Review on the Selection of Aptamers and Application in Paper-Based Sensors
Abstract
1. Aptamers
1.1. Aptamer Screening Technologies
1.1.1. Magnetic Bead-SELEX (Mag-SELEX)
1.1.2. Capture-SELEX
| Methods | Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Mag-SELEX | Targets or ssDNAs are coupled to the surfaces of magnetic beads by a chemical coupling reaction, and the binding sequences are separated by a magnetic field. | Convenient and rapid magnetic separation. Various magnetic bead surface modifications for efficient target fixation, such as modifications using carboxyl, amino, and streptavidin. | Few functional groups available for coupling in some molecules. Complicated coupling process. | [21] |
| Capture-SELEX | A short-chain nucleotide is designed as a bridging sequence and is located on a solid phase carrier. The oligonucleotide library is fixed on the carrier based on the principle of base complementary pairing. When the target specifically recognizes the nucleotide sequence, the conformation of the oligonucleotide library is induced to change, and then the oligonucleotide library is detached from the solid phase carrier. | Long random sequences in oligonucleotide libraries that can form complex structures and bind targets with high specificity. A superior design that facilitates the conformational changes of aptamers and induces the oligonucleotide sequences to detach from the solid carrier. | Low screening efficiency due to the “false positive” result that is caused by the dissociation from targets of unbound sequences in immobilized libraries. Low abundance and diversity of libraries during the screening process. Complex and non-uniform equipment. | [22] |
| CE-SELEX | The bound and unbound oligonucleotide sequences are separated in a free solution according to their mobility differences. | Highly efficient separation. High selectivity. Low screening round numbers (generally only 2–4 rounds), and this leads to a high enrichment rate of the library and greatly improves the screening efficiency. | Not suitable for target molecules without high electrophoretic mobilities and commonly used for aptamer screening of large targets. High screening cost due to the expensive capillary electrophoresis apparatus. | [23] |
| GO-SELEX | Free ssDNAs or RNAs are adsorbed by the π–π stacking interaction, while oligonucleotides with structural changes, such as aptamer-target complexes, are not absorbed. | Effective guarantee of the natural conformations of targets or oligonucleotides. Reduction in the spatial impedance (no fixed-induced structural changes). Less non-specific adsorption of the solid substrate used to fix targets or libraries. | Potential self-desorption of the oligonucleotide sequences adsorbed on the GO surface by interaction. This results in an increase in non-specific sequences. Not suitable for aptamer screening of target molecules that can be adsorbed by GO. | [24] |
