An Overview on Coinage Metal Nanocluster-Based Luminescent Biosensors via Etching Chemistry
Abstract
1. Introduction
2. Etching Chemistry of Metal NCs
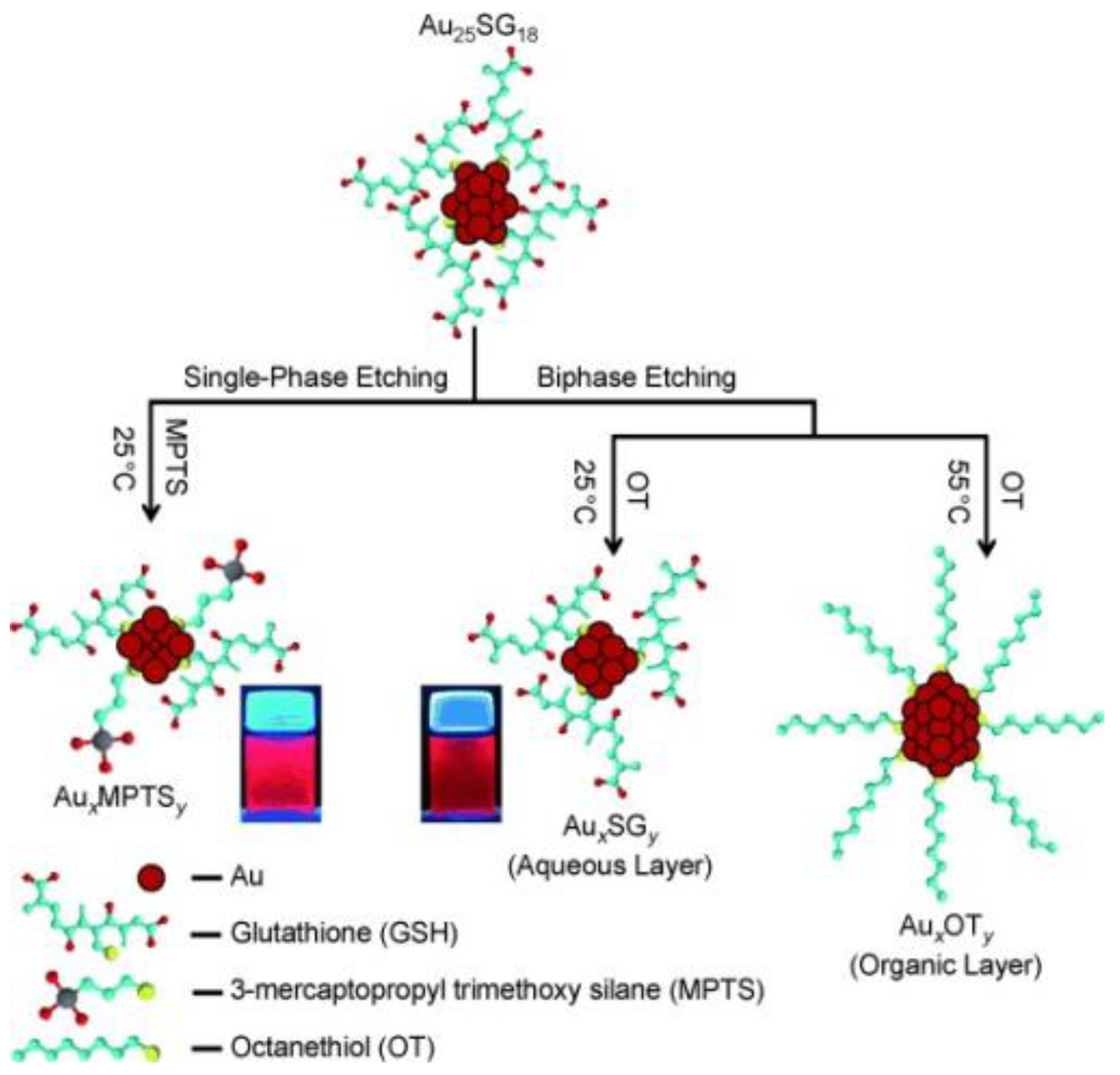
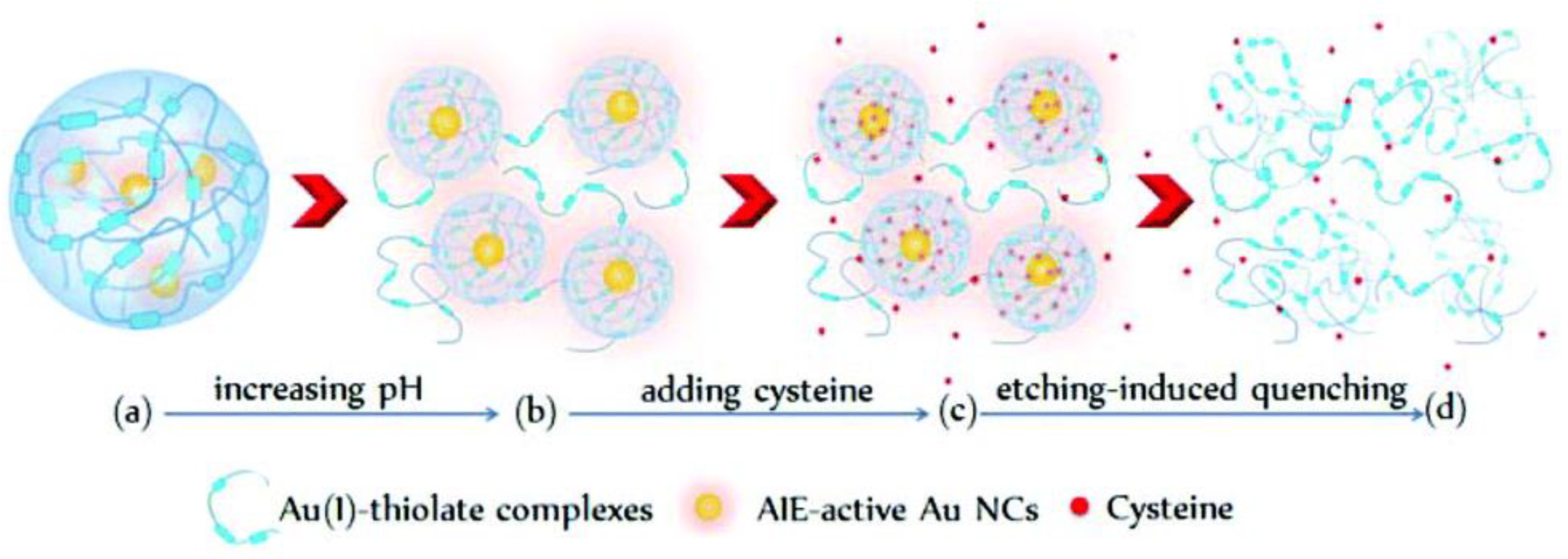
2.1. Thiol-Induced Etching

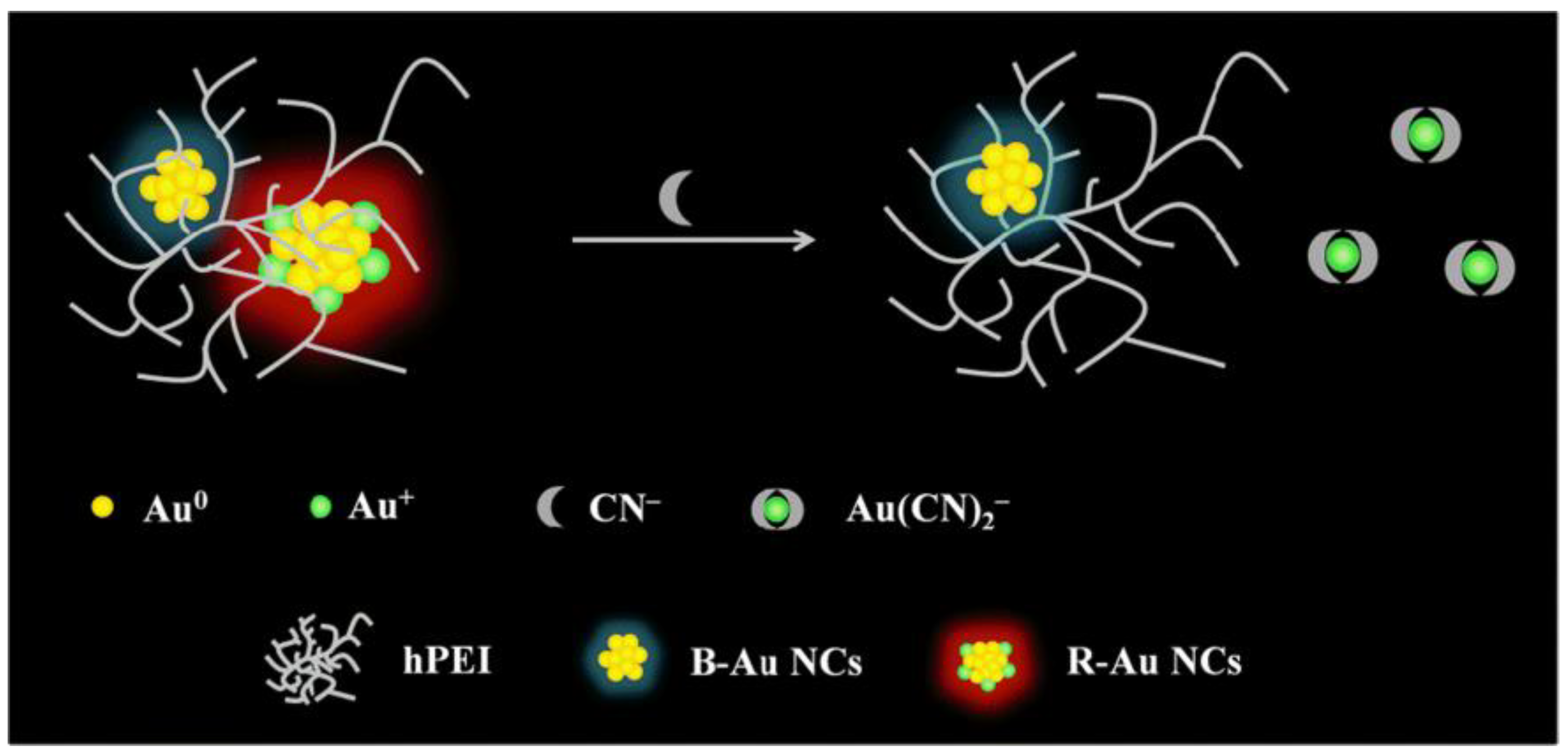
2.2. Cyanide-Induced Etching
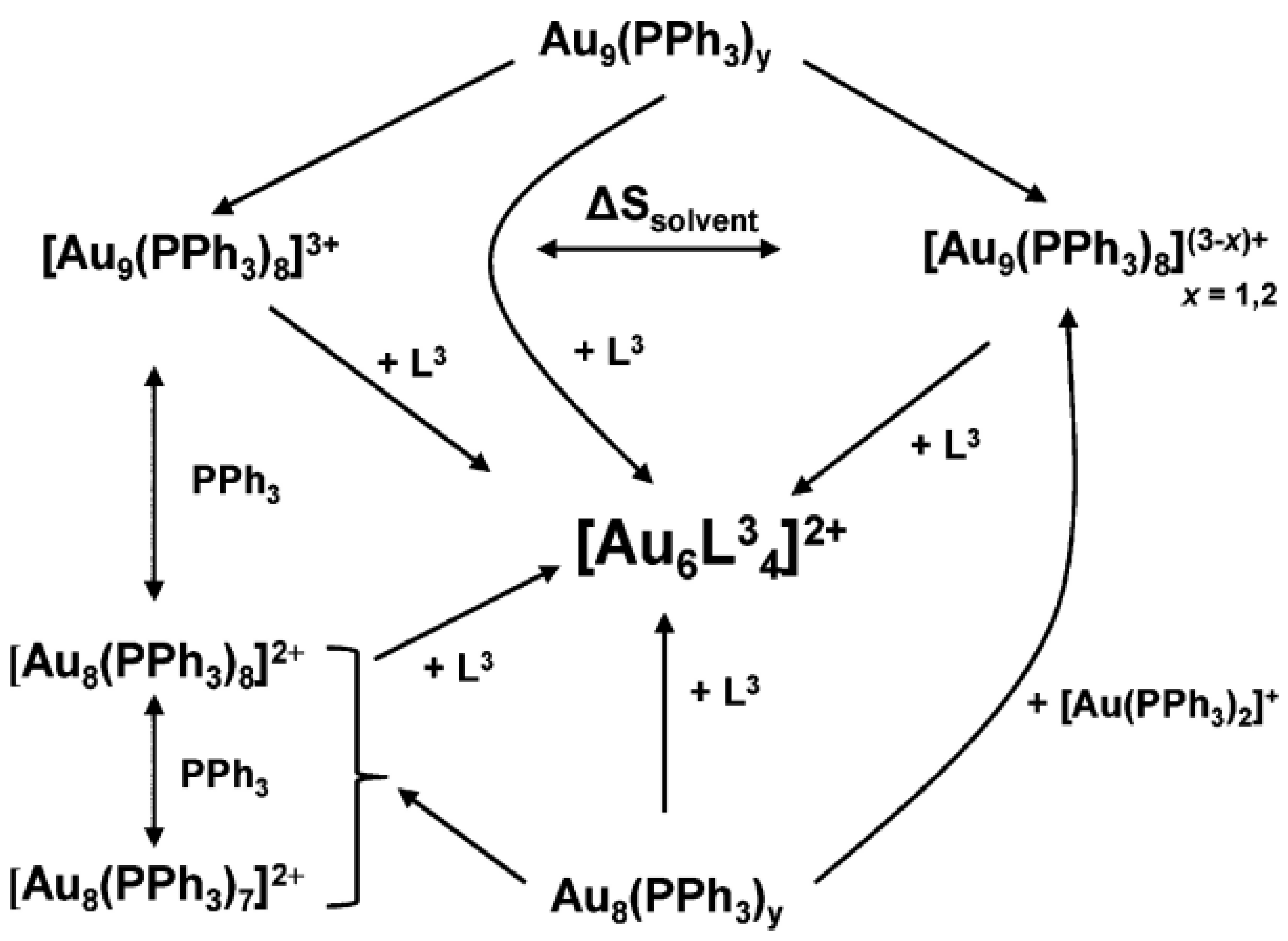
2.3. Phosphine Compound-Induced Etching
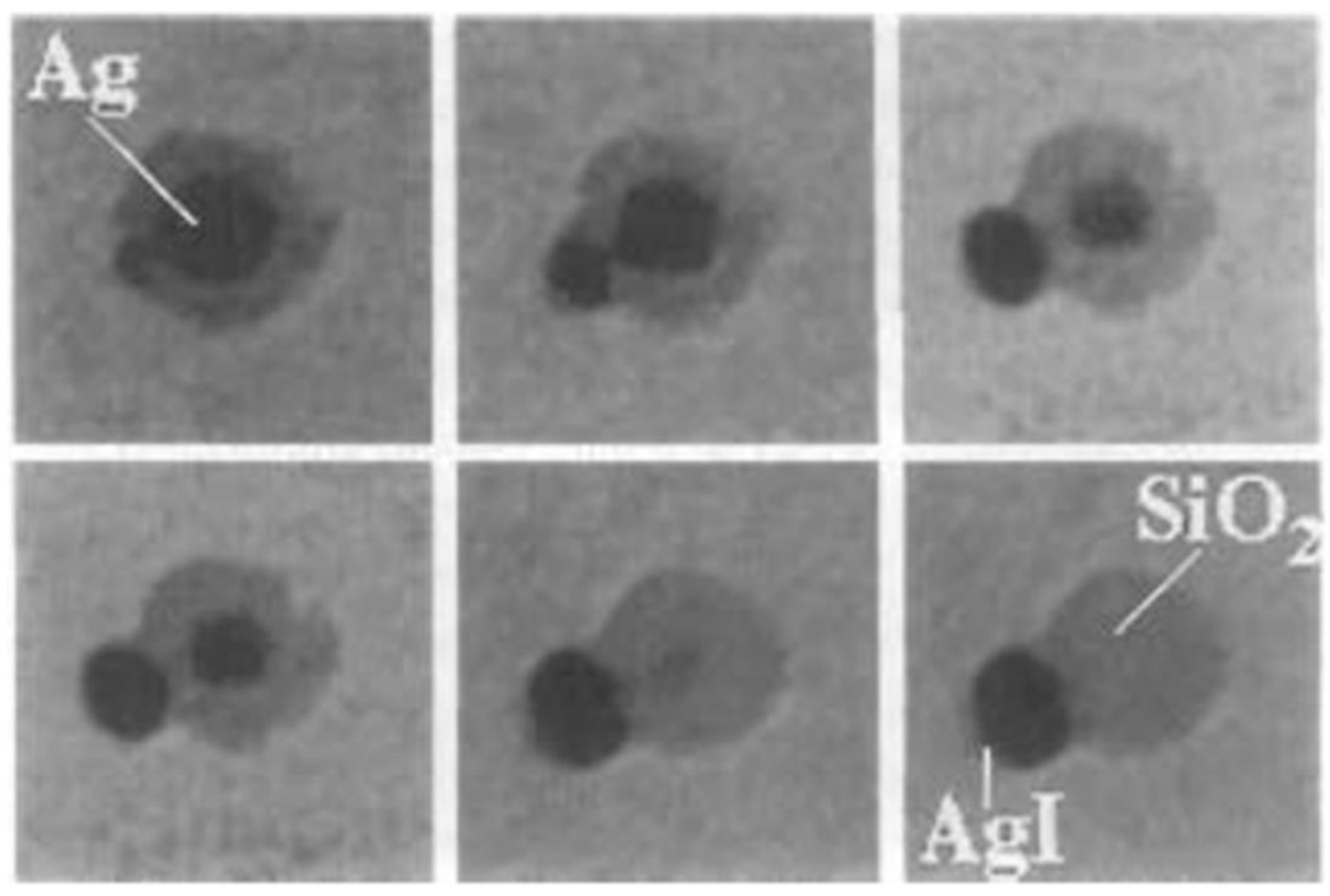
2.4. Iodine Compound-Induced Etching
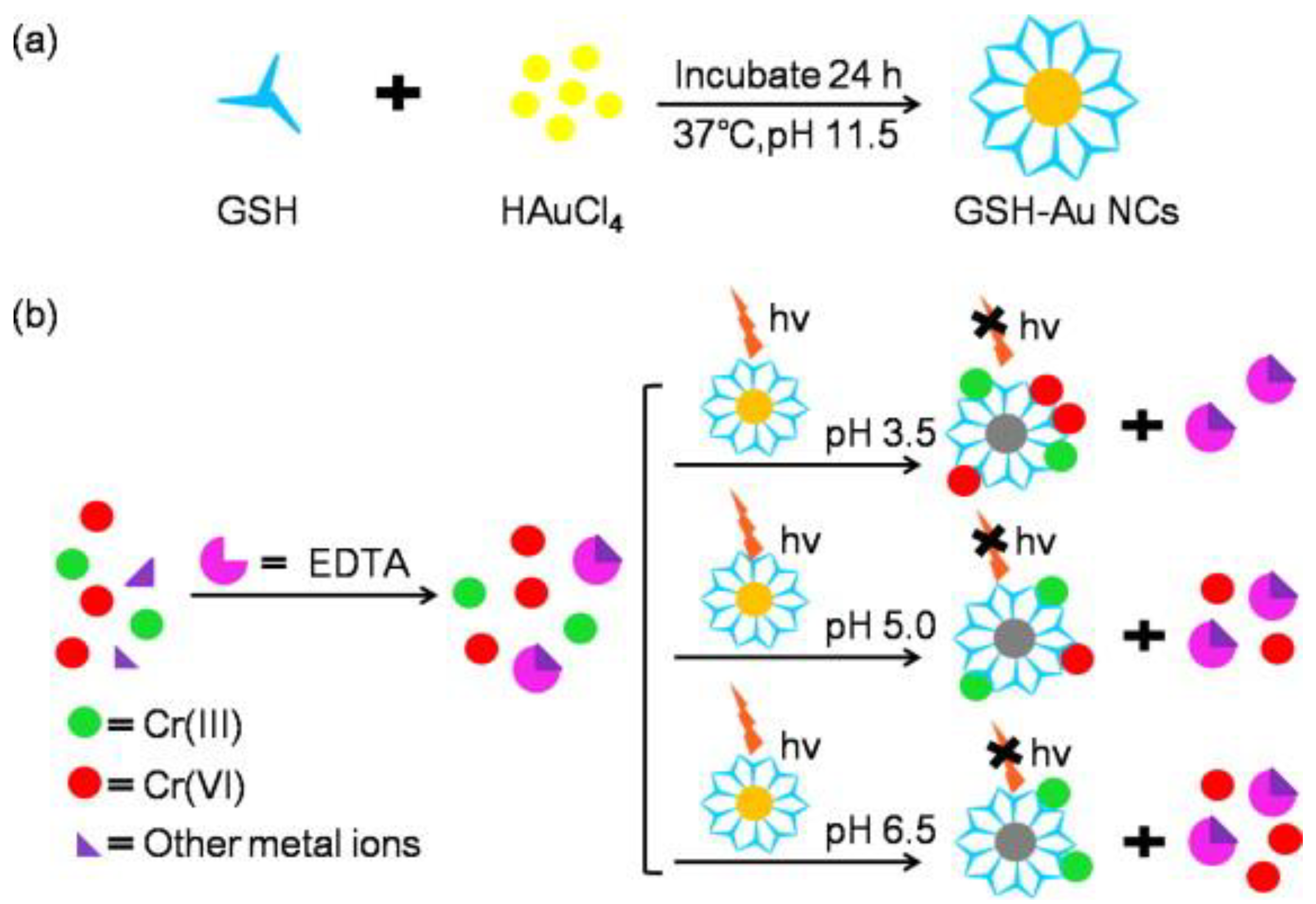
2.5. Heavy Metal Ion-Induced Etching
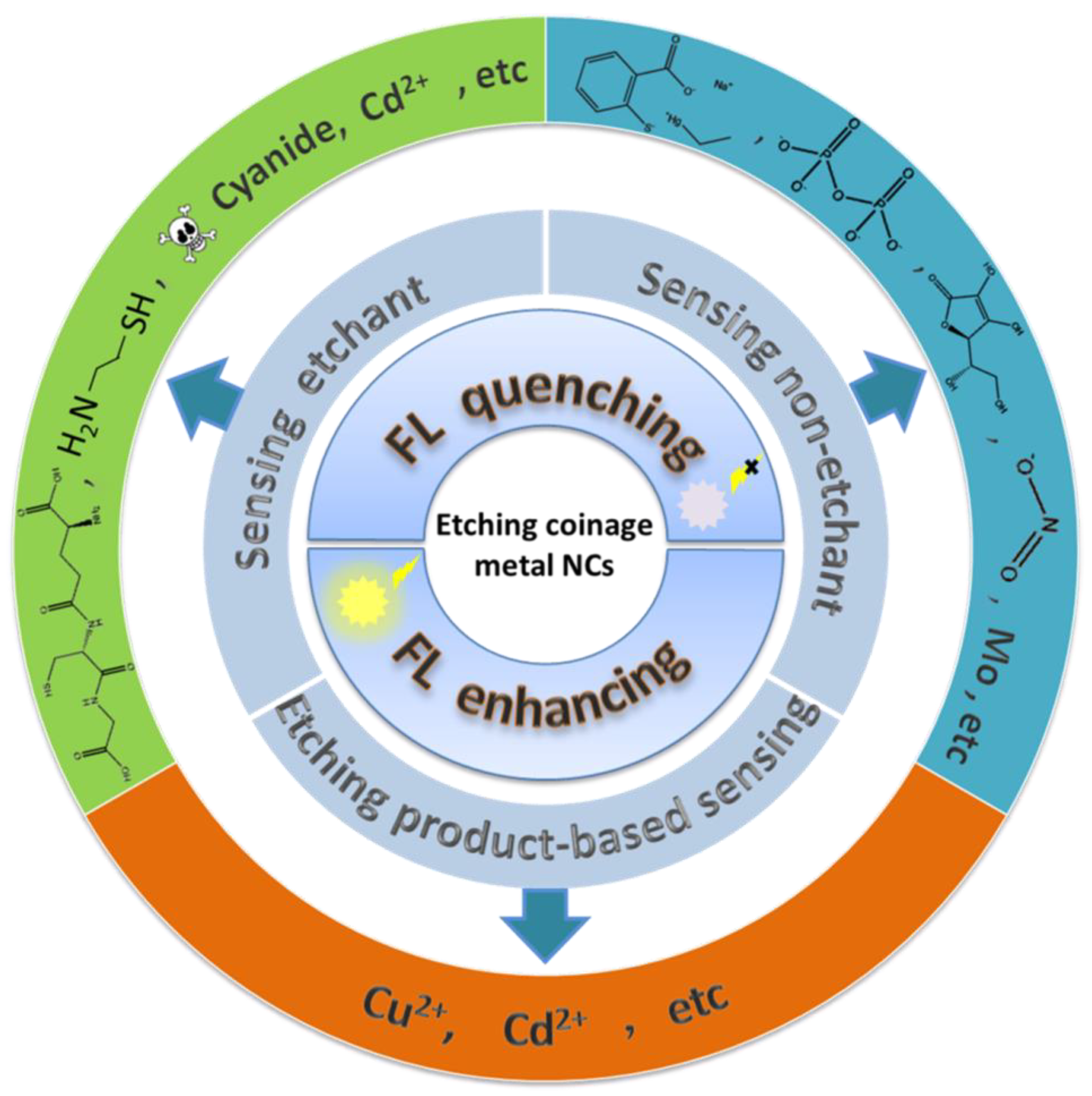
3. Sensor Construction Based on Metal Nanocluster Etching
3.1. Etchant Detection
3.2. Indirect Sensing Method Using Etchant
3.3. Etching Product-Based Sensors
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, J.; Duan, G.; Lu, X.; Mak, T.C.W. High-nuclearity silver(I) chalcogenide clusters: A novel class of supramolecular assembly. Coord. Chem. Rev. 2017, 331, 54–72. [Google Scholar] [CrossRef]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly Fluorescent Noble-Metal Quantum Dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Nan, Z.; Wang, Q. Superatomic Orbital Splitting in Coinage Metal Nanoclusters. J. Phys. Chem. Lett. 2022, 13, 291–295. [Google Scholar] [CrossRef]
- Hao, Y.; Huixin, X.; Jiaohu, L.; Ranran, C.; Yongqi, Y.; Yunhu, H.; Chuanhao, Y. The Factors Dictating Properties of Atomically Precise Metal Nanocluster Electrocatalysts. Small (Weinh. Bergstr. Ger.) 2022, 18, 2200812. [Google Scholar]
- Yang, J.; Yang, F.; Zhang, C.; He, X.; Jin, R. Metal Nanoclusters as Biomaterials for Bioapplications: Atomic Precision as the Next Goal. ACS Mater. Lett. 2022, 4, 1279–1296. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Tiankai, C.; Hongbin, L.; Yitao, C.; Qiaofeng, Y.; Jianping, X. Interactions of Metal Nanoclusters with Light: Fundamentals and Applications (Adv. Mater. 25/2022). Adv. Mater. 2022, 34, 2270187. [Google Scholar]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Development of Copper Nanoclusters for In Vitro and In Vivo Theranostic Applications. Adv. Mater. (Deerfield Beach Fla) 2020, 32, 1906872. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Han, W.; Dong, X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhu, M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef]
- Wan, X.K.; Xu, W.W.; Yuan, S.F.; Gao, Y.; Zeng, X.C.; Wang, Q.M. A Near-Infrared-Emissive Alkynyl-Protected Au24 Nanocluster. Angew. Chem. Int. Ed. Engl. 2015, 54, 9683–9686. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Hasegawa, S.; Suyama, M.; Tsukuda, T. Hydride Doping of Chemically Modified Gold-Based Superatoms. Acc. Chem. Res. 2018, 51, 3074–3083. [Google Scholar] [CrossRef]
- Konishi, K.; Iwasaki, M.; Shichibu, Y. Phosphine-Ligated Gold Clusters with Core+ exo Geometries: Unique Properties and Interactions at the Ligand-Cluster Interface. Acc. Chem. Res. 2018, 51, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Phipps, M.L.; Werner, J.H.; Chakraborty, S.; Martinez, J.S. DNA Templated Metal Nanoclusters: From Emergent Properties to Unique Applications. Acc. Chem. Res. 2018, 51, 2756–2763. [Google Scholar] [CrossRef]
- Yan, J.; Teo, B.K.; Zheng, N. Surface Chemistry of Atomically Precise Coinage-Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis. Acc. Chem. Res. 2018, 51, 3084–3093. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward Total Synthesis of Thiolate-Protected Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yuan, X.; Chen, T.; Leong, D.T.; Xie, J. Engineering Functional Metal Materials at the Atomic Level. Adv. Mater. 2018, 30, e1802751. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, D.M.; Raich, L.; Rovira, C.; Das, A.; Luo, Z.; Yao, Q.; Chatt, A.; Xie, J.; Jin, R.; Akola, J.; et al. Molecular-Scale Ligand Effects in Small Gold-Thiolate Nanoclusters. J. Am. Chem. Soc. 2018, 140, 15430–15436. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, S.; Song, Y.; Jin, S.; Sun, G.; Yu, H.; Zhu, M. Bimetallic Au2Cu6 Nanoclusters: Strong Luminescence Induced by the Aggregation of Copper(I) Complexes with Gold(0) Species. Angew. Chem. Int. Ed. Engl. 2016, 55, 3611–3614. [Google Scholar] [CrossRef] [PubMed]
- Price, R.C.; Whetten, R.L. All-Aromatic, Nanometer-Scale, Gold-Cluster Thiolate Complexes. J. Am. Chem. Soc. 2005, 127, 13750–13751. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Tsunoyama, H.; Suzuki, M.; Kawamura, N.; Matsushita, M.M.; Maruyama, K.; Sugawara, T.; Yokoyama, T.; Tsukuda, T. X-ray Magnetic Circular Dichroism of Size-Selected, Thiolated Gold Clusters. J. Am. Chem. Soc. 2006, 128, 12034–12035. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem. Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef]
- Sun, J.; Jin, Y. Fluorescent Au nanoclusters: Recent progress and sensing applications. J. Mater. Chem. C 2014, 2, 8000–8011. [Google Scholar] [CrossRef]
