Elevated Plasma Oligomeric Amyloid β-42 Is Associated with Cognitive Impairments in Cerebral Small Vessel Disease
Abstract
:1. Introduction
2. Materials and Methods
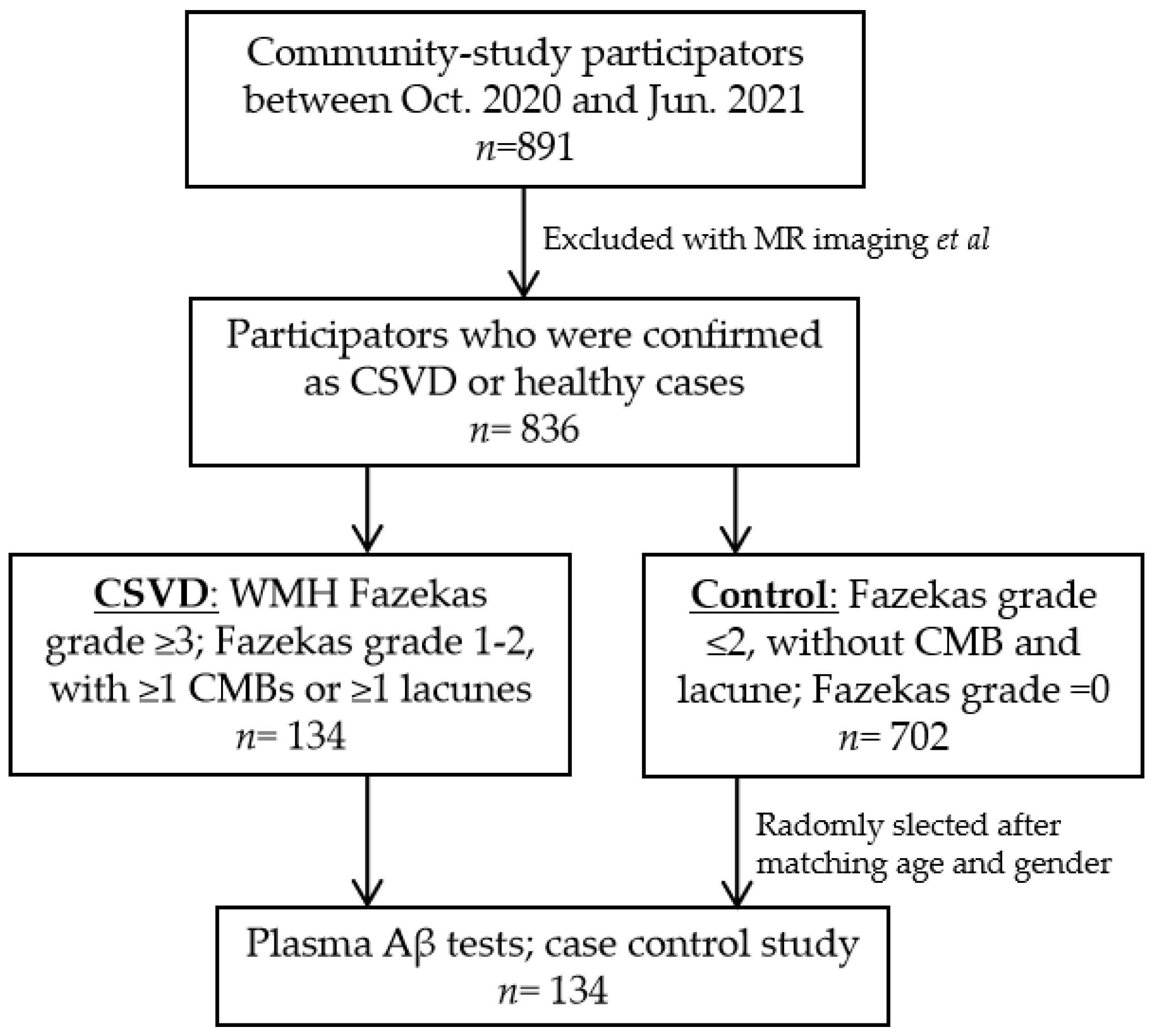
2.1. Study Populations
2.2. Brain MR Imaging
2.3. Groups
2.4. Demographics and Vascular Risk Factors
2.5. Evaluation of Neuropsychological Function
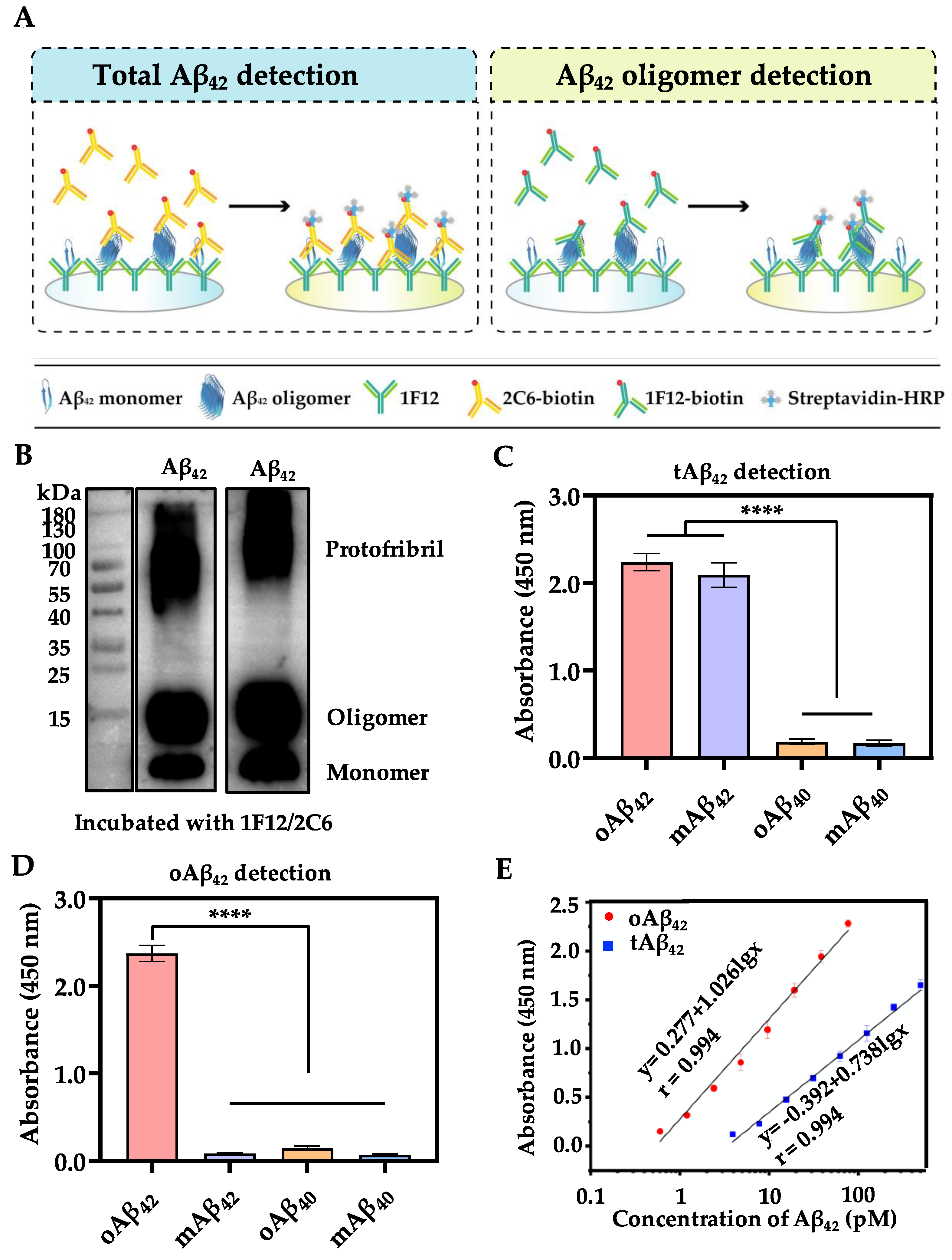
2.6. The Sandwich ELISA for Plasma Aβ Measurements
2.7. Statistical Analysis
3. Results
3.1. Assay Principle of the Proposed Sandwich ELISA
| Method | Target | Specificity | Sensitivity | Ref. |
|---|---|---|---|---|
| ELISA | tAβ42, oAβ42, mAβ42 | High | 3.9, 0.6 pM | Current method |
| ELISA | pAβ42 | High | < 0.68 pM | [20] |
| MSD | oAβ42 | No | 1.5 pM | [33] |
| TES | oAβ42, | NA | 4 pM | [34] |
| fAβ42 | ||||
| Simoa | oAβ42 | High | 0.22 nM | [35] |
| Aptamer | oAβ42 | Moderate | 12.5 nM | [21] |
| SERS | oAβ42 | No | 10 nM | [23] |
| SWV | oAβ42 | No | 48 pM | [36] |
| LSV | oAβ42 | High | 8 pM | [24] |
| DPV | oAβ42 | High | 0.1 nM | [25] |
| FL | oAβ42 | High | 0.2 nM | [22] |
3.2. Characterization of Sandwich ELISA
3.3. Clinical Characteristics
3.4. Plasma Aβ42 and CSVD
3.5. Plasma Aβ42 and Cognition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Leeuw, F.E.; de Groot, J.C.; Achten, E.; Oudkerk, M.; Ramos, L.M.; Heijboer, R.; Hofman, A.; Jolles, J.; van Gijn, J.; Breteler, M.M. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, K.W.; Kwon, H.M.; Lim, J.S.; Han, M.K.; Nam, H.; Lee, Y.S. The presence and severity of cerebral small vessel disease increases the frequency of stroke in a cohort of patients with large artery occlusive disease. PLoS ONE 2017, 12, e0184944. [Google Scholar] [CrossRef] [Green Version]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Shan, Y.; Cai, W.; Liu, S.; Hu, M.; Liao, S.; Huang, X.; Zhang, B.; Wang, Y.; et al. Cerebral small vessel disease: Neuroimaging markers and clinical implication. J. Neurol. 2019, 266, 2347–2362. [Google Scholar] [CrossRef]
- van Leijsen, E.M.C.; Kuiperij, H.B.; Kersten, I.; Bergkamp, M.I.; van Uden, I.W.M.; Vanderstichele, H.; Stoops, E.; Claassen, J.; van Dijk, E.J.; de Leeuw, F.E.; et al. Plasma Abeta (Amyloid-beta) Levels and Severity and Progression of Small Vessel Disease. Stroke 2018, 49, 884–890. [Google Scholar] [CrossRef]
- Hilal, S.; Akoudad, S.; van Duijn, C.M.; Niessen, W.J.; Verbeek, M.M.; Vanderstichele, H.; Stoops, E.; Ikram, M.A.; Vernooij, M.W. Plasma Amyloid-beta Levels, Cerebral Small Vessel Disease, and Cognition: The Rotterdam Study. J. Alzheimer’s Dis. JAD 2017, 60, 977–987. [Google Scholar] [CrossRef]