| Microfluidic chip SELEX | A SELEX technology based on a microfluidic system: a micro platform capable of automated phylogenetic screening of ligands on a single-chip basis. | Small device. Small sample size. Automatic screening. | Unstable injection volume. Limitation of the hydrophilicity of the microchannel. Not commercially available and time-consuming to build. | [25] |
1.1.3. Capillary Electrophoresis-SELEX (CE-SELEX)
1.1.4. Graphene Oxide-SELEX (GO-SELEX)
1.1.5. Microfluidic Chip SELEX
1.2. Optimization Strategy of the Nucleic Acid Aptamer Sequence
1.2.1. Truncation of Nucleic Acid Aptamer Sequences
1.2.2. Cleavage of Nucleic Acid Aptamer Sequences
1.2.3. Splicing of Nucleic Acid Aptamer Sequences
2. Paper-Based Analytical Methods
2.1. Carrier Materials of Paper-Based Analytical Methods
2.1.1. Cellulose
2.1.2. Modified or Compounded Cellulose
2.1.3. Non-Cellulose
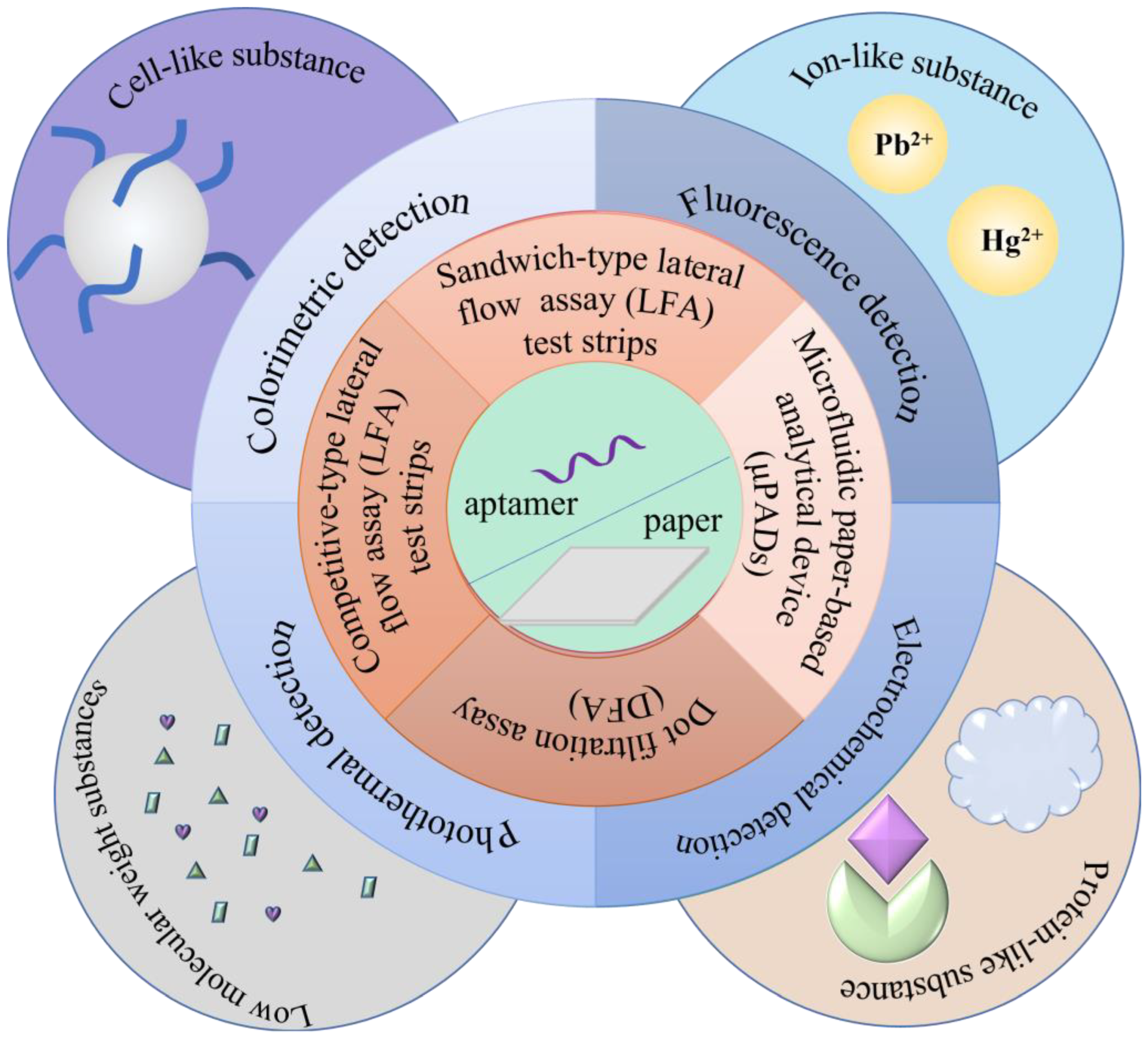
2.2. Type of Paper-Based Analytical Methods
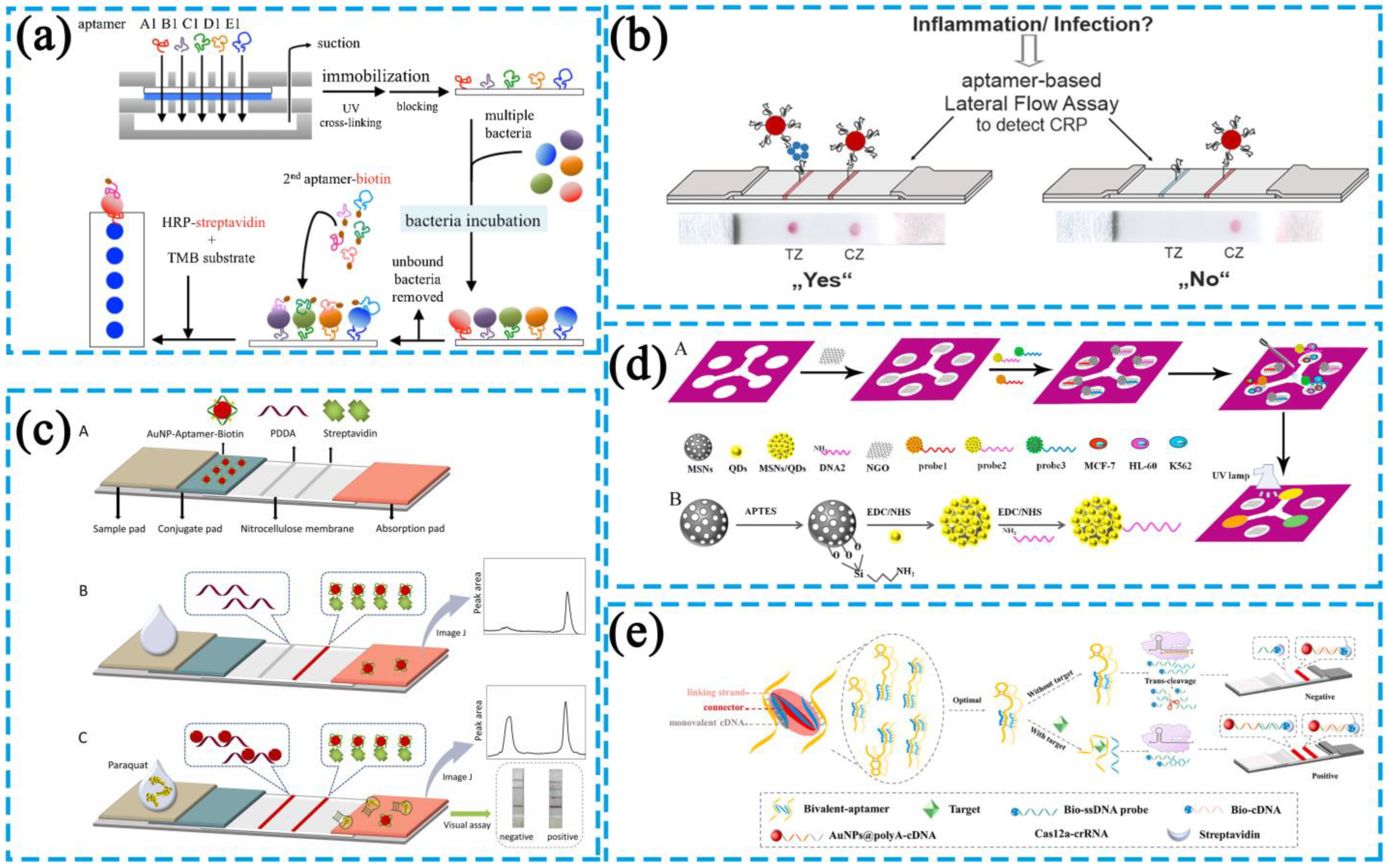
2.2.1. Test Strips
| Paper-Based Sensors | Description | Characteristics | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|---|
| Test strips | DFA | The DFA system is a plastic cartridge with a nitrocellulose membrane placed under the central hole of the cap, an absorbent paper placed at the bottom of the cartridge, and aptamers wrapped in the center of the membrane. | The test strips are divided into competitive- and sandwich-type test strips. The sandwich method is used for the detection of large analytes and analytes with multiple specific binding sites, and occasionally where there are two aptamers specific to a target. The competitive method is used for the detection of small analytes with a low molecular weight, analytes with single specific binding sites, and occasions in which there is only one aptamer specific to an analyte. | Concentrated color due to the dropwise addition of materials to be measured to the detection area. Circular detection area for other convenient subsequent processing and high detection sensitivity. | Not suitable for complex matrices because the test strips must be pretreated. Need for further confirmation in a positive test. Limited application; not yet applied on a large scale and for the market. Only for qualitative analysis. Quantitative analysis must be used in combination with other detection methods, and sensitivity is low. | [44] |
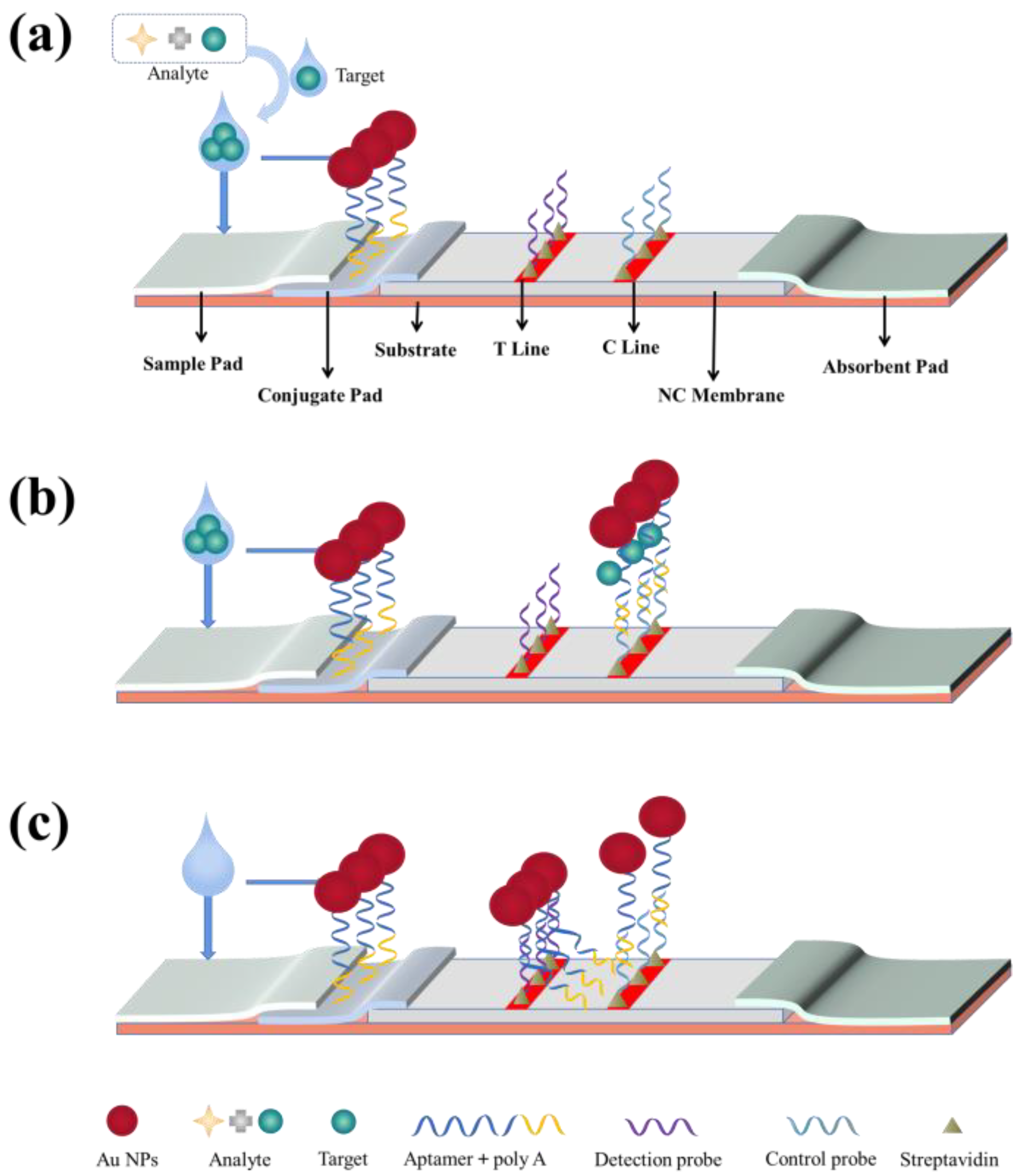
| LFA | Targets and markers are adsorbed on aptamers. The microscopic changes in the detection line and quality control line are achieved by the specific recognition between aptamers and targets with the help of the characteristics of markers, such as color and fluorescence, thus achieving the detection of targets. | Low development cost. Easy to produce. Readily observed results. | [45] | |||
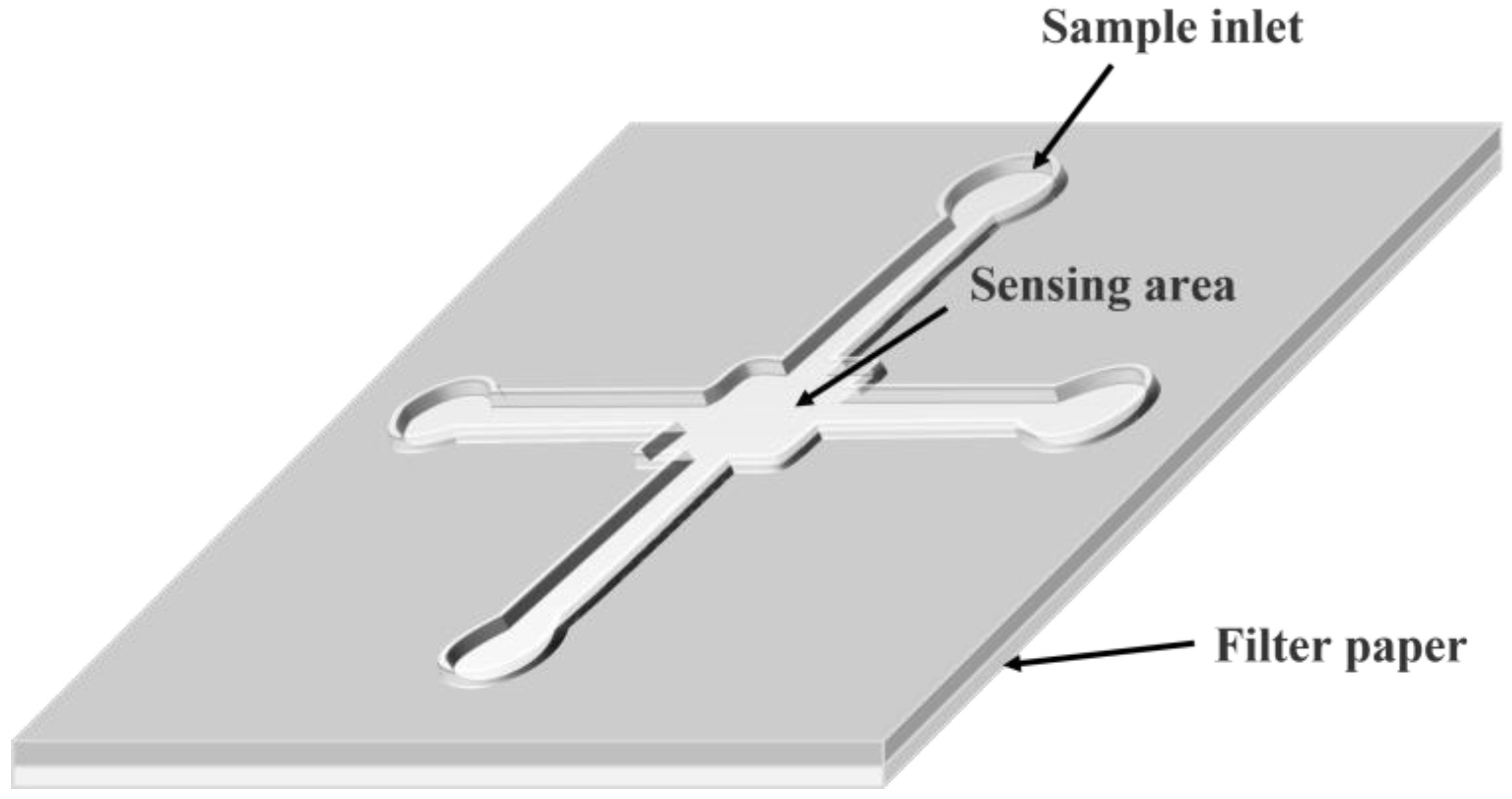