- Guo, C.; Irudayaraj, J. Fluorescent Ag clusters via a protein-directed approach as a Hg(II) ion sensor. Anal. Chem. 2011, 83, 2883–2889. [Google Scholar] [CrossRef]
- Huang, C.C.; Yang, Z.S.; Lee, K.H.; Chang, H.T. Synthesis of Highly Fluorescent Gold Nanoparticles for Sensing Mercury(II). Angew. Chem. 2007, 119, 6948–6952. [Google Scholar] [CrossRef]
- Huang, C.C.; Hung, Y.L.; Shiang, Y.C.; Lin, T.Y.; Lin, Y.S.; Chen, C.T.; Chang, H.T. Photoassisted synthesis of luminescent mannose-Au nanodots for the detection of thyroglobulin in serum. Chem. Asian J. 2010, 5, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hou, C.; Yang, M.; Huo, D.; Fa, H.B. Ultra-sensitive fluorescence determination of chromium(vi) in aqueous solution based on selectively etching of protein-stabled gold nanoclusters. RSC Adv. 2016, 6, 104693–104698. [Google Scholar]
- Zhang, H.; Liu, Q.; Wang, T.; Yun, Z.; Li, G.; Liu, J.; Jiang, G. Facile preparation of glutathione-stabilized gold nanoclusters for selective determination of chromium (III) and chromium (VI) in environmental water samples. Anal. Chim. Acta 2013, 770, 140–146. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+-Au+ interactions. Chem. Commun. (Camb.) 2010, 46, 961–963. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Wu, X.; Jiang, L.; Burda, C.; Zhu, J.J. Rapid sonochemical synthesis of highly luminescent non-toxic AuNCs and Au@AgNCs and Cu (II) sensing. Chem. Commun. (Camb.) 2011, 47, 4237–4239. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, M.; Yang, J.; Zheng, X.; Cai, W.; Meng, G.; Qian, H.; Wang, H.; Jin, R. Well-defined nanoclusters as fluorescent nanosensors: A case study on Au25(SG)18. Small 2012, 8, 2028–2035. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Cheng, X.; Huo, L.; Lu, L. Gold-Nanocluster-Based Fluorescent Sensors for Highly Sensitive and Selective Detection of Cyanide in Water. Adv. Funct. Mater. 2010, 20, 951–956. [Google Scholar] [CrossRef]
- Zhang, G.; Qiao, Y.; Xu, T.; Zhang, C.; Zhang, Y.; Shi, L.; Shuang, S.; Dong, C. Highly selective and sensitive nanoprobes for cyanide based on gold nanoclusters with red fluorescence emission. Nanoscale 2015, 7, 12666–12672. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Ren, T.; Tong, Y.; Yang, B.; Li, Y. Novel bimetallic gold-silver nanoclusters with “Synergy”-enhanced fluorescence for cyanide sensing, cell imaging and temperature sensing. Talanta 2017, 170, 530–539. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Liu, S.; Zhan, X.; Zhou, H.; Li, X.; Yuan, Z. Ratiometric and sensitive cyanide sensing using dual-emissive gold nanoclusters. Anal. Bioanal. Chem. 2020, 412, 5819–5826. [Google Scholar] [CrossRef]
- Zhou, T.; Rong, M.; Cai, Z.; Yang, C.J.; Chen, X. Sonochemical synthesis of highly fluorescent glutathione-stabilized Ag nanoclusters and S2− sensing. Nanoscale 2012, 4, 4103–4106. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Tseng, W.L. (Lysozyme type VI)-stabilized Au8 clusters: Synthesis mechanism and application for sensing of glutathione in a single drop of blood. Small 2012, 8, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P.; Lv, Y.; Hou, X. Ultrasensitive fluorescence detection of glutaraldehyde in water samples with bovine serum albumin-Au nanoclusters. Microchem. J. 2011, 99, 327–331. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.-W.; Wu, Y. A highly selective and sensitive fluorescent probe for lactate dehydrogenase based on ultrabright adenosine monophosphate capped gold nanoclusters. RSC Adv. 2017, 7, 13438–13443. [Google Scholar] [CrossRef]
- Shu, T.; Su, L.; Wang, J.; Li, C.; Zhang, X. Chemical etching of bovine serum albumin-protected Au25 nanoclusters for label-free and separation-free detection of cysteamine. Biosens. Bioelectron. 2015, 66, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, X.; Su, L.; Yin, J.; Shu, T.; Zhang, X. Chemical etching of pH-sensitive aggregation-induced emission-active gold nanoclusters for ultra-sensitive detection of cysteine. Nanoscale 2018, 11, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Çakır, O. Chemical etching of aluminium. J. Mater. Process. Technol. 2008, 199, 337–340. [Google Scholar] [CrossRef]
- Jin, R.; Qian, H.; Wu, Z.; Zhu, Y.; Zhu, M.; Mohanty, A.; Garg, N. Size Focusing: A Methodology for Synthesizing Atomically Precise Gold Nanoclusters. J. Phys. Chem. Lett. 2010, 1, 2903–2910. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, J.; Ruditskiy, A.; Liu, M.; Xia, Y. Oxidative Etching and Its Role in Manipulating the Nucleation and Growth of Noble-Metal Nanocrystals. Chem. Mater. 2014, 26, 22–33. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Li, W.Y.; Zeng, J.; McKiernan, M.; Xie, Z.X.; Xia, Y.N. Synthesis of small silver nanocubes in a hydrophobic solvent by introducing oxidative etching with Fe(III) species. J. Mater. Chem. 2010, 20, 3586–3589. [Google Scholar] [CrossRef]
