A Synergistic Dual-Channel Sensor for Ultrasensitive Detection of Pseudomonas aeruginosa by DNA Nanostructure and G-Quadruplex
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Fabrication of the DNA Probe on Gold Electrode
2.3. Implementation of RCA
2.3.1. Preparation of Circular DNA Template
2.3.2. Synthesis of G-Quadruplex
2.4. Establishment of Dual-Channel Detection Platform
2.4.1. Electrochemical Biosensor Platform
2.4.2. Colorimetric Biosensor Platform
2.5. Agarose Gel Electrophoresis
2.6. Detection of Real Samples
3. Results and Discussion
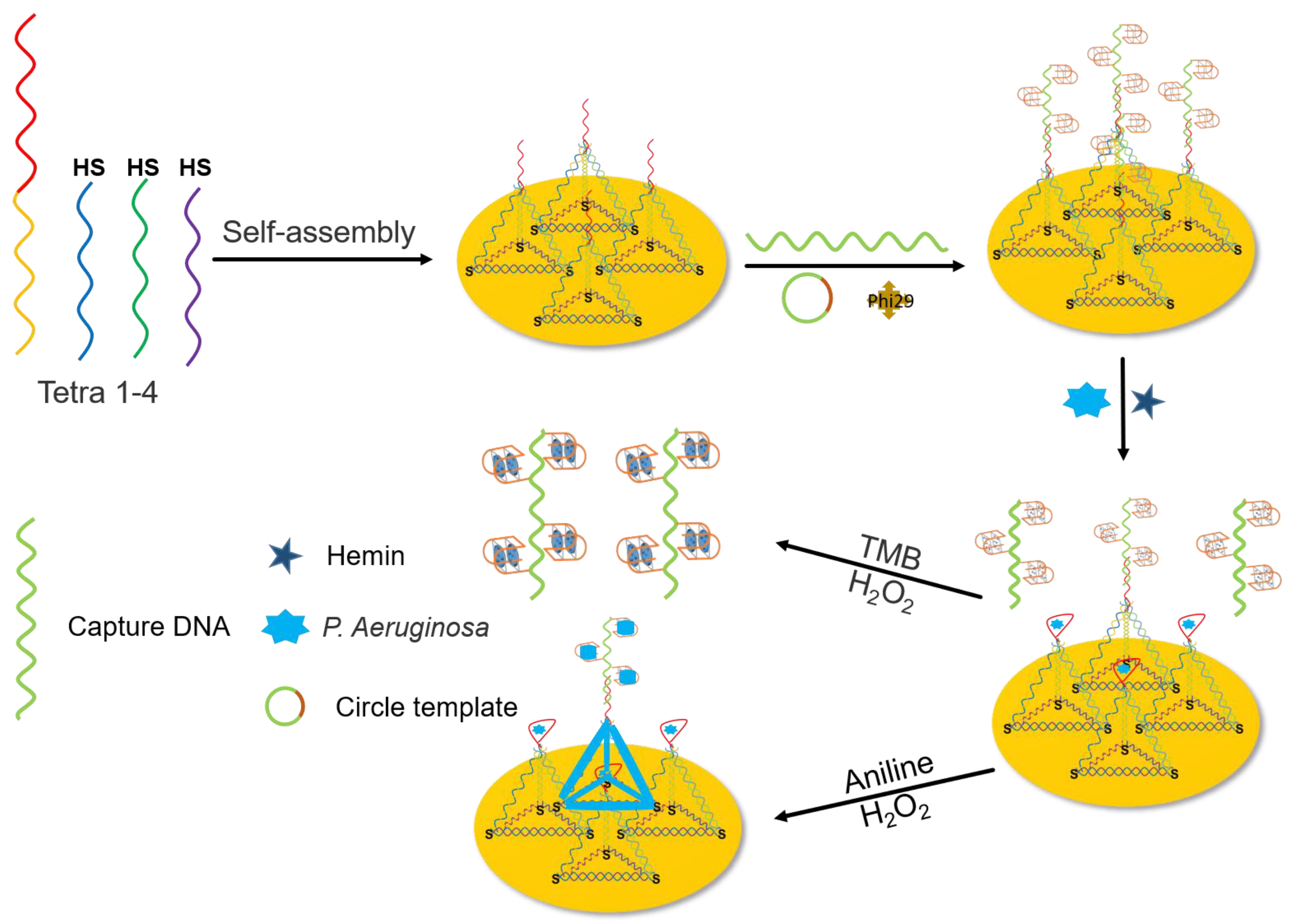
3.1. Principle of Dual-Channel Biosensor
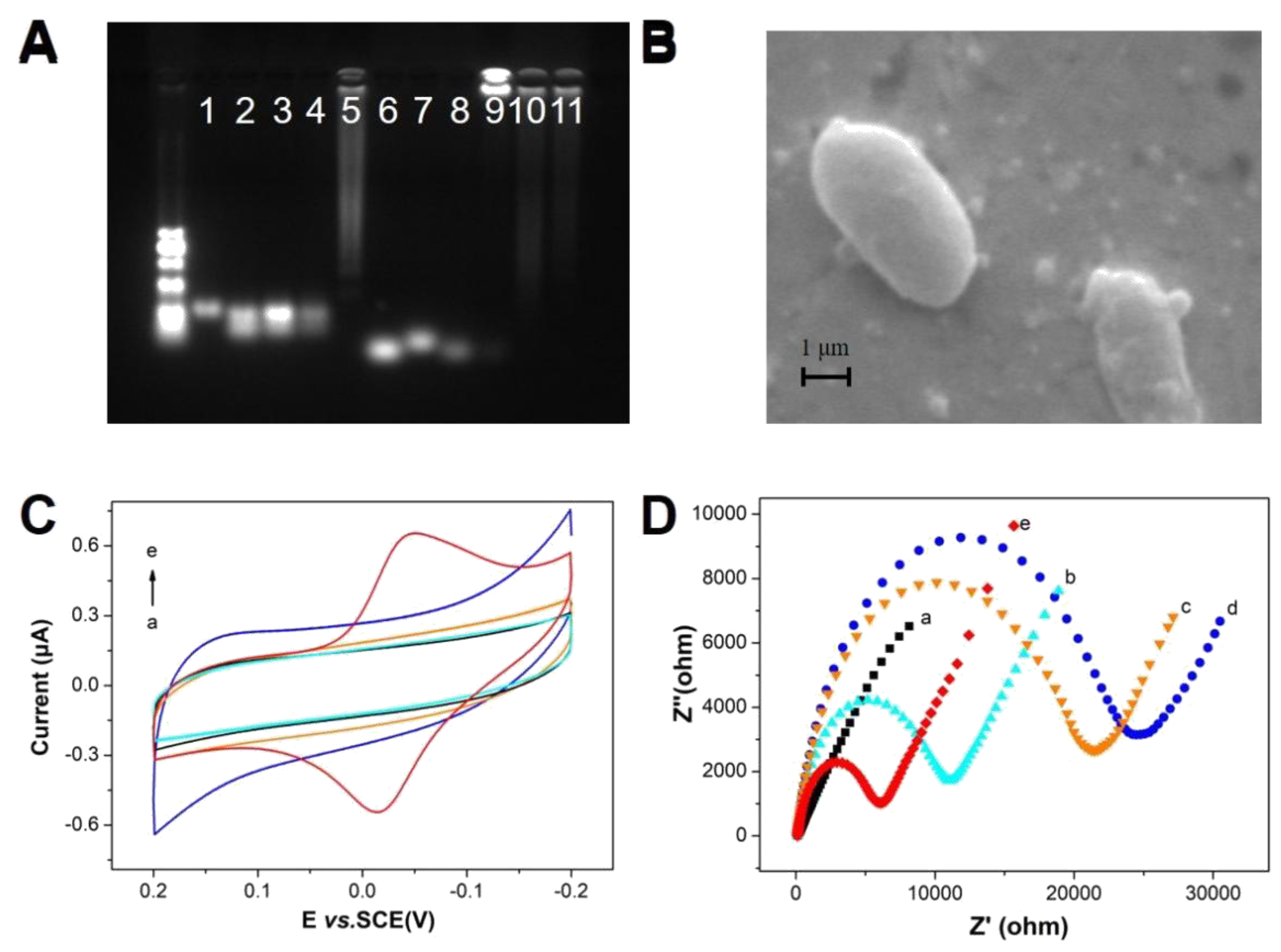
3.2. Characterization of the DNA Probe/G-Quadruplex
3.3. Comparison of Different Sensing Strategies
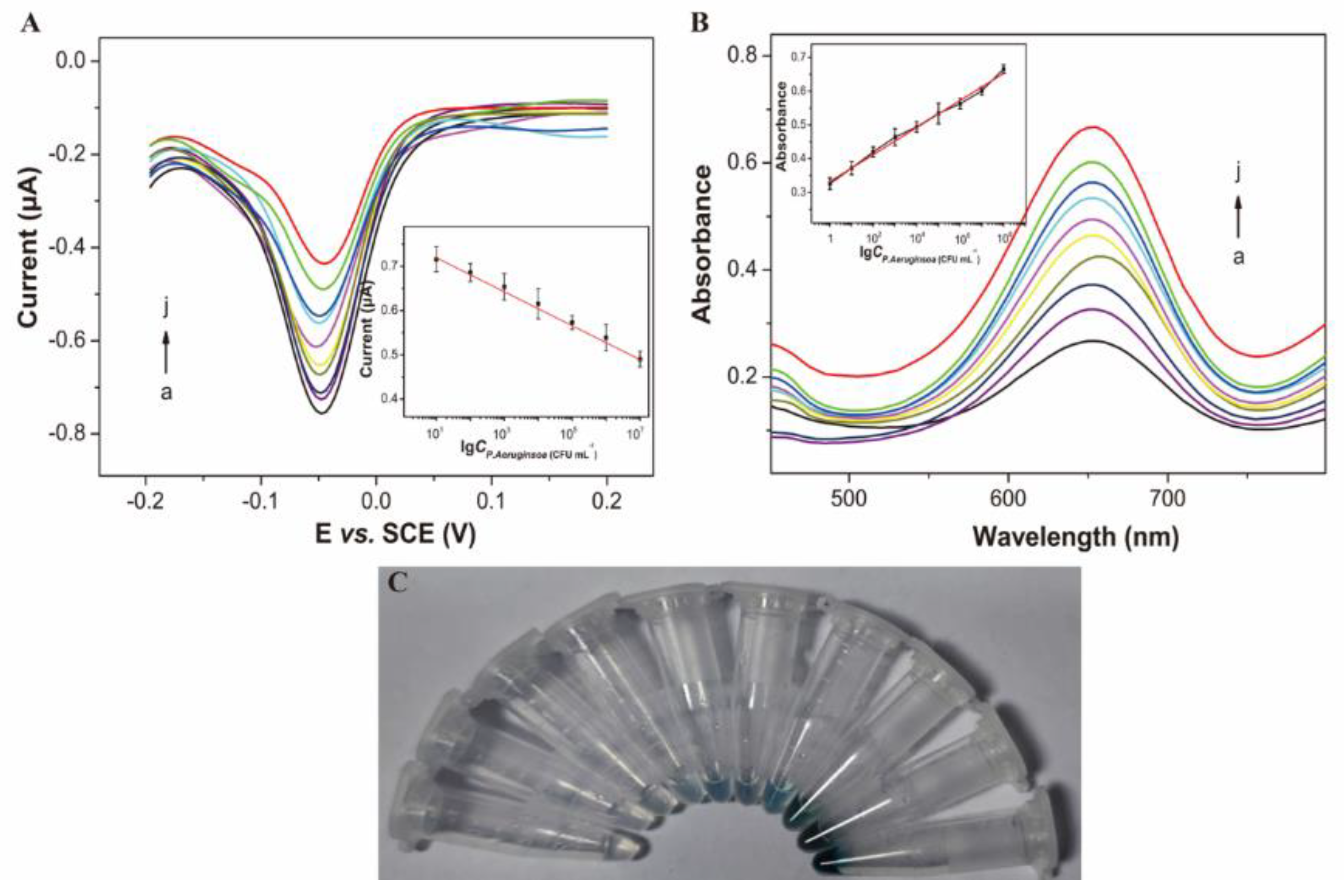
3.4. Dual-Channel Detection of P. aeruginosa
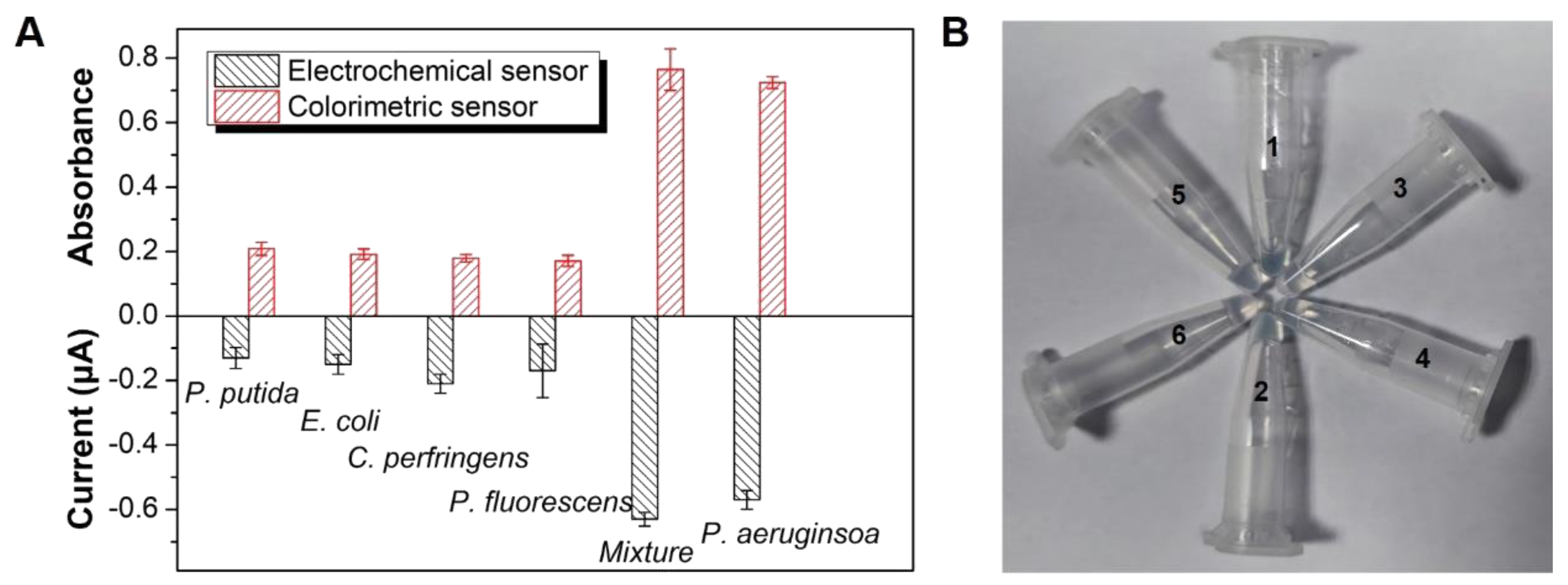
3.5. Selectivity
3.6. Analysis of P. aeruginosa in Meat Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellairaja, S.; Krithiga, N.; Ponmariappan, S.; Vasantha, V.S. Novel Pyrimidine Tagged Silver Nanoparticle Based Fluorescent Immunoassay for the Detection of Pseudomonas aeruginosa. J. Agric. Food Chem. 2021, 65, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Harada, L.K.; Junior, W.B.; Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Junior, J.M.O.; Tubino, M.; Vila, M.M.D.C.; Balcao, V.M. Bacteriophage-based biosensing of Pseudomonas aeruginosa: An integrated approach for the putative real-time detection of multi-drug-resistant strains. Biosensors 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Bai, X.J.; Zhang, X.W.; Fu, Y.C.; Li, Y.B.; Li, X.M.; Kokini, J.L. A low-field magnetic resonance imaging aptasensor for the rapid and visual sensing of Pseudomonas aeruginosa in food, juice, and water. Anal. Chem. 2021, 93, 8631–8637. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, L.L.; Wang, Z.X.; Xu, X.X.; Ding, H.L.; Song, S.S.; Xu, L.G.; Hua, K.; Xu, C.L. Gold nanoparticle-based immunochromatographic assay for detection Pseudomonas aeruginosa in water and food samples. Food Chem. X 2021, 9, 100117. [Google Scholar] [CrossRef] [PubMed]