| Microfluidic Chips | The μPADs, referred to as paper chips, use paper as the carrier. The reaction occurs by controlling the direction and speed of liquid flow in the paper chip channels, thus realizing the purpose of building a “micro-laboratory” on a paper base. | The structure of paper-based microfluidic devices can be broadly classified into two-dimensional (2D) and three-dimensional (3D) devices. The main difference between them is in the structural design, functional integration, and multi-target and multi-step analysis capability. | Portability. Simple and reliable reaction system. High efficiency of nucleic acid molecule detection in complex systems with the combined use of 2D and 3D paper-based structures. | No complete laboratory supporting equipment or a related quality control system. Limited detection resolution due to the current microfabrication methods that cannot achieve high throughput and accurate quantification on a limited paper-based reaction space. | [46] | |
Dot Filtration Assay
Lateral Flow Assay
Sandwich-Type LFA Test Strips
Competitive-Type LFA Test Strips
2.2.2. Microfluidic Paper-Based Analytical Devices
2.3. Detection Method of the Paper-Based Analytical Assay
2.3.1. Colorimetric Detection
2.3.2. Fluorescence Detection
2.3.3. Electrochemical Detection
2.3.4. Photothermal Detection

3. Applications of the Aptamer and Paper-Based Analytical Methods
3.1. Cell-like Substance Detection
3.2. Protein-like Substance Detection
3.3. Detection of Low Molecular Weight Substances
3.4. Ion-like Substance Detection
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Aptamer-based lateral flow assay on-site biosensors. Biosens. Bioelectron. 2021, 186, 113279. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Kalra, P.; Bansal, V.; Bruno, J.G.; Sharma, T.K. Aptamer-based point-of-care diagnostic platforms. Sens. Actuators B Chem. 2017, 246, 535–553. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Duan, N.; Wu, S.; Wang, Z.; Wei, X.; Wang, Y. Selection and characterization of DNA aptamers against Staphylococcus aureus enterotoxin C1. Food Chem. 2015, 166, 623–629. [Google Scholar] [CrossRef]
- Rasoulinejad, S.; Gargari, S.L.M. Aptamer-nanobody based ELASA for specific detection of Acinetobacter baumannii isolates. J. Biotechnol. 2016, 231, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Reinemann, C.; Freiin von Fritsch, U.; Rudolph, S.; Strehlitz, B. Generation and characterization of quinolone-specific DNA aptamers suitable for water monitoring. Biosens. Bioelectron. 2016, 77, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.; Byun, J.Y.; Shim, W.B.; Kim, M.G. A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal. Chim. Acta 2015, 886, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Li, F.; Shen, S.; Tyrrell, D.L.; Le, X.C.; Li, X.F. DNase-mediated single-cycle selection of aptamers for proteins blotted on a membrane. Anal. Chem. 2012, 84, 7603–7606. [Google Scholar] [CrossRef]
