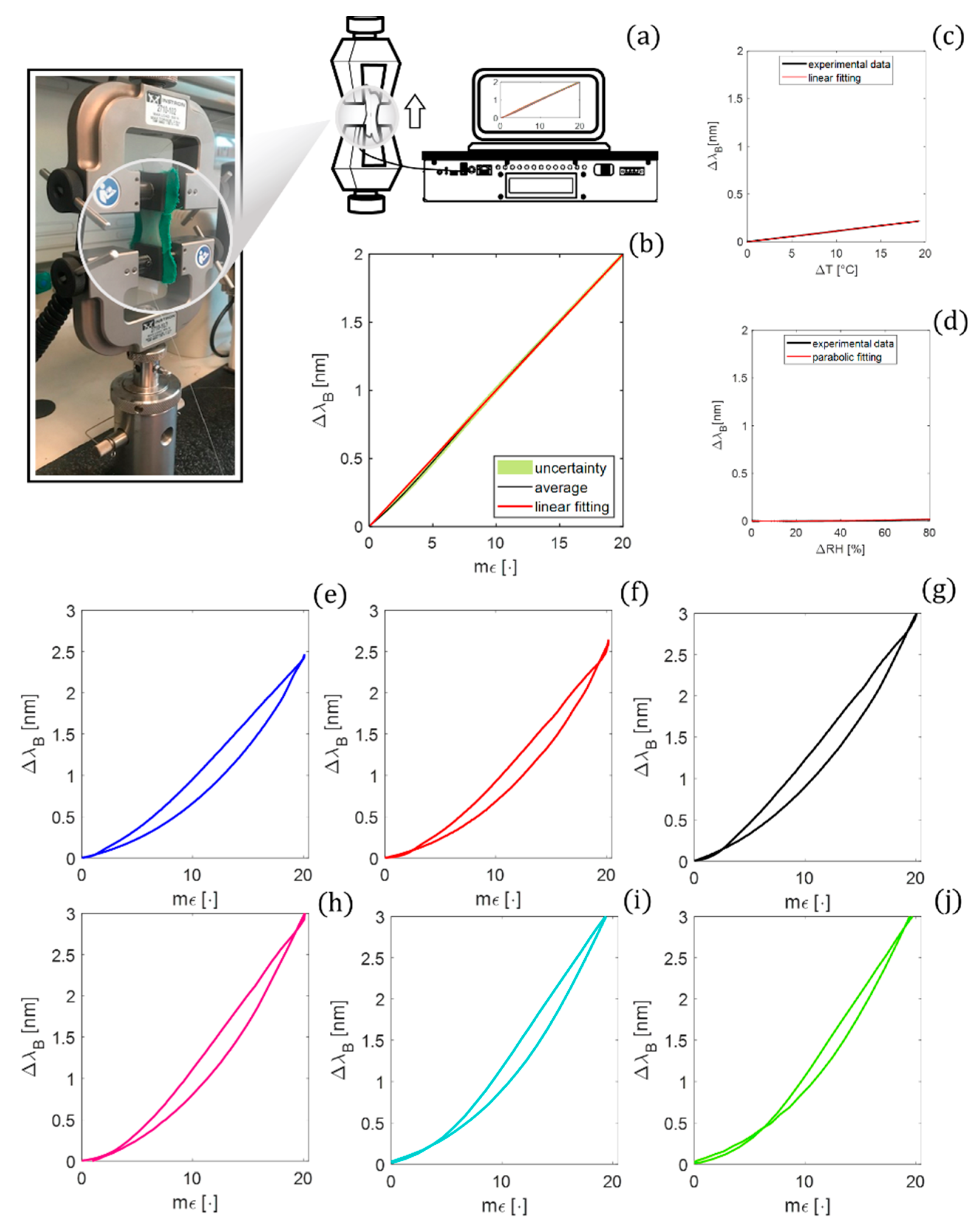
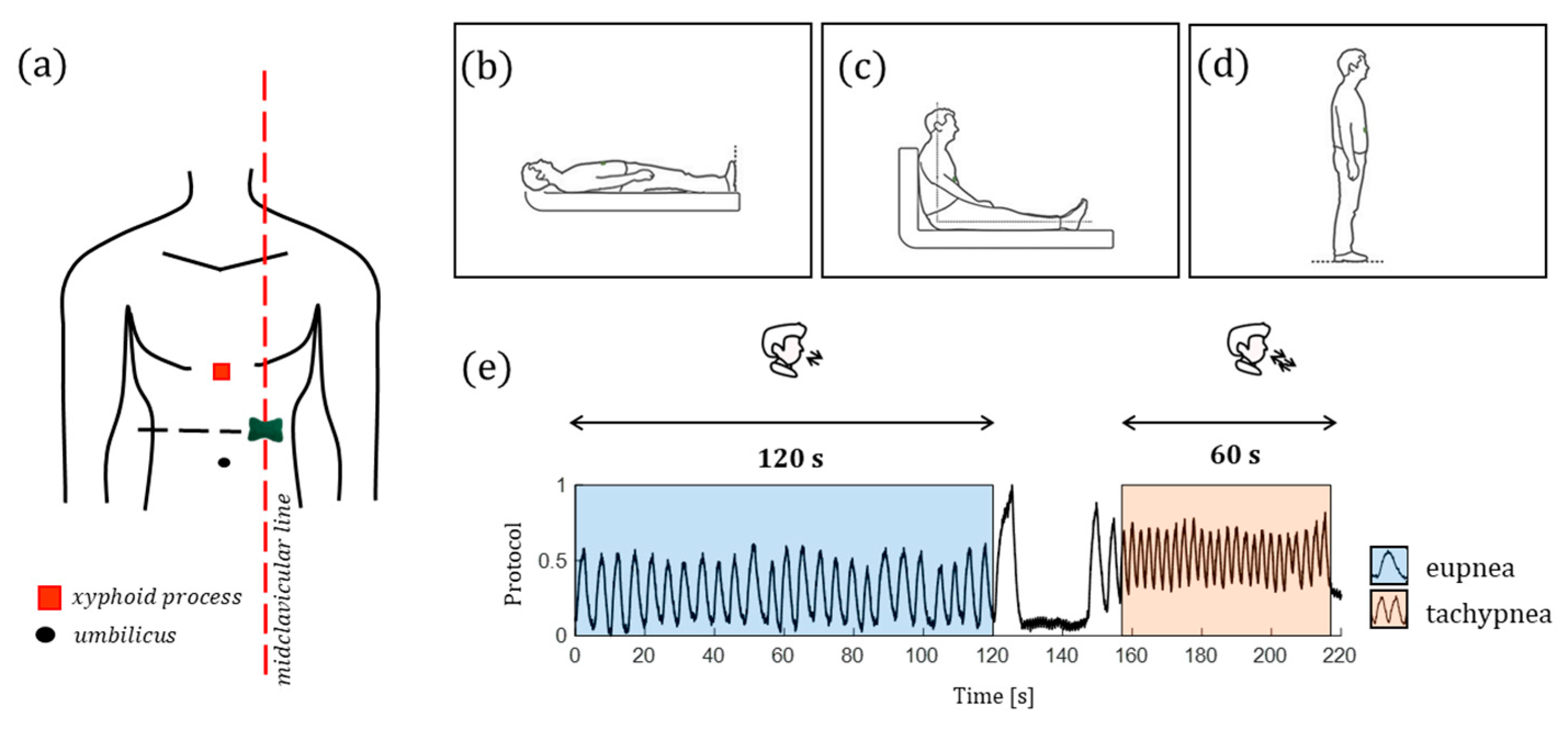

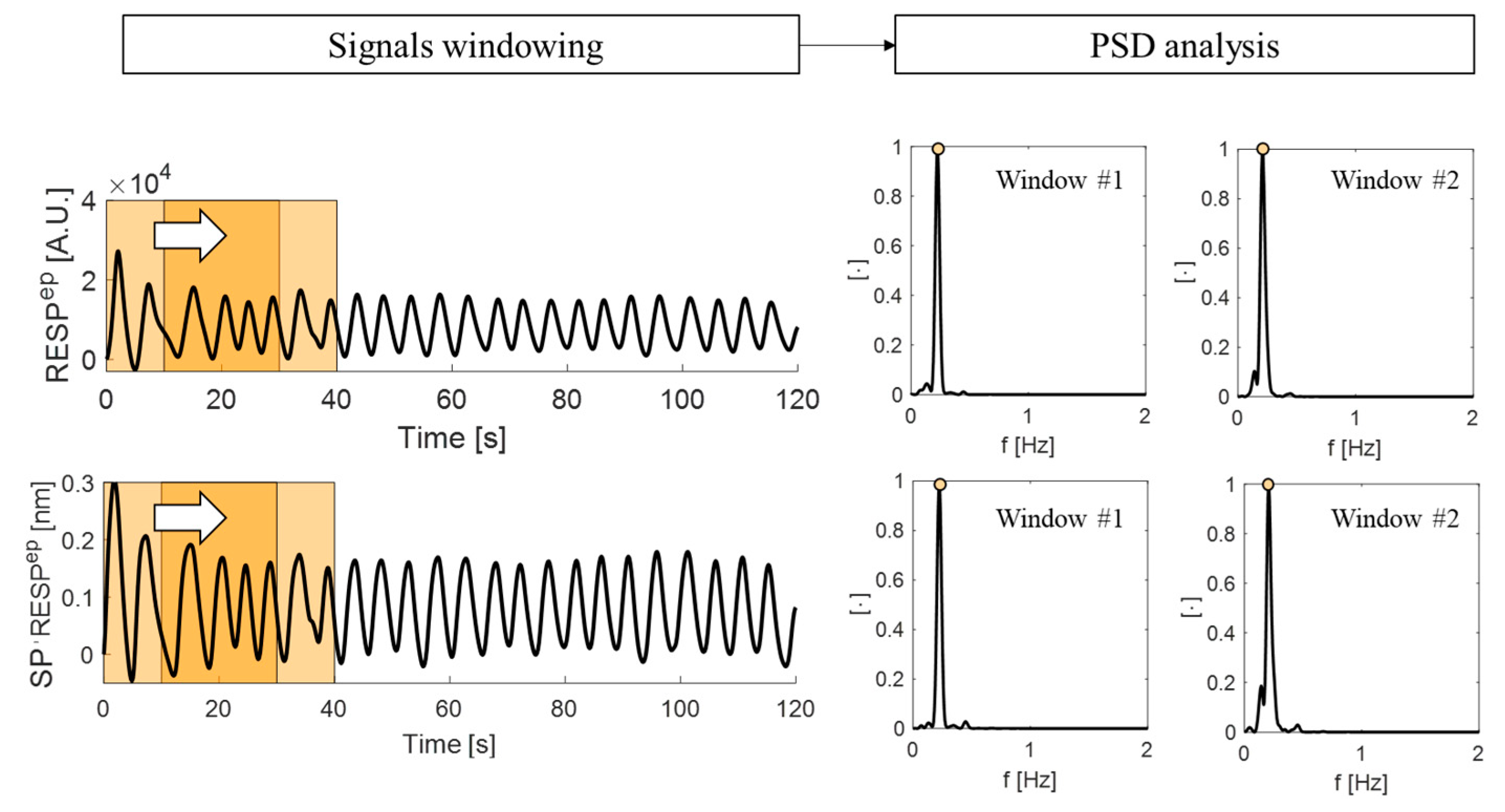
| Model Parameters | Value |
|---|---|
| Skin geometry model | 200 × 120 × 5 [mm] |
| Stretch boundary condition | (2, 4, 6, 8, 10, 12, 14) [mm] |
| Shape function r(x,t) | −0.5·x2 |
| Skin E and ν values | (130 × 103, 0.49) [Pa, -] |
| Fabric liner E and ν | (2 × 104, 0.3) [Pa, -] |
| Dragon Skin silicone E and ν | (45 × 105, 0.49) [Pa, -] |
| RR and HR Values | [%] | |
|---|---|---|
| RR Value | 12 apm | 14.08% |
| 24 apm | 15.84% | |
| 36 apm | 19.32% | |
| HR Value | 60 bpm | 22.38% |