- Capatina, D.; Lupoi, T.; Feier, B.; Olah, D.; Cirstea, C.; Oprean, R. Highly sensitive detection of PQS quorum sensing in Pseudomonas aeruginosa using screen-printed electrodes modified with nanomaterials. Biosensors 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Chen, Y.; Li, M.M.; Jia, L.Z.; Zhang, L.B.; Zhu, J.T. Gelatin-based photonic hydrogels for visual detection of pathogenic Pseudomonas aeruginosa. Sens. Actuators B Chem. 2021, 329, 129137. [Google Scholar] [CrossRef]
- Shi, X.H.; Zhang, J.L.; He, F.J. A new aptamer/polyadenylated DNA interdigitated gold electrode piezoelectric sensor for rapid detection of Pseudomonas aeruginosa. Biosens. Bioelectron. 2019, 132, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Lv, F.T.; Qi, R.L.; Senthikumar, T.; Zhao, H.; Chen, Y.Y.; Liu, L.B.; Wang, S. Forster resonance energy transfer mediated rapid and synergistic discrimination of bacteria over fungi using a cationic conjugated glycopolymer. Appl. Biomater. 2020, 3, 20–28. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, G.M.; Gou, D.; Luo, P.; Yao, Y.; Chen, H. A novel enzyme-free electrochemical biosensor for rapid detection of Pseudomonas aeruginosa based on high catalytic Cu-ZrMOF and conductive Super P. Biosens. Bioelectron. 2019, 142, 111486. [Google Scholar] [CrossRef]
- Kunze, A.; Dilcher, M.; Wahed, A.A.E.; Hufert, F.; Niessner, R.; Seidel, M. On-chip isothermal nucleic acid amplification on flow-based chemiluminescence microarray analysis platform for the detection of viruses and bacteria. Anal. Chem. 2016, 88, 898–905. [Google Scholar] [CrossRef]
- Gall, L.F.; Berre, R.L.; Rosec, S.; Hardy, J.; Gouriou, S.; Gastrin, S.B.; Vallet, S.; Rault, G.; Payan, C.; Arnaud, G.H. Proposal of a quantitative PCR-based protocol for an optimal Pseudomonas aeruginosa detection in patients with cystic fibrosis. BMC Microbiol. 