A Low-Cost Device for Measurement of Exhaled Breath for the Detection of Obstructive Lung Disease
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Subjects
2.2. Protocol
2.3. Health Status
2.4. Experimental Setup
2.4.1. Device Components
2.4.2. Working of MQ Sensor
2.4.3. Interfacing Components
2.5. Data Analysis Statistics
3. Results
3.1. Statistical Comparison Analysis
3.1.1. Exhaled Hydrogen Sulfide
3.1.2. Exhaled Alcohol
3.1.3. Exhaled Ammonia
3.1.4. Exhaled Acetone
4. Discussion
Statistical Correlation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kou, L.; Zhang, D.; You, J.; Jiang, Y. Breath Analysis for Detecting Diseases on Respiratory, Metabolic and Digestive System. J. Biomed. Sci. Eng. 2019, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Gouma, P.; Prasad, A.; Stanacevic, S. A selective nanosensor device for exhaled breath analysis. J. Breath Res. 2011, 5, 037110. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; van Berkel, J.J.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef] [PubMed]
- Zubaydi, F.; Sagahyroon, A.; Aloul, F.; Mir, H. MobSpiro: Mobile based spirometry for detecting COPD. In Proceedings of the 2017 IEEE 7th Annual Computing and Communication Workshop and Conference (CCWC), Las Vegas, NV, USA, 9 January 2017; pp. 1–4. [Google Scholar]
- National Heart, Lung, Blood Institute; National Asthma Education Program; Expert Panel on the Management of Asthma. Guidelines for the Diagnosis and Management of Asthma; National Asthma Education Program; Office of Prevention, Education, and Control, National Heart, Lung, and Blood Institute, National Institutes of Health: Bethesda, MD, USA, 1991. [Google Scholar]
- Braman, S.S. The global burden of asthma. Chest 2006, 130, 4S–12S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennergren, G. Inflammatory mediators in blood and urine. Paediatr. Respir. Rev. 2000, 1, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Jutel, M.; Akdis, C.A.; Klimek, L.; Pfaar, O.; Nadeau, K.C.; Eiwegger, T.; Bedbrook, A.; Ansotegui, I.J.; Anto, J.M.; et al. ARIA-EAACI statement on asthma and COVID-19 (2 June 2020). Allergy 2021, 76, 689–697. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Munakata, M.; Shibata, Y. Hydrogen sulfide as a novel biomarker of asthma and chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 181–189. [Google Scholar] [CrossRef]
- Horváth, I.; Donnelly, L.E.; Kiss, A.; Kharitonov, S.A.; Lim, S.; Chung, K.F.; Barnes, P.J. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am. J. Respir. Crit. Care Med. 1998, 158, 1042–1046. [Google Scholar] [CrossRef] [Green Version]
- Zayasu, K.; Sekizawa, K.; Okinaga, S.; Yamaya, M.; Ohrui, T.; Sasaki, H. Increased carbon monoxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1997, 156, 1140–1143. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.A.; Shin, Y.S.; Park, H.S. Adult asthma biomarkers. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 49–54. [Google Scholar] [CrossRef]