- Xiaoyun, Y.L.; Lin, Y.Y.; Chen, Q.; Belessiotis-Richards, A.; Stevens, M.M.; Thomas, M.R. Iodide-Mediated Rapid and Sensitive Surface Etching of Gold Nanostars for Biosensing. Angew. Chem. Int. Ed. 2021, 60, 9891–9896. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. Engl. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Cortie, M.B.; McDonagh, A.M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 2011, 111, 3713–3735. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Jones, M.R.; Frechette, L.B.; Chen, Q.; Powers, A.S.; Ercius, P.; Dunn, G.; Rotskoff, G.M.; Nguyen, S.C.; Adiga, V.P.; et al. Single-particle mapping ofnonequilibrium nanocrystal transformations. Science 2016, 354, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huo, D.; Goines, S.; Yang, T.H.; Lyu, Z.; Zhao, M.; Gilroy, K.D.; Wu, Y.; Hood, Z.D.; Xie, M.; et al. Enabling Complete Ligand Exchange on the Surface of Gold Nanocrystals through the Deposition and Then Etching of Silver. J. Am. Chem. Soc. 2018, 140, 11898–11901. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, M.; Qin, H.; Peng, X. Ag Nanocrystals with Nearly Ideal Optical Quality: Synthesis, Growth Mechanism, and Characterizations. J. Am. Chem. Soc. 2018, 140, 17734–17742. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Prasad, B.L.V.; Stoeva, S.I.; Sorensen, C.M.; Kenneth, K.J. Digestive-Ripening Agents for Gold Nanoparticles: Alternatives to Thiols. Chem. Mater. 2003, 15, 935–942. [Google Scholar] [CrossRef]
- Dreier, T.A.; Ackerson, C.J. Radicals Are Required for Thiol Etching of Gold Particles. Angew. Chem. Int. Ed. Engl. 2015, 54, 9249–9252. [Google Scholar] [CrossRef]
- Shu, T.; Su, L.; Wang, J.; Lu, X.; Liang, F.; Li, C.; Zhang, X. Value of the Debris of Reduction Sculpture: Thiol Etching of Au Nanoclusters for Preparing Water-Soluble and Aggregation-Induced Emission-Active Au(I) Complexes as Phosphorescent Copper Ion Sensor. Anal. Chem. 2016, 88, 6071–6077. [Google Scholar] [CrossRef]
- Lu, X.; Wang, T.; Shu, T.; Qu, X.; Zhang, X.; Liang, F.; Su, L. Combination of chemical etching of gold nanoclusters with aggregation-induced emission for preparation of new phosphors for the development of UV-driven phosphor-converted white light-emitting diodes. J. Mater. Chem. C 2016, 4, 11482–11487. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H. Bioconjugation Using Thiols: Old Chemistry Rediscovered to Connect Polymers with Nature’s Building Blocks. ACS Macro Lett. 2012, 2, 14–18. [Google Scholar] [CrossRef]
- Yin, C.; Huo, F.; Zhang, J.; Martínez-Máñez, R.; Yang, Y.; Lv, H.; Li, S. Thiol-addition reactions and their applications in thiol recognition. Chem. Soc. Rev. 2013, 42, 6032–6059. [Google Scholar] [CrossRef]
- Yuan, X.; Tay, Y.; Dou, X.; Luo, Z.; Leong, D.T.; Xie, J. Glutathione-Protected Silver Nanoclusters as Cysteine-Selective Fluorometric and Colorimetric Probe. Anal. Chem. 2013, 85, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.A.H.; Verma, P.K.; Pal, S.K.; Kumar, R.C.A.; Paul, S.; Omkumar, R.V.; Pradeep, T. Bright, NIR-Emitting Au23 from Au25: Characterization and Applications Including Biolabeling. Chem.—Eur. J. 2009, 15, 10110–10120. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Hamaguchi, K.; Osaka, I.; Arakawa, R. ph-Dependent Synthesis of Pepsin-Mediated Gold Nanoclusters with Blue Green and Red Fluorescent Emission. Adv. Funct. Mater. 2011, 21, 3508–3515. [Google Scholar] [CrossRef]
- Guo, W.; Yuan, J.; Wang, E. Organic-soluble fluorescent Au8 clusters generated from heterophase ligand-exchange induced etching of gold nanoparticles and their electrochemiluminescence. Chem. Commun. 2012, 48, 3076–3078. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, X.; Li, J.; Liu, Y.N. Synthesis of Ag nanoclusters by a pH-dependent etching method in aqueous solution. Nanoscale 2013, 5, 6261–6264. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Yuan, Z.; Yang, Y.-Y.; Huang, C.-C.; Chang, H.-T. Photoluminescent gold nanodots: Role of the accessing ligands. RSC Adv. 2014, 4, 33629–33635. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, Y.; Yang, X.; Chen, J.; Jiang, Y.; Yu, C.; Lin, Q. Cysteine-directed fluorescent gold nanoclusters for the sensing of pyrophosphate and alkaline phosphatase. J. Mater. Chem. C 2014, 2, 4080–4085. [Google Scholar] [CrossRef]
- Yuan, X.; Setyawati, M.I.; Tan, A.S.; Ong, C.N.; Leong, D.T.; Xie, J. Highly luminescent silver nanoclusters with tunable emissions: Cyclic reduction–decomposition synthesis and antimicrobial properties. NPG Asia Mater. 2013, 5, e39. [Google Scholar] [CrossRef]
- Qian, H.F.; Zhu, Y.; Jin, R.C. Size-focusing synthesis, optical and electrochemical properties of monodisperse Au38(SC2H4Ph)24 nanoclusters. ACS Nano 2009, 3, 3795–3803. [Google Scholar] [CrossRef]
- Dharmaratne, A.C.; Krick, T.; Dass, A. Nanocluster size evolution studied by mass spectrometry in room temperature Au25(SR)18 synthesis. J. Am. Chem. Soc. 2009, 131, 13604–13605. [Google Scholar] [CrossRef]