2013, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Yang, Q.Q.; Zhang, Q.L.; Jiang, X.Y.; Wang, X.C.; Li, Y.; Zhao, J.; Qu, K.M. Rapid detection of cytochrome cd1-containing nitrite reductase encoding gene nirS of denitrifying bacteria with loop-mediated isothermal amplification assay. Sci. Rep. 2020, 10, 16484. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Zhu, Y.Z.; Li, N.; Lu, Y.; Cheng, J.; Xu, Y.C. A portable microfluidic analyzer for integrated bacterial detection using visible loop-mediated amplification. Sens. Actuators B Chem. 2020, 310, 127834. [Google Scholar] [CrossRef]
- Chen, S.M.; Wu, H.J.; Song, Y.X.; Peng, W.; Liu, Y. A fiber-optic surface plasmon resonance sensor for bio-detection in visible to near-infrared images. Biosensors 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Dhiman, A.; Kapil, V.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; Tang, P.; Xing, X.Y.; Cheng, W.; Liu, S.D.; Lu, X.X.; Zhong, L.Y. Colorimetry/SERS dual-sensor of H2O2 constructed via TMB-Fe3O4@AuNPs. Talanta 2022, 240, 123118. [Google Scholar] [CrossRef] [PubMed]
- Sarabaegi, M.; Roushani, M. A nano-sized chitosan particle based electrochemical aptasensor for sensitive detection of P. aeruginosa. Anal. Methods 2019, 11, 5591–5597. [Google Scholar] [CrossRef]
- Simoska, O.; Sans, M.; Fitzpatrick, M.D.; Crittenden, C.M.; Eberlin, L.S.; Shear, J.B.; Stevenson, K.J. Real-time electrochemical detection of Pseudomonas aeruginosa phenazine metabolites using transparent carbon ultramicroelectrode arrays. ACS Sens. 2019, 4, 170–179. [Google Scholar] [CrossRef]
- Sundhoro, M.; Agnihotra, S.R.; Amberger, B.; Augustus, K.; Khan, N.D.; Barnes, A.; BelBruno, J.; Mendecki, L. An electrochemical molecularly imprinted polymer sensor for rapid and selective food allergen detection. Food Chem. 2021, 344, 128648. [Google Scholar] [CrossRef]
- Qian, J.; Ren, C.C.; Wang, C.Q.; Keqi, A.; Cui, H.N.; Hao, N.; Wang, K. Gold nanoparticles mediated designing of versatile aptasensor for colorimetric/electrochemical dual-channel detection of aflatoxin B1. Biosens. Bioelectron. 2020, 166, 112443. [Google Scholar] [CrossRef]