- Kaffashian, S.; Tzourio, C.; Soumare, A.; Dufouil, C.; Zhu, Y.; Crivello, F.; Maillard, P.; Schraen-Maschke, S.; Mazoyer, B.; Buee, L.; et al. Plasma beta-amyloid and MRI markers of cerebral small vessel disease: Three-City Dijon study. Neurology 2014, 83, 2038–2045. [Google Scholar] [CrossRef]
- Findeis, M.A. The role of amyloid beta peptide 42 in Alzheimer’s disease. Pharmacol. Ther. 2007, 116, 266–286. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liang, X.; Zhang, Z.; Luo, H. Cerebrospinal fluid and blood biomarkers in the diagnostic assays of Alzheimer’s disease. J. Innov. Opt. Health Sci. 2022, 15, 2230001. [Google Scholar] [CrossRef]
- O’Brien, E.P.; Okamoto, Y.; Straub, J.E.; Brooks, B.R.; Thirumalai, D. Thermodynamic perspective on the dock-lock growth mechanism of amyloid fibrils. J. Phys. Chem. B 2009, 113, 14421–14430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, E.P.; Takeda, T.; Barsegov, V.; Klimov, D.K. Mechanical unbinding of abeta peptides from amyloid fibrils. J. Mol. Biol. 2007, 373, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M. Fibril formation from the amyloid-β peptide is governed by a dynamic equilibrium involving association and dissociation of the monomer. Biophys. Rev. 2017, 9, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Wang, N.; Liu, X. Resveratrol and Amyloid-Beta: Mechanistic Insights. Nutrients 2017, 9, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irizarry, M.C. Biomarkers of Alzheimer disease in plasma. NeuroRx J. Am. Soc. Exp. Neurother. 2004, 1, 226–234. [Google Scholar] [CrossRef]
- Graff-Radford, N.R.; Crook, J.E.; Lucas, J.; Boeve, B.F.; Knopman, D.S.; Ivnik, R.J.; Smith, G.E.; Younkin, L.H.; Petersen, R.C.; Younkin, S.G. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2007, 64, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Mayeux, R.; Honig, L.S.; Tang, M.X.; Manly, J.; Stern, Y.; Schupf, N.; Mehta, P.D. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: Relation to age, mortality, and risk. Neurology 2003, 61, 1185–1190. [Google Scholar] [CrossRef]
- Pomara, N.; Willoughby, L.M.; Sidtis, J.J.; Mehta, P.D. Selective reductions in plasma Abeta 1-42 in healthy elderly subjects during longitudinal follow-up: A preliminary report. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2005, 13, 914–917. [Google Scholar] [CrossRef]