- MacGregor, G.; Ellis, S.; Andrews, J.; Imrie, M.; Innes, A.; Greening, A.P.; Cunningham, S. Breath condensate ammonium is lower in children with chronic asthma. Eur. Respir. J. 2005, 26, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, D.H.; Kharitonov, S.A.; Robbins, R.A.; Thomas, P.S.; Barnes, P.J. The effect of alcohol ingestion on exhaled nitric oxide. Eur. Respir. J. 1996, 9, 1130–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinues, P.M.; Meier, L.; Berchtold, C.; Ivanov, M.; Sievi, N.; Camen, G.; Kohler, M.; Zenobi, R. Breath analysis in real time by mass spectrometry in chronic obstructive pulmonary disease. Respiration 2014, 87, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinakar, C. Exhaled nitric oxide in the clinical management of asthma. Curr. Allergy Asthma Rep. 2004, 4, 454–459. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Faster rise of exhaled breath temperature in asthma: A novel marker of airway inflammation? Am. J. Respir. Crit. Care 2002, 165, 181–184. [Google Scholar] [CrossRef]
- Macker, A.; Shukla, A.K.; Dey, S.; Agarwal, J. ARDUINO Based LPG Gas Monitoring… Automatic Cylinder Booking with Alert System. In Proceedings of the 2018 2nd International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 11 May 2018; pp. 1209–1212. [Google Scholar]
- Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Chen, Y.; Yao, W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology 2014, 19, 1165–1169. [Google Scholar] [CrossRef]
- Kinoyama, M.; Nitta, H.; Watanabe, A.; Ueda, H. Acetone and isoprene concentrations in exhaled breath in healthy subjects. J. Health Sci. 2008, 54, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.J.; Španěl, P.; Smith, D. Breath analysis of ammonia, volatile organic compounds and deuterated water vapor in chronic kidney disease and during dialysis. Bioanalysis 2014, 6, 843–857. [Google Scholar] [CrossRef]
- Dutta, R.; Hines, E.L.; Gardner, J.W.; Boilot, P. Bacteria classification using Cyranose 320 electronic nose. Biomed. Eng. Online 2002, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, V.R.; Mohapatra, A.K.; Lukose, J.; Kartha, V.B.; Chidangil, S. Post-COVID syndrome screening through breath analysis using electronic nose technology. Anal. Bioanal. Chem. 2022, 414, 3617–3624. [Google Scholar]




| S.N | Dependent Variable | Independent Variables (Exhale Breath Compounds) | p-Value | Conclusion |
|---|---|---|---|---|
| 1 | Obstructive Lung Disease | Alcohol (C2H5OH) | p > 0.05 | Insignificant difference |
| 2 | Acetone (CH3COCH3) | p < 0.001 | Significant Difference | |
| 4 | Ammonia (NH3) | p < 0.0001 | Significant Difference | |
| 5 | Hydrogen Sulfide (H2S) | p < 0.0001 | Significant Difference |
| Information Group | Patients | Healthy |
|---|---|---|
| Number | 60 | 45 |
| Gender (M/F) | 45/15 | 42/3 |
| Average Age (Years) | 45.66 ± 15.08 | 42.22 ± 14.84 |
| Average Weight (Kg) | 67.76 ± 11.51 | 70.5 ± 12.41 |
| Smoking Status(Smoker/Non-smoker) | 12/48 | 11/34 |
| Overall Average level of exhaled gases | NH3 (68.83 ± 35.25ppb), H2S (32.29 ± 20.83 ppb), C2H6CO (0.50 ± 0.26 ppm), C2H5OH (0.96 ± 0.52 ppm) | NH3 (103.49 ± 35.01 ppb), H2S (62.71 ± 22.20 ppb), C2H6CO (0.66 ± 0.31 ppm), C2H5OH (1.09 ± 0.51 ppm) |