- Cai, R.F.; Zhang, S.X.; Chen, L.; Li, M.L.; Zhang, Y.T.; Zhou, N.D. Self-assembled DNA nanoflowers triggered by a DNA walker for highly sensitive electrochemical detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2021, 13, 4905–4914. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Karimi, F.; Sanati, A.L.; Alizadeh, M.; Karaman, G.; Orooji, Y. Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 2021, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Nameghi, M.A.; Gerayelou, G. A novel electrochemical aptasensor for ochratoxin a sensing in spiked food using strand-displacement polymerase reaction. Talanta 2021, 223, 121705. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.H.; Yuan, W.; Li, Y.F.; Lu, Y.C.; Xiong, X.; Li, Y.; Liu, Y.J.; Lu, L.X. Sensitive electrochemical detection of aflatoxin B1 using DNA tetrahedron-nanostructure as substrate of antibody ordered assembly and template of aniline polymerization. Food Chem. 2020, 331, 127368. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, L.X.; Lu, Y.C.; Xiong, X.; Li, Y.; Cui, X.W.; Liu, Y.J.; Xiong, X.H. Synergistic effects of DNA structure for ultrasensitive detecting OTA in grains. Food Anal. Methods 2021, 14, 2308–2316. [Google Scholar] [CrossRef]
- Wang, W.Z.; Yuan, W.; Wang, D.B.; Mai, X.T.; Wang, D.Y.; Zhu, Y.Z.; Liu, F.; Sun, Z.L. Dual-mode sensor based on the synergy of magnetic separation and functional probes for the ultrasensitive detection of Clostridium perfringens. RSC Adv. 2022, 12, 25744. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Wei, M.; Liu, X.; Wei, W.; Zhao, H.Y.; Zhang, Y.J.; Liu, S.Q. Label-free ultrasensitive detection of telomerase activity via multiple telomeric hemin/G-quadruplex triggered polyaniline deposition and a DNA tetrahedron-structure regulated signal. Chem. Commun. 2016, 52, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Lin, H.Y.; Lv, W.X.; Gai, P.P.; Li, F. Equipment-free and visual detection of multiple biomarkers via an aggregation induced emission luminogen-based paper biosensor. Biosens. Bioelectron. 2020, 165, 112336. [Google Scholar] [CrossRef]