- Kinghorn, A.B.; Fraser, L.A.; Liang, S.L.; Shiu, S.C.C.; Tanner, J.A. Aptamer Bioinformatics. Int. J. Mol. Sci. 2017, 18, 2516. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhang, Y.L.; Tian, F.Y.; Liu, X.Y.; Du, S.Y.; Ren, G.C. Overview of Rapid Detection Methods for Salmonella in Foods: Progress and Challenges. Foods 2021, 10, 2402. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Liu, Y.; Mukama, O.; Han, X.; Huang, H.; Li, S.; Zhou, P.; Lu, X.; Li, Z. A signal-enhanced and sensitive lateral flow aptasensor for the rapid detection of PDGF-BB. RSC Adv. 2020, 10, 18601–18607. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, L.; Duan, N.; Li, Q.; Zhou, Y.; Wang, Z. Aptamer-Based Lateral Flow Test Strip for Rapid Detection of Zearalenone in Corn Samples. J. Agric. Food Chem. 2018, 66, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, S.; Wang, S.; Liu, J.; Dong, Y. Development of Lateral Flow Immunochromatographic Strips for Micropollutants Screening Using Colorants of Aptamer Functionalized Nanogold Particles Part I Methodology and Optimization. J. AOAC Int. 2018, 101, 1402–1407. [Google Scholar] [CrossRef]
- Stojanovic, M.N.; Landry, D.W. Aptamer-based colorimetric probe for cocaine. J. Am. Chem. Soc. 2002, 124, 9678–9679. [Google Scholar] [CrossRef]
- Lin, C.; Katilius, E.; Liu, Y.; Zhang, J.; Yan, H. Self-assembled signaling aptamer DNA arrays for protein detection. Angew. Chem. Int. Ed. Engl. 2006, 45, 5296–5301. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Xu, E.; Jiao, A.; Chughtai, M.F.J.; Jin, Z. Rapid detection of beta-conglutin with a novel lateral flow aptasensor assisted by immunomagnetic enrichment and enzyme signal amplification. Food Chem. 2018, 269, 375–379. [Google Scholar] [CrossRef]
- Riccitelli, N.J.; Luptak, A. Computational discovery of folded RNA domains in genomes and in vitro selected libraries. Methods 2010, 52, 133–140. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal. Bioanal. Chem. 2005, 383, 83–91. [Google Scholar] [CrossRef]
- Yan, J.; Xiong, H.; Cai, S.; Wen, N.; He, Q.; Liu, Y.; Peng, D.; Liu, Z. Advances in aptamer screening technologies. Talanta 2019, 200, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Khan, I.M.; Wang, Z. Capture-SELEX for aptamer selection: A short review. Talanta 2021, 229, 122274. [Google Scholar] [CrossRef] [PubMed]
- Mosing, R.K.; Bowser, M.T. Isolating aptamers using capillary electrophoresis-SELEX (CE-SELEX). Methods Mol. Biol. 2009, 535, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Kwon, Y.S.; Kim, J.H.; Gu, M.B. Multiple GO-SELEX for efficient screening of flexible aptamers. Chem. Commun. 2014, 50, 10513–10516. [Google Scholar] [CrossRef]
- Jiang, H.; Lyu, X.F.; Zhao, K.X. Progress of Aptamer Screening Techniques Based on Microfluidic Chips. Chin. J. Anal. Chem. 2020, 48, 590–600. [Google Scholar] [CrossRef]
- Khatri, K.; Klein, J.A.; Haserick, J.R.; Leon, D.R.; Costello, C.E.; McComb, M.E.; Zaia, J. Microfluidic Capillary Electrophoresis Mass Spectrometry for Analysis of Monosaccharides, Oligosaccharides, and Glycopeptides. Anal. Chem. 2017, 89, 6645–6655. [Google Scholar] [CrossRef]