- van Oijen, M.; Hofman, A.; Soares, H.D.; Koudstaal, P.J.; Breteler, M.M. Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: A prospective case-cohort study. Lancet Neurol. 2006, 5, 655–660. [Google Scholar] [CrossRef]
- Englund, H.; Sehlin, D.; Johansson, A.S.; Nilsson, L.N.; Gellerfors, P.; Paulie, S.; Lannfelt, L.; Pettersson, F.E. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J. Neurochem. 2007, 103, 334–345. [Google Scholar] [CrossRef]
- Liu, L.; Chang, Y.; Yu, J.; Jiang, M.; Xia, N. Two-in-one polydopamine nanospheres for fluorescent determination of beta-amyloid oligomers and inhibition of beta-amyloid aggregation. Sens. Actuators B Chem. 2017, 251, 359–365. [Google Scholar] [CrossRef]
- Xia, N.; Zhou, B.; Huang, N.; Jiang, M.; Zhang, J.; Liu, L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 2016, 85, 625–632. [Google Scholar] [CrossRef]
- D’Urso, L.; Condorelli, M.; Puglisi, O.; Tempra, C.; Lolicato, F.; Compagnini, G.; La Rosa, C. Detection and characterization at nM concentration of oligomers formed by hIAPP, Aβ(1-40) and their equimolar mixture using SERS and MD simulations. Phys. Chem. Chem. Phys. 2018, 20, 20588–20596. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Zhou, B.; Wu, Y.; Mao, W.; Liu, L. Electrochemical Detection of Amyloid-β Oligomers Based on the Signal Amplification of a Network of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 19303–19311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Liu, L.; Li, C.; Chang, Z.; Zhu, X.; Ye, B.; Xu, M. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci. Rep. 2016, 6, 35186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, C.; Li, Y.; Niu, S.; Liang, X.; Zhang, Z.; Luo, Q.; Luo, H. Dynamic Changes in the Levels of Amyloid-beta42 Species in the Brain and Periphery of APP/PS1 Mice and Their Significance for Alzheimer’s Disease. Front. Mol. Neurosci. 2021, 14, 723317. [Google Scholar] [CrossRef]
- Zhang, L.; Du, X.; Su, Y.; Niu, S.; Li, Y.; Liang, X.; Luo, H. Quantitative assessment of AD markers using naked eyes: Point-of-care testing with paper-based lateral flow immunoassay. J. Nanobiotechnol. 2021, 19, 366. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Wilson, D.; Ambler, G.; Banerjee, G.; Shakeshaft, C.; Cohen, H.; Yousry, T.; Al-Shahi Salman, R.; Lip, G.Y.H.; Houlden, H.; et al. Small Vessel Disease and Ischemic Stroke Risk during Anticoagulation for Atrial Fibrillation after Cerebral Ischemia. Stroke 2021, 52, 91–99. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, E.; Brambilla, M.; Cova, I.; Pomati, S.; Pantoni, L. Cognitive evaluation in cerebral small vessel disease: Towards an evidence-based identification of the reference standards. Part 1. A systematic review and qualitative data synthesis. J. Neurol. 2021, 268, 4563–4572. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kwak, H.; Lawton, T.L.; Jin, S.X.; Meunier, A.L.; Dang, Y.; Ostaszewski, B.; Pietras, A.C.; Stern, A.M.; Selkoe, D.J. An ultra-sensitive immunoassay detects and quantifies soluble Aβ oligomers in human plasma. Alzheimer’s Dement. 2022, 18, 1186–1202. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, T.; Peng, Q.; Kong, L.; Li, C.; Tang, D.; Yin, X. Simultaneous Monitoring of Amyloid-β (Aβ) Oligomers and Fibrils for Effectively Evaluating the Dynamic Process of Aβ Aggregation. ACS Sens. 2019, 4, 471–478. [Google Scholar] [CrossRef]
- Chan, H.N.; Xu, D.; Ho, S.L.; Wong, M.S.; Li, H.W. Ultra-sensitive detection of protein biomarkers for diagnosis of Alzheimer’s disease. Chem. Sci. 2017, 8, 4012–4018. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xie, H.; Cao, Y.; Ding, X.; Yin, Y.; Li, G. A general way to assay protein by coupling peptide with signal reporter via supermolecule formation. Anal. Chem. 2013, 85, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Yoo, Y.K.; Kim, H.Y.; Roh, J.H.; Kim, J.; Baek, S.; Lee, J.C.; Kim, H.J.; Chae, M.S.; Jeong, D.; et al. Comparative analyses of plasma amyloid-β levels in heterogeneous and monomerized states by interdigitated microelectrode sensor system. Sci. Adv. 2019, 5, eaav1388. [Google Scholar] [CrossRef]
- Hatami, A.; Albay, R., 3rd; Monjazeb, S.; Milton, S.; Glabe, C. Monoclonal antibodies against Aβ42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain. J. Biol. Chem. 2014, 289, 32131–32143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayed, R.; Head, E.; Sarsoza, F.; Saing, T.; Cotman, C.W.; Necula, M.; Margol, L.; Wu, J.; Breydo, L.; Thompson, J.L.; et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2007, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease-one peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wong, A.; Law, A.C.; Mok, V.C. Cerebrovascular disease, amyloid plaques, and dementia. Stroke 2015, 46, 1402–1407. [Google Scholar] [CrossRef] [Green Version]
- Saridin, F.N.; Hilal, S.; Villaraza, S.G.; Reilhac, A.; Gyanwali, B.; Tanaka, T.; Stephenson, M.C.; Ng, S.L.; Vrooman, H.; van der Flier, W.M.; et al. Brain amyloid beta, cerebral small vessel disease, and cognition: A memory clinic study. Neurology 2020, 95, e2845–e2853. [Google Scholar] [CrossRef]