- Guidez, E.B.; Hadley, A.; Aikens, C.M. Initial Growth Mechanisms of Gold-Phosphine Clusters. J. Phys. Chem. C 2011, 115, 6305–6316. [Google Scholar] [CrossRef]
- Barngrover, B.M.; Aikens, C.M. The golden pathway to thiolate-stabilized nanoparticles: Following the formation of gold(I) thiolate from gold(III) chloride. J. Am. Chem. Soc. 2012, 134, 12590–12595. [Google Scholar] [CrossRef][Green Version]
- Schaaff, T.G.; Whetten, R.L. Controlled Etching of Au:SR Cluster Compounds. J. Phys. Chem. B 1999, 103, 9394–9396. [Google Scholar] [CrossRef]
- Edinger, K.; Gölzhäuser, A.; Demota, K.; Wöll, C.; Grunze, M. Formation of self-assembled monolayers of n-alkanethiols on gold: A scanning tunneling microscopy study on the modification of substrate morphology. Langmuir 1993, 9, 4–8. [Google Scholar] [CrossRef]
- Shichibu, Y.; Negishi, Y.; Tsunoyama, H.; Kanehara, M.; Teranishi, T.; Tsukuda, T. Extremely high stability of glutathionate-protected Au25 clusters against core etching. Small 2007, 3, 835–839. [Google Scholar] [CrossRef]
- Nimmala, P.R.; Dass, A. Au36(SPh)23 nanomolecules. J. Am. Chem. Soc. 2011, 133, 9175–9177. [Google Scholar] [CrossRef]
- Zeng, C.; Qian, H.; Li, T.; Li, G.; Rosi, N.L.; Yoon, B.; Barnett, R.N.; Whetten, R.L.; Landman, U.; Jin, R. Total structure and electronic properties of the gold nanocrystal Au36(SR)24. Angew. Chem. Int. Ed. Engl. 2012, 51, 13114–13118. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.F.; Zhu, M.Z.; Andersen, U.N.; Jin, R.C. Facile, large-scale synthesis of dodecanethiol-stabilized Au38 clusters. J. Phys. Chem. A 2009, 113, 4281–4284. [Google Scholar] [CrossRef] [PubMed]
- Qian, H. Thiolate-protected Au38(SR)24 nanocluster: Size-focusing synthesis, structure determination, intrinsic chirality, and beyond. Pure Appl. Chem. 2014, 86, 27–37. [Google Scholar] [CrossRef]
- Nimmala, P.R.; Dass, A. Au99(SPh)42 nanomolecules: Aromatic thiolate ligand induced conversion of Au144(SCH2CH2Ph)60. J. Am. Chem. Soc. 2014, 136, 17016–17023. [Google Scholar] [CrossRef] [PubMed]
- Nimmala, P.R.; Jupally, V.R.; Dass, A. Core size conversion: Route for exclusive synthesis of Au38 or Au40 nanomolecules. Langmuir 2014, 30, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Udaya Bhaskara Rao, T.; Pradeep, T. Luminescent Ag7 and Ag8 clusters by interfacial synthesis. Angew. Chem. Int. Ed. Engl. 2010, 49, 3925–3929. [Google Scholar] [CrossRef]
- Ionita, P.; Gilbert, B.C.; Chechik, V. Radical Mechanism of a Place-Exchange Reaction of Au Nanoparticles. Angew. Chem. 2005, 117, 3786–3788. [Google Scholar] [CrossRef]
- Tan, K.J.; Wille, U. Activation of molecular oxygen by S-radicals: Experimental and computational studies on a novel oxidation of alkynes to alpha-diketones. Chem. Commun. (Camb.) 2008, 46, 6239–6241. [Google Scholar] [CrossRef]
- Nauser, T.K.; Koppenol, W.H.; Schoneich, C. Reversible hydrogen transfer reactions in thiyl radicals from cysteine and related molecules: Absolute kinetics and equilibrium constants determined by pulse radiolysis. J. Phys. Chem. B 2012, 116, 5329–5341. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.; Liu, T.; Chen, T.; Zhang, B.; Jiang, D.E.; Xie, J. Revealing the etching process of water-soluble Au25 nanoclusters at the molecular level. Nat. Commun. 2021, 12, 3212. [Google Scholar] [CrossRef]
- Ke, C.-Y.; Chen, T.-H.; Lu, L.-C.; Tseng, W.-L. Understanding thiol-induced etching of luminescent gold nanoclusters. RSC Adv. 2014, 4, 26050–26056. [Google Scholar] [CrossRef]
- Wang, X.B.; Wang, Y.L.; Yang, J.; Xing, X.P.; Li, J.; Wang, L.S. Evidence of significant covalent bonding in Au(CN)2−. J. Am. Chem. Soc. 2009, 131, 16368–16370. [Google Scholar] [CrossRef] [PubMed]
- Giersig, M.; Ung, Y.; Liz-Marzan, L.M.; Mulvaney, P. Direct Observation of Chemical Reactions in Silica-Coated Gold and Silver Nanoparticles. Adv. Mater. 1997, 9, 570–575. [Google Scholar]
- Zeng, J.B.; Cao, Y.Y.; Chen, J.J.; Wang, X.D.; Yu, J.F.; Yu, B.B.; Yan, Z.F.; Chen, X. Au@Ag core/shell nanoparticles as colorimetric probes for cyanide sensing. Nanoscale 2014, 6, 9939–9943. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Qin, C.; Jin, L.; Wang, L.; Dong, S. Turn-on fluorescent detection of cyanide based on the inner filter effect of silver nanoparticles. Analyst 2009, 134, 1477–1482. [Google Scholar] [CrossRef]
- Zhai, J.; Zhai, Y.; Dong, S. Direct dissolution of Au nanoparticles induced by potassium ferricyanide. Colloids Surf. A Physicochem. Eng. Asp. 2009, 335, 207–210. [Google Scholar] [CrossRef]
- Peng, J.; Ling, J.; Wen, Q.-L.; Li, Y.; Cao, Q.-E.; Huang, Z.-J.; Ding, Z.-T. The presence of a single-nucleotide mismatch in linker increases the fluorescence of guanine-enhanced DNA-templated Ag nanoclusters and their application for highly sensitive detection of cyanide. RSC Adv. 2018, 8, 41464–41471. [Google Scholar] [CrossRef]