- Mi, L.; Sun, Y.D.; Shi, L.; Li, T. Hemin-bridged MOF interface with double amplification of G-quadruplex payload and DNAzyme catalysis: Ultrasensitive lasting chemiluminescence MicroRNA imaging. ACS Appl. Mater. Interfaces 2020, 12, 7879–7887. [Google Scholar] [CrossRef]
- Tang, X.Q.; Wang, Y.; Zhou, L.; Zhang, W.Q.; Yang, S.; Yu, L.; Zhao, S.; Chang, K.; Chen, M. Strand displacement-triggered G-quadruplex/rolling circle amplification strategy for the ultra-sensitive electrochemical sensing of exosomal microRNAs. Microchim. Acta 2020, 187, 172. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Q.; Yuan, H.; Zhao, Q.; Xing, L.; Zheng, X.Y.; Wang, W.G.; Zhao, Y.L.; Yang, Y.; Hu, L.; Yao, W.L. Recent developments of nanoenzyme-based colorimetric sensors for heavy metal detection and the interaction mechanism. Analyst 2020, 145, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gao, G.Z.; Zhai, K.F.; Xu, L.; Zhang, D.G. A visual Hg2+ detection strategy based on distance as readout by G-quadruplex DNAzyme on microfluidic paper. Food Chem. 2020, 331, 127208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.R.; Zhao, L.; Feng, H.D.; Chen, X.L.; Zhang, H.L.; Bai, Y.F.; Feng, F.; Shuang, S.M. A label-free fluorescent aptasensor based on HCR and G-quadruplex DNAzymes for the detection of prostate-specific antigen. Analyst 2021, 146, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, J.; Hu, X.L.; Gyimah, E.; Salome, Y.B.; Wang, K.; Wu, X.Y.; Zhang, Z. Electrochemical biosensor based on tetrahedral DNA nanostructures and G-quadruplex-hemin conformation for the ultrasensitive detection of MicroRNA-21 in serum. Anal. Chem. 2019, 91, 7353–7359. [Google Scholar] [CrossRef]
- Li, H.B.; Han, M.; Weng, X.; Zhang, Y.Y.; Li, J. DNA-tetrahedral-nanostructure-based entropy-driven amplifier for high-performance photoelectrochemical biosensing. ACS Nano 2021, 15, 1710–1717. [Google Scholar] [CrossRef]