- Yang, J.; Bowser, M.T. Capillary Electrophoresis–SELEX Selection of Catalytic DNA Aptamers for a Small-Molecule Porphyrin Target. Anal. Chem. 2013, 85, 1525–1530. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Duan, N.; Wu, S.; Xia, Y.; Ma, X.; Zhu, C.; Jiang, Y.; Wang, Z. Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J. Agric. Food Chem. 2014, 62, 10368–10374. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, X.; Jiao, B.; Wang, L. Post-SELEX optimization of aptamers. Anal. Bioanal. Chem. 2016, 408, 4567–4573. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.M.; Chikkanna, A.; Chen, S.X.; Brusius, I.; Sbuh, N.; Veedu, R.N. Development of nucleic acid aptamer-based lateral flow assays: A robust platform for cost-effective point-of-care diagnosis. Theranostics 2021, 11, 5174–5196. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, G.; Huang, Y.; Yang, S.; Ren, S.; Gao, Z.; Chen, A. Dual-competitive lateral flow aptasensor for detection of aflatoxin B1 in food and feedstuffs. J. Hazard. Mater. 2018, 344, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.G.; Xing, Y.; Zhou, X.; Wang, R.; Liu, L.; Zeng, S.; He, M.; Ma, M. Ultrasensitive colorimetric aptasensor for Hg2+ detection using Exo-III assisted target recycling amplification and unmodified AuNPs as indicators. J. Hazard. Mater. 2020, 384, 120948. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Shi, Q.; Du, D.; Liu, D.; Luo, Y.; Xu, W.; Lin, Y. Au@Pd Nanopopcorn and Aptamer Nanoflower Assisted Lateral Flow Strip for Thermal Detection of Exosomes. Anal. Chem. 2019, 91, 13986–13993. [Google Scholar] [CrossRef]
- Freeman, R.; Sharon, E.; Tel-Vered, R.; Willner, I. Supramolecular Cocaine-Aptamer Complexes Activate Biocatalytic Cascades. J. Am. Chem. Soc. 2009, 131, 5028–5029. [Google Scholar] [CrossRef] [PubMed]
- Patel, D. Structural analysis of nucleic acid aptamers. Curr. Opin. Chem. Biol. 1997, 1, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Mallikaratchy, P.R.; Ruggiero, A.; Gardner, J.R.; Kuryavyi, V.; Maguire, W.F.; Heaney, M.L.; McDevitt, M.R.; Patel, D.J.; Scheinberg, D.A. A multivalent DNA aptamer specific for the B-cell receptor on human lymphoma and leukemia. Nucleic Acids Res. 2011, 39, 2458–2469. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.-P.; Ku, C.-H.; Yang, R.-J. Porosity estimation using electric current measurements for paper-based microfluidics. Microfluid. Nanofluidics 2019, 23, 59. [Google Scholar] [CrossRef]
- Shih, C.M.; Chang, C.L.; Hsu, M.Y.; Lin, J.Y.; Kuan, C.M.; Wang, H.K.; Huang, C.T.; Chung, M.C.; Huang, K.C.; Hsu, C.E.; et al. Paper-based ELISA to rapidly detect Escherichia coli. Talanta 2015, 145, 2–5. [Google Scholar] [CrossRef]