- Kapasi, A.; Leurgans, S.E.; Arvanitakis, Z.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Abeta (Amyloid Beta) and Tau Tangle Pathology Modifies the Association between Small Vessel Disease and Cortical Microinfarcts. Stroke 2021, 52, 1012–1021. [Google Scholar] [CrossRef]
- Koncz, R.; Wen, W.; Makkar, S.R.; Lam, B.C.P.; Crawford, J.D.; Rowe, C.C.; Sachdev, P.; Alzheimer’s Disease Neuroimaging, I. The Interaction between Vascular Risk Factors, Cerebral Small Vessel Disease, and Amyloid Burden in Older Adults. J. Alzheimer’s Dis. 2022, 86, 1617–1628. [Google Scholar] [CrossRef]
- Roseborough, A.; Ramirez, J.; Black, S.E.; Edwards, J.D. Associations between amyloid beta and white matter hyperintensities: A systematic review. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 1154–1167. [Google Scholar] [CrossRef]
- Yatawara, C.; Ng, K.P.; Cristine Guevarra, A.; Wong, B.; Yong, T.; Kandiah, N. Small Vessel Disease and Associations with Cerebrospinal Fluid Amyloid, Tau, and Neurodegeneration (ATN) Biomarkers and Cognition in Young Onset Dementia. J. Alzheimer’s Dis. JAD 2020, 77, 1305–1314. [Google Scholar] [CrossRef]
- Kettunen, P.; Bjerke, M.; Eckerstrom, C.; Jonsson, M.; Zetterberg, H.; Blennow, K.; Svensson, J.; Wallin, A. Blood-brain barrier dysfunction and reduced cerebrospinal fluid levels of soluble amyloid precursor protein-beta in patients with subcortical small-vessel disease. Alzheimer’s Dement. 2022, 14, e12296. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Thijssen, E.; Koelewijn, J.; Mauroo, K.; Vanbrabant, J.; de Wilde, A.; Zwan, M.D.; Verfaillie, S.C.J.; Ossenkoppele, R.; Barkhof, F.; et al. Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimer’s Res. Ther. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Tan Hehir, C.A.; Baker, D.; et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Okereke, O.I.; Yang, T.; Blacker, D.; Selkoe, D.J.; Grodstein, F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: A systematic review and meta-analysis. Arch. Neurol. 2012, 69, 824–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H. Amyloid-beta and cognition in aging and Alzheimer’s disease: Molecular and neurophysiological mechanisms. J. Alzheimer’s Dis. JAD 2013, 33 (Suppl. S1), S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Konijnenberg, E.; den Braber, A.; Ten Kate, M.; Tomassen, J.; Mulder, S.D.; Yaqub, M.; Teunissen, C.E.; Lammertsma, A.A.; van Berckel, B.N.M.; Scheltens, P.; et al. Association of amyloid pathology with memory performance and cognitive complaints in cognitively normal older adults: A monozygotic twin study. Neurobiol. Aging 2019, 77, 58–65. [Google Scholar] [CrossRef]