- Shojaeifard, Z.; Hemmateenejad, B.; Shamsipur, M. Efficient On–Off Ratiometric Fluorescence Probe for Cyanide Ion Based on Perturbation of the Interaction between Gold Nanoclusters and a Copper(II)-Phthalocyanine Complex. ACS Appl. Mater. Interfaces 2016, 8, 15177–15186. [Google Scholar] [CrossRef]
- Cang, J.; Wang, C.-W.; Chen, P.-C.; Lin, Y.-J.; Li, Y.-C.; Chang, H.-T. Control of pH for separated quantitation of nitrite and cyanide ions using photoluminescent copper nanoclusters. Anal. Methods 2017, 9, 5254–5259. [Google Scholar] [CrossRef]
- Rader, R.A.; McMillin, D.R.; Buckner, M.T.; Matthews, T.G.; Casadonte, D.J.; Lengel, R.K.; Whittaker, S.B.; Darmon, L.M.; Lytle, F.E. Photostudies of [Cu(bpy)(PPh3)2]+, [Cu(phen)(PPh3)2]+, and [Cu(dmp)(PPh3)2]+ in Solution and in Rigid, Low-Temperature Glasses. Simultaneous Multiple Emissions from Intraligand and Charge-Transfer States. J. Am. Chem. SOC 1981, 103, 5906–5912. [Google Scholar] [CrossRef]
- McKeage, M.; Maharaj, L.; Berners-Price, S.J. Mechanisms of cytotoxicity and antitumor activity of gold(I) phosphine complexes: The possible role of mitochondria. Coord. Chem. Rev. 2002, 232, 127–135. [Google Scholar] [CrossRef]
- Meyer, A.; Bagowski, C.P.; Kokoschka, M.; Stefanopoulou, M.; Alborzinia, H.; Can, S.; Vlecken, D.H.; Sheldrick, W.S.; Wolfl, S.; Ott, I. On the biological properties of alkynyl phosphine gold(I) complexes. Angew. Chem. Int. Ed. Engl. 2012, 51, 8895–8899. [Google Scholar] [CrossRef] [PubMed]
- Nell, B.P.; Tyler, D.R. Synthesis, reactivity, and coordination chemistry of secondary phosphines. Coord. Chem. Rev. 2014, 279, 23–42. [Google Scholar] [CrossRef]
- Hall, K.P.; Mingos, D.M.P. Homo- and Heteronuclear Cluster Compounds of Gold. Prog. Inorg. Chem. 1984, 32, 237–325. [Google Scholar]
- Teo, B.K.; Shi, X.B.; Zhang, H. Pure Gold Cluster of 1:9:9:1:9:9:1 Layered Structure: A Novel 39-Metal-Atom Cluster [(Ph3P)14Au39Cl6]Cl2 with an Interstitial Gold Atom in a Hexagonal Antiprismatic Cage. J. Am. Chem. Soc. 1992, 114, 2743–2745. [Google Scholar] [CrossRef]
- Nunokawa, K.; Ito, M.; Sunahara, T.; Onaka, S.; Ozeki, T.; Chiba, H.; Funahashi, Y.; Masuda, H.; Yonezawa, T.; Nishihara, H.; et al. A new 19-metal-atom cluster [(Me2PhP)10Au12Ag7(NO3)9] with a nearly staggered-staggered M5 ring configuration. Dalton Trans. 2005, 16, 2726–2730. [Google Scholar] [CrossRef]
- Das, A.; Li, T.; Nobusada, K.; Zeng, Q.; Rosi, N.L.; Jin, R. Total structure and optical properties of a phosphine/thiolate-protected Au24 nanocluster. J. Am. Chem. Soc. 2012, 134, 20286–20289. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Negishi, Y.; Fujihara, H.; Tsukuda, T. Chiroptical Activity of BINAP-Stabilized Undecagold Clusters. J. Phys. Chem. B 2006, 110, 11611–11614. [Google Scholar] [CrossRef]
- Shichibu, Y.; Konishi, K. HCl-induced nuclearity convergence in diphosphine-protected ultrasmall gold clusters: A novel synthetic route to “magic-number” Au13 clusters. Small 2010, 6, 1216–1220. [Google Scholar] [CrossRef]
- Wan, X.K.; Yuan, S.F.; Lin, Z.W.; Wang, Q.M. A chiral gold nanocluster Au20 protected by tetradentate phosphine ligands. Angew. Chem. Int. Ed. Engl. 2014, 53, 2923–2926. [Google Scholar] [CrossRef]
- Britvin, S.N.; Lotnyk, A. Water-Soluble Phosphine Capable of Dissolving Elemental Gold: The Missing Link between 1,3,5-Triaza-7-phosphaadamantane (PTA) and Verkade’s Ephemeral Ligand. J. Am. Chem. Soc. 2015, 137, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Pettibone, J.; Hudgens, J. Gold cluster formation with phosphine ligands: Etching as a size-selective synthetic pathway for small clusters? ACS Nano 2011, 5, 2989–3002. [Google Scholar] [CrossRef] [PubMed]
- Hudgens, J.W.; Pettibone, J.M.; Senftle, T.P.; Bratton, R.N. Reaction mechanism governing formation of 1,3-bis(diphenylphosphino)propane-protected gold nanoclusters. Inorg. Chem. 2011, 50, 10178–10189. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Shichibu, Y.; Konishi, K. Generation of small gold clusters with unique geometries through cluster-to-cluster transformations: Octanuclear clusters with edge-sharing gold tetrahedron motifs. Angew. Chem. Int. Ed. Engl. 2011, 50, 7442–7445. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, J.W.A.; Bour, J.J.; Steggerda, J.J.; Beurskens, P.T.; Roseboom, M.; Noordik, J.H. Gold clusters. Tetrakis[1,3-bis(diphenylphosphino)propane]hexagold dinitrate: Preparation, X-ray analysis, and gold-197 Moessbauer and phosphorus-31{proton} NMR spectra. Inorg. Chem. 1982, 21, 4321–4324. [Google Scholar] [CrossRef]
- Shu, T.; Wang, J.; Su, L.; Zhang, X. Chemical Etching of Bovine Serum Albumin-Protected Au25 Nanoclusters for Label-Free and Separation-Free Ratiometric Fluorescent Detection of Tris(2-carboxyethyl)phosphine. Anal. Chem. 2016, 88, 11193–11198. [Google Scholar] [CrossRef]
- Su, Z.; Wang, X.; Luo, M.; Li, L.; Tu, Y.; Yan, J. Fluorometric determination of nitrite through its catalytic effect on the oxidation of iodide and subsequent etching of gold nanoclusters by free iodine. Microchim. Acta 2019, 186, 619. [Google Scholar] [CrossRef]