- Lu, L.X.; Yuan, W.; Xiong, Q.; Wang, M.H.; Liu, Y.J.; Cao, M.; Xiong, X.H. One-step grain pretreatment for ochratoxin A detection based on bipolar electrode-electrochemiluminescence biosensor. Anal. Chim. Acta 2021, 1141, 83–90. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wang, D.B.; Sun, Z.L.; Liu, F.; Du, L.H.; Wang, D.Y. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef]
- Hu, Y.L.; Xie, Q.; Chang, L.; Tao, X.Q.; Tong, C.Y.; Liu, B.; Wang, W. A radar-like DNA monitor for RNase H-targeted natural compounds screening and RNase H activity in situ detection. Analyst 2021, 146, 5980–5987. [Google Scholar] [CrossRef]
- Hong, C.Y.; Zhang, X.X.; Ye, S.S.; Yang, H.F.; Huang, Z.Y.; Yang, D.; Cai, R.; Tan, W.H. Aptamer-pendant DNA tetrahedron nanostructure probe for ultrasensitive detection of tetracycline by coupling target-triggered rolling circle amplification. ACS Appl. Mater. Interfaces 2021, 13, 19695–19700. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.X.; Chen, H.M.; Zhong, Q.P.; Hou, Y.Q.; Chen, W.J.; Chen, W.X. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Yan, Y.C.; Li, J.; Li, W.H.; Wang, Y.; Song, W.L.; Bi, S. DNA flower-encapsulated horseradish peroxidase with enhanced biocatalytic activity synthesized by an isothermal one-pot method based on rolling circle amplification. Nanoscale 2018, 10, 22456–22465. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Ranjbar, S. Development of a sensitive diagnostic device based on zeolitic imidazolate frameworks-8 using ferrocene-graphene oxide as electroactive indicator for Pseudomonas aeruginosa detection. ACS Sustain. Chem. Eng. 2019, 7, 12760. [Google Scholar] [CrossRef]
- Gao, R.; Zhong, Z.T.; Gao, X.M.; Jia, L. Graphene oxide quantum dots assisted construction of fluorescent aptasensor for rapid detection of Pseudomonas aeruginosa in food samples. J. Agric. Food Chem. 2018, 66, 10898–10905. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, M.G.; Fan, E.C.; Ouyang, H.; Yue, H.; Su, X.X.; Liao, G.J.; Wang, L.; Lu, S.G.; Fu, Z.F. Highly specific bacteriophage-affinity strategy for rapid separation and sensitive detection of viable Pseudomonas aeruginosa. Anal. Chem. 2017, 89, 1916–1921. [Google Scholar] [CrossRef]