- Tonkinson, J.L.; Stillman, B.A. Nitrocellulose: A tried and true polymer finds utility as a post-genomic substrate. Front. Biosci. 2002, 7, C1–C12. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, M.; Fang, Z.; Wan, P.; Yu, G. A Wearable Transient Pressure Sensor Made with MXene Nanosheets for Sensitive Broad-Range Human-Machine Interfacing. Nano Lett. 2019, 19, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiong, L.; Pu, Y.; Quan, Y.; Li, S. High-Performance Paper-Based Capacitive Flexible Pressure Sensor and Its Application in Human-Related Measurement. Nanoscale Res. Lett. 2019, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoci, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Lv, X.; Wang, M.; Jiang, X.; Jiang, Y.; Wang, H.; Zhao, Y.; Xia, L. Paper-based immune-affinity arrays for detection of multiple mycotoxins in cereals. Anal. Bioanal. Chem. 2018, 410, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ge, Y.; Wang, X.; Lu, Z.; Wang, T.; Zhang, H.; Du, S. Rapid and sensitive multimode detection of Salmonella typhimurium based on the photothermal effect and peroxidase-like activity of MoS2@Au nanocomposite. Sens. Actuators B Chem. 2021, 326, 128807. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Song, S.; Park, S.; Joo, C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef]
- Zhang, D.; Li, C.; Ji, D.; Wang, Y. Paper-Based Microfluidic Sensors for Onsite Environmental Detection: A Critical Review. Crit. Rev. Anal. Chem. 2022, 52, 1432–1449. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Huang, Y.; Dandapat, A.; Dai, L.; Zhang, G.; Lu, X.; Zhang, J.; Lai, W.; Chen, T. Hollow Au-Ag Nanoparticles Labeled Immunochromatography Strip for Highly Sensitive Detection of Clenbuterol. Sci. Rep. 2017, 7, 41419. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Zhan, L.; Liu, Y.; Qin, Z. Signal amplification and quantification on lateral flow assays by laser excitation of plasmonic nanomaterials. Theranostics 2020, 10, 4359–4373. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Zhang, S.; Liu, J.H.; Dong, Y.Y. State of the art: Lateral flow assay (LFA) biosensor for on-site rapid detection. Chin. Chem. Lett. 2018, 29, 1567–1577. [Google Scholar] [CrossRef]
- Jauset-Rubio, M.; El-Shahawi, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C.K. Advances in aptamers-based lateral flow assays. TrAC-Trends Anal. Chem. 2017, 97, 385–398. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Sobolev, A.M.; Tessier, M.D.; Hens, Z.; Goryacheva, I.Y.; De Saeger, S. Fluorescently labelled multiplex lateral flow immunoassay based on cadmium-free quantum dots. Methods 2017, 116, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, X.; An, Q.; Zhou, R.; Xu, W.; Xu, D.; Lin, Q.; Cao, X. Gold nanostar as an ultrasensitive colorimetric probe for picomolar detection of lead ion. Anal. Chim. Acta 2021, 1160, 338380. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Giovannoli, C.; Passini, C.; Baggiani, C. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal. Bioanal. Chem. 2013, 405, 9859–9867. [Google Scholar] [CrossRef]