| 90 bpm | 23.94% | |
| 120 bpm | 24.78% | |
| Supine | Sitting | Standing | ||||
|---|---|---|---|---|---|---|
| RR [apm] | HR [bpm] | RR [apm] | HR [bpm] | RR [apm] | HR [bpm] | |
| MOD | 0.0059 | 0.071 | 0.012 | −0.5515 | −0.006 | 0.1757 |
| LOA1 | 0.1796 | 2.6174 | 0.1983 | 5.9152 | 0.3046 | 3.4398 |
| LOA2 | −0.1679 | −1.3797 | −0.1739 | −7.0182 | −0.3167 | −3.0885 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, D.; Bianchi, D.; Massaroni, C.; Gizzi, A.; Schena, E. A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring. Biosensors 2022, 12, 363. https://doi.org/10.3390/bios12060363
Lo Presti D, Bianchi D, Massaroni C, Gizzi A, Schena E. A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring. Biosensors. 2022; 12(6):363. https://doi.org/10.3390/bios12060363
Chicago/Turabian StyleLo Presti, Daniela, Daniele Bianchi, Carlo Massaroni, Alessio Gizzi, and Emiliano Schena. 2022. "A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring" Biosensors 12, no. 6: 363. https://doi.org/10.3390/bios12060363
APA StyleLo Presti, D., Bianchi, D., Massaroni, C., Gizzi, A., & Schena, E. (2022). A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring. Biosensors, 12(6), 363. https://doi.org/10.3390/bios12060363