- Morley, J.E.; Farr, S.A. The role of amyloid-beta in the regulation of memory. Biochem. Pharmacol. 2014, 88, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Llado-Saz, S.; Atienza, M.; Cantero, J.L. Increased levels of plasma amyloid-beta are related to cortical thinning and cognitive decline in cognitively normal elderly subjects. Neurobiol. Aging 2015, 36, 2791–2797. [Google Scholar] [CrossRef]
- Mroczko, B.; Groblewska, M.; Litman-Zawadzka, A.; Kornhuber, J.; Lewczuk, P. Amyloid beta oligomers (AbetaOs) in Alzheimer’s disease. J. Neural Transm. 2018, 125, 177–191. [Google Scholar] [CrossRef]
- Wang, M.J.; Yi, S.; Han, J.Y.; Park, S.Y.; Jang, J.W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric forms of amyloid-beta protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chan, K.H.; Chu, L.W.; Kwan, J.S.; Song, Y.Q.; Chen, L.H.; Ho, P.W.; Cheng, O.Y.; Ho, J.W.; Lam, K.S. Plasma amyloid-beta oligomers level is a biomarker for Alzheimer’s disease diagnosis. Biochem. Biophys. Res. Commun. 2012, 423, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Huang, L.C.; Hsieh, S.W.; Huang, L.J. Dynamic Blood Concentrations of Abeta(1-40) and Abeta(1-42) in Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, L.; Paquet, C.; Guey, S.; Zarea, A.; Martinaud, O.; Rotharmel, M.; Maltete, D.; Quillard-Muraine, M.; Nicolas, G.; Charbonnier, C.; et al. Cerebrospinal Fluid Profile of Tau, Phosphorylated Tau, Abeta42, and Abeta40 in Probable Cerebral Amyloid Angiopathy. J. Alzheimer’s Dis. JAD 2022, 87, 791–802. [Google Scholar] [CrossRef] [PubMed]


| Control (n = 134) | CSVD (n = 134) | p | |
|---|---|---|---|
| Age (y) | 67.0 ± 6.1 | 67.5 ± 6.1 | 0.994 |
| Male (%) | 70 (52.2) | 70 (52.2) | 1.000 |
| Obesity (%) | 65 (48.5) | 71 (53.0) | 0.463 |
| Hypertension (%) | 53 (39.6) | 84 (62.7) | 0.000* |
| Diabetes (%) | 28 (20.9) | 32 (23.9) | 0.558 |
| Hyperlipemia (%) | 31 (23.1) | 33 (24.6) | 0.774 |
| Smoke (%) | 31 (23.1) | 33 (24.6) | 0.774 |
| Drinking (%) | 28 (20.9) | 31 (23.1) | 0.658 |
| Previous stroke (%) | 12 (9.0) | 35 (26.1) | 0.000 * |
| Education years (y) | 12.0 [9.0–13.3] | 9.0 [9.0–12.0] | 0.023 * |
| tAβ42 (pg/mL) | 58.32 [49.22–79.80] | 73.47 [51.75–112.03] | 0.000 * |