- Wang, Z.L.; Sun, Y.Z.; Liang, D.M.; Zeng, Y.Y.; He, S.; Mari, G.M.; Peng, T.; Jiang, H.Y. Highly sensitive chromatographic time-resolved fluoroimmunoassay for rapid onsite detection of streptomycin in milk. J. Dairy Sci. 2020, 103, 8750–8760. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Zhao, Y.; Fan, J.; Han, T.T.; Si, Y.A.; Zhou, B.; Zhang, J.; Hu, Z.G.; Xie, M.H. Dual-label time-resolved fluoroimmunoassay for simultaneous measurement of human epidermal growth factor receptor 2 and human epididymis protein 4 in serum. J. Anal. Sci. Technol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Lu, Z.; Tao, X. Comparative Study of Time-Resolved Fluorescent Nanobeads, Quantum Dot Nanobeads and Quantum Dots as Labels in Fluorescence Immunochromatography for Detection of Aflatoxin B1 in Grains. Biomolecules 2020, 10, 575. [Google Scholar] [CrossRef]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B1 and Aflatoxin B1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef]
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal. Chem. 2018, 107, 60–77. [Google Scholar] [CrossRef]
- Lu, C.X.; Gao, X.X.; Chen, Y.; Ren, J.T.; Liu, C.B. Aptamer-Based Lateral Flow Test Strip for the Simultaneous Detection of Salmonella typhimurium, Escherichia coli O157:H7 and Staphylococcus aureus. Anal. Lett. 2020, 53, 646–659. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Z.Y.; Xi, X.X.; Cao, T.W.; Wen, W.; Zhang, X.H.; Wang, S.F. An aptamer-based hook-effect-recognizable three-line lateral flow biosensor for rapid detection of thrombin. Biosens. Bioelectron. 2019, 133, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, W.; Du, D.; Zhang, X.H.; Wang, S.F.; Lin, Y.H. A universal lateral flow biosensor for proteins and DNAs based on the conformational change of hairpin oligonucleotide and its use for logic gate operations. Biosens. Bioelectron. 2014, 61, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liang, Z.; Zhou, N. Design strategies for aptamer-based biosensors. Sensors 2010, 10, 4541–4557. [Google Scholar] [CrossRef] [PubMed]
- Kurth, T.; Witt, S.; Bolten, S.; Waniek, J.J.; Kortmann, C.; Lavrentieva, A.; Scheper, T.; Walter, J.G. Development of Aptamer-Based TID Assays Using Thermophoresis and Microarrays. Biosensors 2019, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Montes, R.J.; Ladd, A.J.C.; Butler, J.E. Transverse migration and microfluidic concentration of DNA using Newtonian buffers. Biomicrofluidics 2019, 13, 044104. [Google Scholar] [CrossRef]
- Li, H.; Sun, D.E.; Liu, Y.; Liu, Z. An ultrasensitive homogeneous aptasensor for kanamycin based on upconversion fluorescence resonance energy transfer. Biosens. Bioelectron. 2014, 55, 149–156. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Stockton, A.M.; Carrilho, E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (mu PADs)—A review. Anal. Chim. Acta 2017, 970, 1–22. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, S.; Tang, D. Novel 3D Printed Device for Dual-Signaling Ratiometric Photoelectrochemical Readout of Biomarker Using λ-Exonuclease-Assisted Recycling Amplification. Anal. Chem. 2019, 91, 10049–10055. [Google Scholar] [CrossRef]