- Wang, X.; Su, Z.; Li, L.; Tu, Y.; Yan, J. Sensitive detection of molybdenum through its catalysis and quenching of gold nanocluster fluorescence. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117909. [Google Scholar] [CrossRef]
- Mo, Q.; Liu, F.; Gao, J.; Zhao, M.; Shao, N. Fluorescent sensing of ascorbic acid based on iodine induced oxidative etching and aggregation of lysozyme-templated silver nanoclusters. Anal. Chim. Acta 2018, 1003, 49–55. [Google Scholar] [CrossRef]
- Wenlong, C.; Shaojun, D.; Erkang, W. Iodine-induced gold-nanoparticle fusion/fragmentation/aggregation and iodine-linked nanostructured assemblies on a glass substrate. Angew. Chem. (Int. Ed. Engl.) 2003, 42, 465–468. [Google Scholar]
- Zhang, Z.; Chen, Z.; Wang, S.; Cheng, F.; Chen, L. Iodine-Mediated Etching of Gold Nanorods for Plasmonic ELISA Based on Colorimetric Detection of Alkaline Phosphatase. ACS Appl. Mater. Interfaces 2015, 7, 27639–27645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Cheng, F.; Zhang, Y.; Chen, L. Iodine-mediated etching of gold nanorods for plasmonic sensing of dissolved oxygen and salt iodine. Analyst 2016, 141, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gao, M.; Lei, G.; Zou, H.; Ma, J.; Huang, C. Visually monitoring the etching process of gold nanoparticles by KI/I2 at single-nanoparticle level using scattered-light dark-field microscopic imaging. Nano Res. 2016, 9, 1125–1134. [Google Scholar] [CrossRef]
- Luo, M.; Di, J.; Li, L.; Tu, Y.; Yan, J. Copper ion detection with improved sensitivity through catalytic quenching of gold nanocluster fluorescence. Talanta 2018, 187, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, P.; Fan, J.; Di, J.; Tu, Y.; Yan, J. Sensitive iodate sensor based on fluorescence quenching of gold nanocluster. Anal. Chim. Acta 2014, 827, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cao, F.; Zheng, W.; Tian, Y.; Xianyu, Y.; Xu, P.; Zhang, W.; Wang, Z.; Deng, K.; Jiang, X. Detection of the nanomolar level of total Cr[(iii) and (vi)] by functionalized gold nanoparticles and a smartphone with the assistance of theoretical calculation models. Nanoscale 2015, 7, 2042–2049. [Google Scholar] [CrossRef]
- Elavarasi, M.; Paul, M.L.; Rajeshwari, A.; Chandrasekaran, N.; Mandal, A.B.; Mukherjee, A. Studies on fluorescence determination of nanomolar Cr(iii) in aqueous solutions using unmodified silver nanoparticles. Anal. Methods 2012, 4, 3407–3412. [Google Scholar] [CrossRef]
- Jian-feng, G.; Chang-jun, H.; Mei, Y.; Dan-qun, H.; Jun-jie, L.; Huan-bao, F.; Hui-bo, L.; Ping, Y. Colorimetric sensing of chromium(vi) ions in aqueous solution based on the leaching of protein-stabled gold nanoparticles. Anal. Methods 2016, 8, 5526–5532. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Wang, X.; Lin, L.; Cai, W.; Lin, X.; Zeng, Y.; Li, Z.; Lin, S. Non-aggregation based label free colorimetric sensor for the detection of Cr (VI) based on selective etching of gold nanorods. Sens. Actuators B Chem. 2011, 155, 817–822. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, F.; Gao, Y.; Feng, Y.; Chen, S.; Wu, A. A rapid colorimetric detection method of trace Cr (VI) based on the redox etching of Ag(core)-Au(shell) nanoparticles at room temperature. Talanta 2012, 101, 122–127. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Jiao, Y.; Jia, R.; Chen, Z. Core-shell Cu@Au nanoparticles as an optical probe for ultrasensitive detection of chromium(VI) via an etching effect. Microchim. Acta 2017, 184, 3817–3823. [Google Scholar] [CrossRef]
- Guo, J.F.; Huo, D.Q.; Yang, M.; Hou, C.J.; Li, J.J.; Fa, H.B.; Luo, H.B.; Yang, P. Colorimetric detection of Cr (VI) based on the leaching of gold nanoparticles using a paper-based sensor. Talanta 2016, 161, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Zeng, A.L.; Luo, H.Q.; Li, N.B. Fluorescent silver nanoclusters for ultrasensitive determination of chromium(VI) in aqueous solution. J. Hazard. Mater. 2016, 304, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, B.; Lanzano, C.; Libassi, M. The cyanide revolution: Efficiency gains and exclusion in artisanal- and small-scale gold mining. Geoforum 2021, 126, 267–276. [Google Scholar] [CrossRef]
- Gonzalez-Valoys, A.C.; Arrocha, J.; Monteza-Destro, T.; Vargas-Lombardo, M.; Esbri, J.M.; Garcia-Ordiales, E.; Jimenez-Ballesta, R.; Garcia-Navarro, F.J.; Higueras, P. Environmental challenges related to cyanidation in Central American gold mining; the Remance mine (Panama). J. Environ. Manag. 2022, 302, 113979. [Google Scholar] [CrossRef] [PubMed]
- Razanamahandry, L.C.; Andrianisa, H.A.; Karoui, H.; Podgorski, J.; Yacouba, H. Prediction model for cyanide soil pollution in artisanal gold mining area by using logistic regression. Catena 2018, 162, 40–50. [Google Scholar] [CrossRef]
- Rosario, C.G.A.; Vallenas-Arévalo, A.T.; Arévalo, S.J.; Espinosa, D.C.R.; Tenório, J.A.S. Biodegradation of cyanide using a Bacillus subtilis strain isolated from artisanal gold mining tailings. Braz. J. Chem. Eng. 2022, 1–8. [Google Scholar] [CrossRef]