| Severity of Lung Disease and Average plus STD level of exhaled Gases | ||
| Mild Obstruction (number, level of exhaled gases) | 21, NH3 (88.11 ± 32.66 ppb), H2S (37 ± 18.33 ppb), C2H6CO (0.51 ± 0.23 ppm), C2H5OH (0.92 ± 0.17 ppm) | same as above |
| Moderate Obstruction (number, level of exhaled gases) | 10, NH3 (65.77 ± 32.07 ppb), H2S (42.36 ± 22.56 ppb), C2H6CO (0.56 ± 0.25 ppm), C2H5OH (1.07 ± 0.57 ppm), | same as above |
| Moderate Severe Obstruction (number, level of exhaled gases) | 10, NH3 (55.18 ± 22.86 ppb), H2S (23.24 ± 15.72 ppb), C2H6CO (0.39 ± 0.28 ppm), C2H5OH (0.84 ± 0.63 ppm) | same as above |
| Severe Obstruction (number, level 0f exhaled gases) | 14, NH3 (58.14 ± 38.66 ppb) H2S (26.31 ± 20.39 ppb) C2H6 CO (0.48 ± 0.28 ppm) C2H5OH (0.0.93 ± 0.42 ppm) | same as above |
| Very Severe Obstruction (number, level of exhaled gases) | 5, NH3 (43.32 ± 21.35 ppb) H2S (21.36 ± 19.52 ppb) C2H6CO (0.404 ± 0.34 ppm) C2H5OH (1.41 ± 0.51 ppm) | same as above |
| Average plus STD level of % of Predicted FVC | 76.93 ± 23.08 | 100.27 ± 10.11 |
| Average plus STD level of % of Predicted FEV1 | 60.60 ± 18.59 | 98.91 ± 12.40 |
| Average plus STD level of % of Predicted FVC/FEV1 | 81.18 ± 17.31 | 101.82 ± 9.19 |
| Average level of % of Predicted PEF | 49.4 ± 19.88 | 88.14 ± 20.14 |
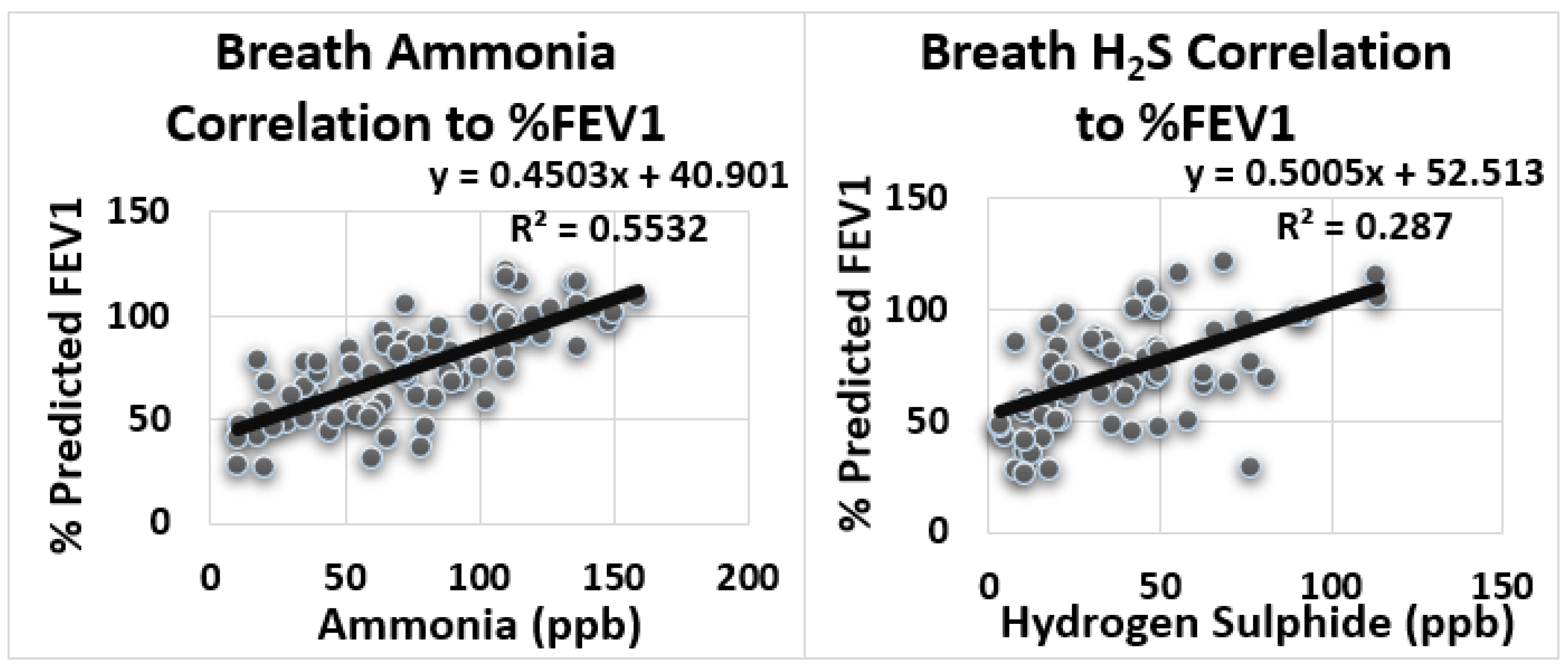
| S.N | Variables | Equation | R (Correlation Coefficient) | R2 (Determination Coefficient) | Summary |
|---|---|---|---|---|---|
| 1 | NH3-FEV1 | y = 0.45x + 40.90 | 0.74 | 0.553 | Large Positive Correlation |
| 2 | H2S-FEV1 | y = 0.50x + 52.51 | 0.54 | 0.287 | Medium Positive Correlation |
| 3 | NH3-FVC | y = 0.34x + 61.27 | 0.61 | 0.377 | Medium Positive Correlation |
| 4 | H2S-FVC | y = 0.36x + 69.67 | 0.41 | 0.172 | Small Positive Correlation |
| 5 | NH3-PEF | y = 0.47x + 31.10 | 0.63 | 0.398 | Medium Positive Correlation |
| 6 | H2S-PEF | y = 0.429x + 45.55 | 0.37 | 0.14 | Small Positive Correlation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, A.A.; Mushtaq, S.; Waris, A.; Gilani, S.O.; Alnuwaiser, M.A.; Jameel, M.; Khan, N.B. A Low-Cost Device for Measurement of Exhaled Breath for the Detection of Obstructive Lung Disease. Biosensors 2022, 12, 409. https://doi.org/10.3390/bios12060409
Shahzad AA, Mushtaq S, Waris A, Gilani SO, Alnuwaiser MA, Jameel M, Khan NB. A Low-Cost Device for Measurement of Exhaled Breath for the Detection of Obstructive Lung Disease. Biosensors. 2022; 12(6):409. https://doi.org/10.3390/bios12060409
Chicago/Turabian StyleShahzad, Adil Ahmad, Shafaq Mushtaq, Asim Waris, Syed Omer Gilani, Maha Abdallah Alnuwaiser, Mohammed Jameel, and Niaz Bahadur Khan. 2022. "A Low-Cost Device for Measurement of Exhaled Breath for the Detection of Obstructive Lung Disease" Biosensors 12, no. 6: 409. https://doi.org/10.3390/bios12060409
APA StyleShahzad, A. A., Mushtaq, S., Waris, A., Gilani, S. O., Alnuwaiser, M. A., Jameel, M., & Khan, N. B. (2022). A Low-Cost Device for Measurement of Exhaled Breath for the Detection of Obstructive Lung Disease. Biosensors, 12(6), 409. https://doi.org/10.3390/bios12060409