- Viswanath, K.B.; Krithiga, N.; Jayachitra, A.; Mideen, A.K.S.; Amali, A.J.; Vasantha, V.S. Enzyme-free multiplex detection of Pseudomonas aeruginosa and Aeromonas hydrophila with ferrocene and thionine-labeled antibodies using ZIF-8/Au NPs as a platform. ACS Omega 2019, 3, 17010–17022. [Google Scholar] [CrossRef]
- Li, H.B.; Liu, M.B.; Zhao, W.H.; Pu, J.M.; Xu, J.G.; Wang, S.Q.; Yu, R.Q. Multi-channel collection of G-quadruplex transducers for amplified signaling of Pax-5 based on target-triggered split-to-intact remodeling of dual-G-rich duplex probe. Sens. Actuator B Chem. 2020, 311, 127913. [Google Scholar] [CrossRef]
- Yu, J.L.; Wu, H.H.; He, L.Y.; Tan, L.; Jia, Z.J.; Gan, N. The universal dual-mode aptasensor for simultaneous determination of different bacteria based on naked eyes and microfluidic-chip together with magnetic DNA encoded probes. Talanta 2021, 225, 122062. [Google Scholar] [CrossRef]

| Sequence | 5′-3′ |
|---|---|
| Tetra-1 | CCCCCGTTGCTTTCGCTTTTCCTTTCGCTTTTGTTCGTTTCGTCCCTGCTTCCTTTCTTG-AAAAA-ACATTCCTAAGTCTGAAACATTACAGCTTGCTACACGAGAAGAGCCGCCATAGTA |
| Tetra-2 | HS-(CH2)6-TATCACCAGGCAGTTGACAGTGTAGCAAGCTGTAATAGATGCGAGGGTCCAATAC |
| Tetra-3 | HS-(CH2)6-TCAACTGCCTGGTGATAAAACGACACTACGTGGGAATCTACTATGGCGGCTCTTC |
| Tetra-4 | HS-(CH2)6-TTCAGACTTAGGAATGTGCTTCCCACGTAGTGTCGTTTGTATTGGACCCTCGCAT |
| Single DNA | CCCCCGTTGCTTTCGCTTTTCCTTTCGCTTTTGTTCGTTTCGTCCCTGCTTCCTTTCTTG-AAAAA-(CH2)6-SH |
| RCA template | PO43-TTCAGGTAGTGCATCACCCTCCCACCCCTCCCACCCCTATATCGGAGC |
| Capture probe | GCGAAAGCAACGGGGCACTACCTGAAGCTCC |
| Method | System | Detection Range | LOD | Reference |
|---|---|---|---|---|
| Electrochemical | A biosensor based on the aptamer immobilization on the surface of engineered ZIFs-8 via carbodiimide cross-linking | 12–1.2 × 107 CFU mL−1 | 1 CFU mL−1 | [42] |
| Fluorescence | Graphene Oxide Quantum Dots Assisted Fluorescent Aptasensor | 1.28 × 103–2 × 107 CFU mL−1 | 100 CFU mL−1 | [43] |
| bioluminescence | firefly luciferase-adenosine triphosphate bioluminescence system | 600–3.0 × 105 CFU mL−1 | 200 CFU mL−1 | [44] |