- Manyuchi, M.M.; Sukdeo, N.; Stinner, W. Potential to remove heavy metals and cyanide from gold mining wastewater using biochar. Phys. Chem. Earth Parts A/B/C 2022, 126, 103110. [Google Scholar] [CrossRef]
- Sharma, S.; Hundal, M.S.; Hundal, G. Dual channel chromo/fluorogenic chemosensors for cyanide and fluoride ions—An example of in situ acid catalysis of the Strecker reaction for cyanide ion chemodosimetry. Org. Biomol. Chem. 2013, 11, 654–661. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, X.; Wei, T.B.; Zhang, Y.M. Reaction-based ratiometric chemosensor for instant detection of cyanide in water with high selectivity and sensitivity. Chem. Asian J. 2013, 8, 3015–3021. [Google Scholar] [CrossRef]
- Pramanik, S.; Bhalla, V.; Kumar, M. Hexaphenylbenzene-based fluorescent aggregates for ratiometric detection of cyanide ions at nanomolar level: Set-reset memorized sequential logic device. ACS Appl. Mater. Interfaces 2014, 6, 5930–5939. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, Y.; Li, D.; Liu, X.; Jiang, C.; Huang, L.; Wen, H.; Hu, J. Ratiometric fluorometric and visual determination of cyanide based on the use of carbon dots and gold nanoclusters. Mikrochim. Acta 2019, 186, 809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, T.; Yang, J.; Zhang, X. A robust gold nanocluster-peroxyoxalate chemiluminescence system for highly sensitive detection of cyanide in environmental water. Sens. Actuators B Chem. 2022, 353, 131038. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, L.; Zhang, M.; Cao, W.; Yang, L.; Ma, L.J. Glutamine-containing “turn-on” fluorescence sensor for the highly sensitive and selective detection of chromium (III) ion in water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 460–465. [Google Scholar] [CrossRef]
- Zhang, Z.; Sha, C.; Liu, A.; Zhang, Z.; Xu, D. Highly selective detection of Cr(VI) in water matrix by a simple 1,8-naphthalimide-based turn-on fluorescent sensor. J. Fluoresc. 2015, 25, 335–340. [Google Scholar] [CrossRef]
- Sui, C.-X.; Liu, Y.-F.; Zhang, W.-H.; Li, P.-A.; Zhang, D. CdTe-CdSe nanocrystals capped with dimethylaminoethanethiol as ultrasensitive fluorescent probes for chromium(VI). Microchim. Acta 2013, 181, 347–353. [Google Scholar] [CrossRef]
- Tsuyumoto, I.; Maruyama, Y. X-ray fluorescence analysis of hexavalent chromium using Kbeta satellite peak observed as counterpart of X-ray absorption near-edge structure pre-edge peak. Anal. Chem. 2011, 83, 7566–7569. [Google Scholar] [CrossRef]
- Chwastowska, J.; Skwara, W.; Sterlinska, E.; Pszonicki, L. Speciation of chromium in mineral waters and salinas by solid-phase extraction and graphite furnace atomic absorption spectrometry. Talanta 2005, 66, 1345–1349. [Google Scholar] [CrossRef]
- Jiang, H.; Su, X.; Zhang, Y.; Zhou, J.; Fang, D.; Wang, X. Unexpected Thiols Triggering Photoluminescent Enhancement of Cytidine Stabilized Au Nanoclusters for Sensitive Assays of Glutathione Reductase and Its Inhibitors Screening. Anal. Chem. 2016, 88, 4766–4771. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Wang, E. Cu nanoclusters with aggregation induced emission enhancement. Small 2013, 9, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, X.; Zheng, Y.; Wang, Y.; Guo, Z.; Jiang, H.; Wang, X. A novel turn-on fluorescent sensor for the sensitive detection of glutathione via gold nanocluster preparation based on controllable ligand-induced etching. Analyst 2020, 145, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B.; Ye, Y.; Chen, W.; Song, X. A ratiometric fluorescent probe for simultaneous detection of Cys/Hcy and GSH. Org. Biomol. Chem. 2019, 17, 9631–9635. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tu, Y.; Wang, R.; Pu, S. A lysosome-targetable fluorescent probe for simultaneous detection and discrimination of Cys/Hcy and GSH by dual channels. Dye. Pigment. 2020, 177, 108270. [Google Scholar] [CrossRef]
- Zong, C.; Zheng, L.R.; He, W.; Ren, X.; Jiang, C.; Lu, L. In Situ Formation of Phosphorescent Molecular Gold(I) Cluster in a Macroporous Polymer Film to Achieve Colorimetric Cyanide Sensing. Anal. Chem. 2014, 86, 1687–1692. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. β-Cyclodextrin protected gold nanoparticle based cotton swabs as an effective candidate for specific sensing of trace levels of cyanide. Anal. Methods 2019, 11, 97–104. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, H.-Y.; Wu, C.-S.; Meena, J.S.; Simon, T.; Ko, F.-H. A highly sensitive and selective cyanide detection using a gold nanoparticle-based dual fluorescence–colorimetric sensor with a wide concentration range. Sens. Actuators B Chem. 2016, 227, 283–290. [Google Scholar] [CrossRef]
- Zheng, A.; Chen, J.; Wu, G.; Wu, G.; Zhang, Y.G.; Wei, H. A novel fluorescent distinguished probe for Cr (VI) in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 265–270. [Google Scholar] [CrossRef]