| oAβ42 (pg/mL) | 32.58 [31.89–33.49] | 33.44 [32.33–35.53] | 0.000 * |
| Factors | OR [95%CI] | p | |
|---|---|---|---|
| History | gender | 0.819 [0.434–1.543] | 0.536 |
| hyperlipidemia | 0.839 [0.456–1.542] | 0.571 | |
| diabetes | 0.947 [0.500–1.793] | 0.866 | |
| age | 1.005 [0.964–1.049] | 0.803 | |
| obesity | 1.002 [0.601–1.669] | 0.995 | |
| smoke | 1.017 [0.485–2.133] | 0.964 | |
| drinking | 1.220 [0.568–2.620] | 0.611 | |
| Previous stroke | 2.692 [1.307–5.545] | 0.007 * | |
| hypertension | 2.374 [1.439–3.917] | 0.001 * | |
| Plasma tests † | tAβ42 quintiles | 1.292 [1.080–1.546] | 0.005 * |
| oAβ42 quintiles | 1.500 [1.242–1.811] | 0.000 * |
| WMH | Lacune | CMB | PVS | ||
|---|---|---|---|---|---|
| tAβ42 quintiles | OR [95%CI] P | 1.336 [1.138–1.640] 0.001 * | 1.202 [0.960–1.506] 0.109 | 1.204 [0.954–1.518] 0.118 | 1.156 [0.917–1.457] 0.221 |
| oAβ42 quintiles | OR [95%CI] p | 1.481 [1.228–1.786] 0.000 * | 1.163 [0.928–1.457] 0.191 | 1.358 [1.068–1.727] 0.012 * | 0.900 [0.713–1.134] 0.371 |
| Digit Span-Forward | Digit Span-Backward | AVLT-n4 | AVLT-n5 | AVLT-n6 | ||
|---|---|---|---|---|---|---|
| Short-Term Memory | Working Memory | Short-Delayed Recall | Long-Delayed Recall | Recognition | ||
| tAβ42 quintiles | OR 95%CI p | 0.998 [0.996–1.001] 0.146 | 0.996 [0.992–1.000] 0.036 * | 0.890 [0.792–1.000] 0.049 * | 1.000 [0.999–1.002] 0.682 | 1.002 [1.000–1.004] 0.050 |
| oAβ42 quintiles | OR 95%CI p | 0.970 [0.932–1.009] 0.124 | 0.974 [0.935–1.015] 0.215 | 0.951 [0.912–0.991] 0.017 * | 0.943 [0.905–0.982] 0.005 * | 0.950 [0.913–0.989] 0.013 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, W.; Zhang, L.; Liang, X.; Yu, Z.; Huang, H.; Zhao, J.; Guo, Y.; Zhou, X.; Xu, S.; Luo, H.; et al. Elevated Plasma Oligomeric Amyloid β-42 Is Associated with Cognitive Impairments in Cerebral Small Vessel Disease. Biosensors 2023, 13, 110. https://doi.org/10.3390/bios13010110
Qu W, Zhang L, Liang X, Yu Z, Huang H, Zhao J, Guo Y, Zhou X, Xu S, Luo H, et al. Elevated Plasma Oligomeric Amyloid β-42 Is Associated with Cognitive Impairments in Cerebral Small Vessel Disease. Biosensors. 2023; 13(1):110. https://doi.org/10.3390/bios13010110
Chicago/Turabian StyleQu, Wensheng, Liding Zhang, Xiaohan Liang, Zhiyuan Yu, Hao Huang, Jing Zhao, Yinping Guo, Xirui Zhou, Shabei Xu, Haiming Luo, and et al. 2023. "Elevated Plasma Oligomeric Amyloid β-42 Is Associated with Cognitive Impairments in Cerebral Small Vessel Disease" Biosensors 13, no. 1: 110. https://doi.org/10.3390/bios13010110