| Fluorescence | Novel Pyrimidine Tagged Silver Nanoparticle-Based Fluorescent Immunoassay | 8–10–1 CFU mL−1 | 1.5 CFU mL−1 | [1] |
| Electrochemical | An immunosensor using ZIF-8/Au NPfilm as a platform | 101–105 CFU mL−1 | 3.53 CFU mL−1 | [45] |
| Electrochemical/colorimetric | Dual channel sensor | 10–106/1–108 CFU mL−1 | 1.7/1.3 CFU mL−1 | This work |
| Sample | Added (CFU g−1) | Traditional Plate Count | The Proposed Dual-Mode Biosensor | |||||
|---|---|---|---|---|---|---|---|---|
| Electrochemical Sensor | Colorimetric Sensor | |||||||
| Found (CFU g−1) | Recovery (%) | Found (CFU g−1) | Recovery (%) | Found (CFU g−1) | Colorimetric Images | Recovery (%) | ||
| Chicken | 99 | >102 | - | 108.1 | 109.2 | 94.7 |  | 95.6 |
| Duck | 112 | >102 | - | 90.9 | 81.2 | 101.6 |  | 90.7 |
| Pork | 103 | >102 | - | 113.5 | 110.2 | 97.5 |  | 94.7 |
| Beef | 86 | >102 | - | 100.1 | 116.4 | 89.5 |  | 104.4 |
| Mutton | 94 | 109 | 115.9 | 87.5 | 93.1 | 100.4 |  | 106.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Wang, X.; Sun, Z.; Liu, F.; Wang, D. A Synergistic Dual-Channel Sensor for Ultrasensitive Detection of Pseudomonas aeruginosa by DNA Nanostructure and G-Quadruplex. Biosensors 2023, 13, 24. https://doi.org/10.3390/bios13010024
Yuan W, Wang X, Sun Z, Liu F, Wang D. A Synergistic Dual-Channel Sensor for Ultrasensitive Detection of Pseudomonas aeruginosa by DNA Nanostructure and G-Quadruplex. Biosensors. 2023; 13(1):24. https://doi.org/10.3390/bios13010024
Chicago/Turabian StyleYuan, Wei, Xinxia Wang, Zhilan Sun, Fang Liu, and Daoying Wang. 2023. "A Synergistic Dual-Channel Sensor for Ultrasensitive Detection of Pseudomonas aeruginosa by DNA Nanostructure and G-Quadruplex" Biosensors 13, no. 1: 24. https://doi.org/10.3390/bios13010024
APA StyleYuan, W., Wang, X., Sun, Z., Liu, F., & Wang, D. (2023). A Synergistic Dual-Channel Sensor for Ultrasensitive Detection of Pseudomonas aeruginosa by DNA Nanostructure and G-Quadruplex. Biosensors, 13(1), 24. https://doi.org/10.3390/bios13010024