- Hui-Lin, T.; Ming-Ze, X.; Qing-Jun, Z.; Xue-Hong, Z.; Shu-Huai, L.; Fu-Xin, Z.; Chao, S.; Xiu-Fen, L. Determination of Cr(vi) in Leersia hexandra Swartz based on the efficient fluorescence energy transfer between CdTe@SiO2 nanoparticles and Rhodamine B. Anal. Methods 2013, 5, 5961–5968. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Y.; Dong, Y.; Jiang, X.; Zhu, N. Colorimetric determination of hexavalent chromium with ascorbic acid capped silver nanoparticles. Anal. Methods 2013, 5, 560–565. [Google Scholar] [CrossRef]
- Trümpler, S.; Lohmann, W.; Meermann, B.; Buscher, W.; Sperling, M.; Karst, U. Interaction of thimerosal with proteins—ethylmercury adduct formation of human serum albumin and β-lactoglobulin A. Metallomics 2009, 1, 87–91. [Google Scholar] [CrossRef]
- Liu, C.S.; Shih, K.; Sun, C.X.; Wang, F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci. Total Environ. 2012, 416, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, L.; Huang, T.; Sun, Z.; Yao, T.; Jiang, Y.; Wei, S. XAFS study on thiol etching of diphosphine-stabilized gold nanoclusters. Radiat. Phys. Chem. 2017, 137, 99–103. [Google Scholar] [CrossRef]
- Shu, T.; Cheng, X.; Wang, J.; Lin, X.; Zhou, Z.; Su, L.; Zhang, X. Synthesis of Luminescent Gold Nanoclusters Embedded Goose Feathers for Facile Preparation of Au(I) Complexes with Aggregation-Induced Emission. ACS Sustain. Chem. Eng. 2018, 7, 592–598. [Google Scholar] [CrossRef]


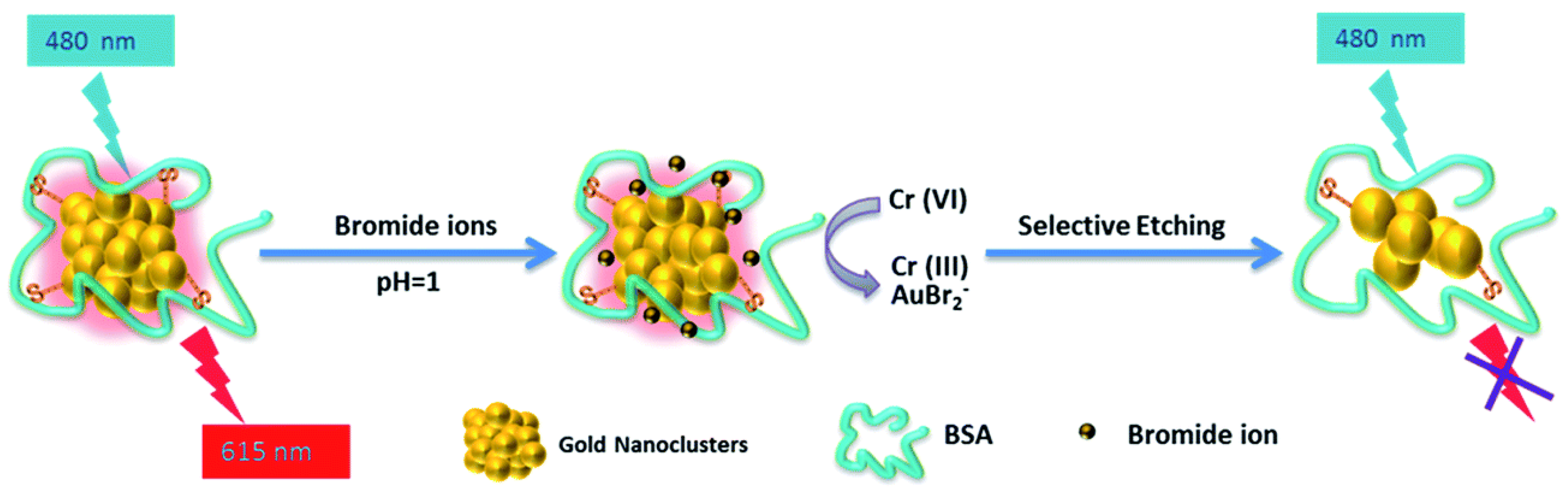
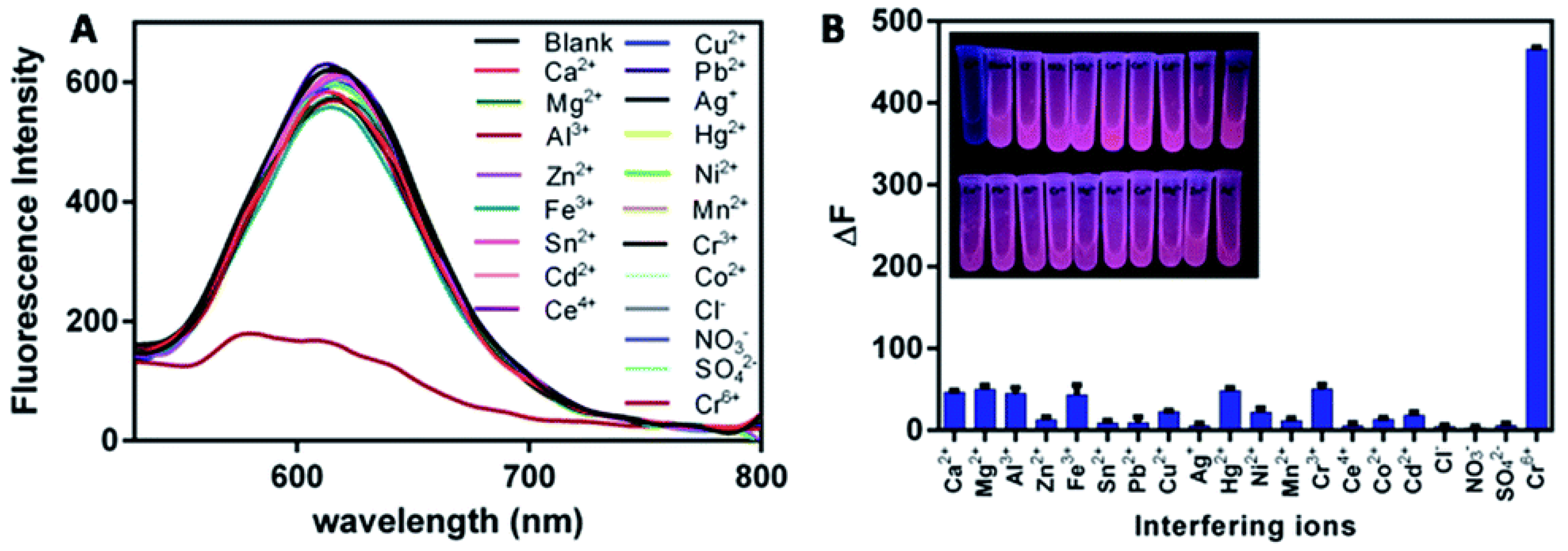
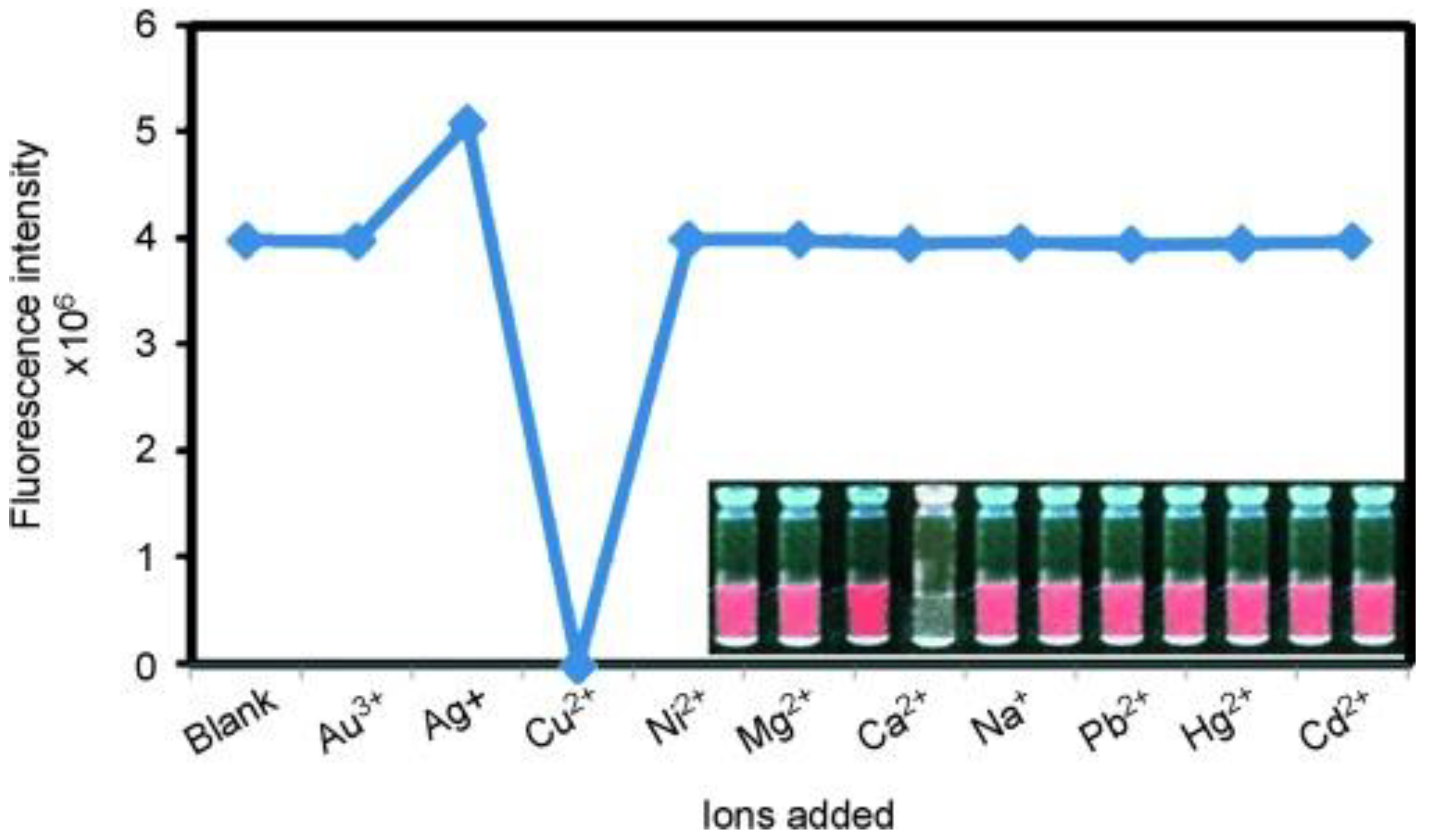
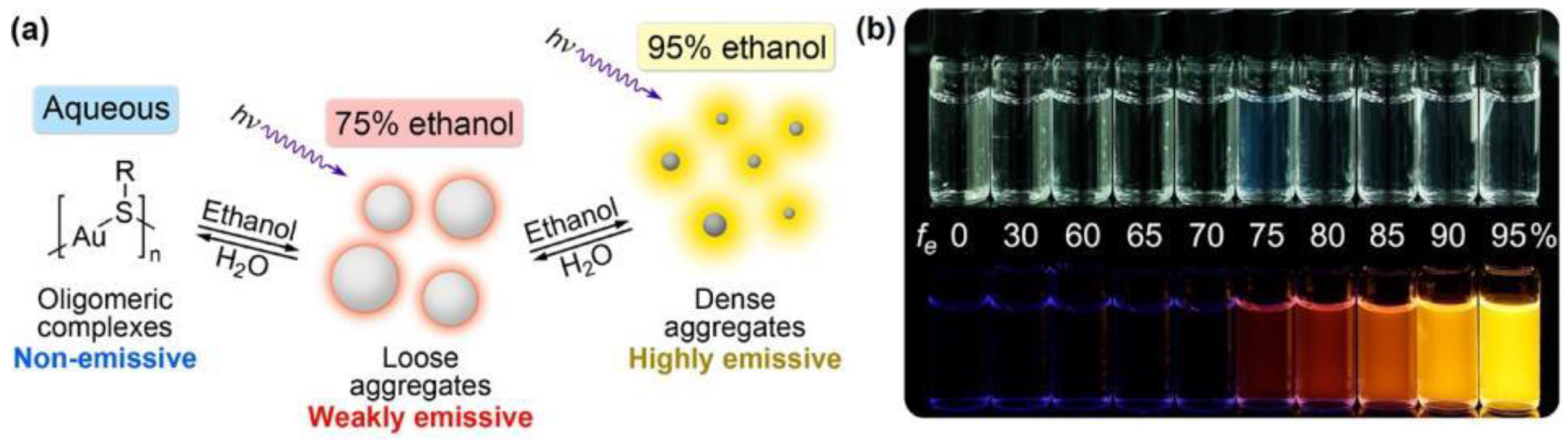
| Target | Probe/Instrument | Strategy | LOD(nM) | Linear Range (μM) | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|---|
| GSH | AuCyt NCs | Fluorescence (GSH etched AuCyt NCs to generate highly fluorescent Au species) | 2 | 0.02–3 | Highly sensitive detection, could be extended to detect the glutathione reductase activity | Unable to detect on-site | [149] |
| CPR | FRET 1 (GSH broke the link between fluorophores, inhibiting FRET) | 30 | None | Sensitive and selective detection | Probe preparation was complex | [153] | |
| Lyso-O-NBD | Fluorescence (GSH reacted with Lyso-O-NBD to produce blue fluorescence) | 39 | 0–5 | Sensitive and selective detection | Probe preparation was complex | [154] | |
| CN− | DE-Au NCs | Ratiometric fluorescence (CN-drove etching DE-Au NCs based on surface valence state) | 10 | 0.02–1 | Highly sensitive and selective detection, could be used for river water and urine sample analysis | Unable to detect on-site | [40] |
| Molecular Au (I) cluster | Fluorescence (In situ formation of phosphorescent molecular gold(I) cluster) | 80 | 0.16–50 | Sensitive detection | Unable to detect on-site | [155] | |
| β-CD-Au NPs 2 | Colorimetric (Cyanide etchedβ-CD-Au NPs) | 93 | 4.5–99 | Could quickly detect water samples on-site | In contrast, it was not sensitive enough | [156] | |
| PS 20-Au NP-FITC 3 | Dual fluorescence–colorimetric assay (cyanide etched PS 20-Au NP-FITC) | 100 | 0–7 | Selective detection, cost-effective | Not sensitive enough | [157] | |
| Cr6+ | BSA-Au NCs | Fluorescence (Cr6+ etched BSA-Au NCs) | 0.6 | 0.001–2.5 | Highly sensitive detection, simple, short detection time | The quantum yield of BSA-Au NCs was not high | [32] |
| X-ray fluorescencespectra | Kβ emission spectra for Cr6+ compounds | None | None | Could detect Cr6+ compounds | high cost | [147] | |
| SRBH 4 | Fluorescence (Reaction between potassium dichromate and non-fluorescent SRBH to produce highly fluorescent Rhodamine B) | 1.5 | 0.01–0.3 | Sensitive detection | Only used to detect CrO42− | [158] | |
| CdTe@SiO2 and RhB 5 | FRET (Cr6+ and RhB electrostatically attract) | 6.2 | 0.02–0.3 | Sensitive detection | Unable to detect on-site | [159] | |
| AA-capped Ag NPs | Colorimetry (Crosslinking of Cr6+ reduction products with AA caused AA-capped Ag NPs to aggregate) | 50 | 0.08–1.84 | Sensitive detection, simple | In contrast, it was not sensitive enough | [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, H.; Shu, T.; Du, X.; Su, L.; Zhang, X. An Overview on Coinage Metal Nanocluster-Based Luminescent Biosensors via Etching Chemistry. Biosensors 2022, 12, 511. https://doi.org/10.3390/bios12070511
Si H, Shu T, Du X, Su L, Zhang X. An Overview on Coinage Metal Nanocluster-Based Luminescent Biosensors via Etching Chemistry. Biosensors. 2022; 12(7):511. https://doi.org/10.3390/bios12070511
Chicago/Turabian StyleSi, Hongxin, Tong Shu, Xin Du, Lei Su, and Xueji Zhang. 2022. "An Overview on Coinage Metal Nanocluster-Based Luminescent Biosensors via Etching Chemistry" Biosensors 12, no. 7: 511. https://doi.org/10.3390/bios12070511
APA StyleSi, H., Shu, T., Du, X., Su, L., & Zhang, X. (2022). An Overview on Coinage Metal Nanocluster-Based Luminescent Biosensors via Etching Chemistry. Biosensors, 12(7), 511. https://doi.org/10.3390/bios12070511