- Gao, X.; Qi, L.; Liu, K.; Meng, C.; Li, Y.; Yu, H.Z. Exonuclease I-Assisted General Strategy to Convert Aptamer-Based Electrochemical Biosensors from "Signal-Off" to "Signal-On". Anal. Chem. 2020, 92, 6229–6234. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Wu, L.; Lu, Y. A coffee-ring effect-based paper sensor chip for the determination of beta-lactoglobulin in foods via a smartphone. Sens. Actuators B Chem. 2023, 374, 132807. [Google Scholar] [CrossRef]
- Liu, Y.; Alkhamis, O.; Liu, X.; Yu, H.; Canoura, J.; Xiao, Y. Aptamer-Integrated Multianalyte-Detecting Paper Electrochemical Device. ACS Appl. Mater. Interfaces 2021, 13, 17330–17339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, S.; Su, S.; Wang, Y.; Zhang, H. Rapid detection method and portable device based on the photothermal effect of gold nanoparticles. Biosens. Bioelectron. 2019, 123, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lin, M.; Chen, G.; Fan, C.; Li, M.; Gu, X.; Cong, S.; Zhao, Z.; Fu, L.; Fang, X.; et al. Photodegradable CuS SERS Probes for Intraoperative Residual Tumor Detection, Ablation, and Self-Clearance. ACS Appl. Mater. Interfaces 2019, 11, 23436–23444. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Zhu, Y.; Xu, K.; Wang, W.; Hou, R.; Li, X. Photothermal Microfluidic Sensing Platform Using Near-Infrared Laser-Driven Multiplexed Dual-Mode Visual Quantitative Readout. Anal. Chem. 2019, 91, 13290–13296. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, X.; Zhang, H. Development of a photothermal-sensing microfluidic paper-based analytical chip (PT-Chip) for sensitive quantification of diethylstilbestrol. Food Chem. 2023, 402, 134128. [Google Scholar] [CrossRef]
- Qin, Z.; Chan, W.C.; Boulware, D.R.; Akkin, T.; Butler, E.K.; Bischof, J.C. Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angew. Chem. Int. Ed Engl. 2012, 51, 4358–4361. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wu, J.-J.; Lee, G.-B. Screening of highly-specific aptamers and their applications in paper-based microfluidic chips for rapid diagnosis of multiple bacteria. Sens. Actuators B Chem. 2019, 284, 395–402. [Google Scholar] [CrossRef]
- Liang, L.; Su, M.; Li, L.; Lan, F.; Yang, G.; Ge, S.; Yu, J.; Song, X. Aptamer-based fluorescent and visual biosensor for multiplexed monitoring of cancer cells in microfluidic paper-based analytical devices. Sens. Actuators B Chem. 2016, 229, 347–354. [Google Scholar] [CrossRef]
- Phung, N.L.; Walter, J.G.; Jonczyk, R.; Seiler, L.K.; Scheper, T.; Blume, C. Development of an Aptamer-Based Lateral Flow Assay for the Detection of C-Reactive Protein Using Microarray Technology as a Prescreening Platform. ACS Comb. Sci. 2020, 22, 617–629. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Xia, L.; Yu, H.; Wang, Q.; Wu, Y. Cationic polyelectrolyte as powerful capture molecule in aptamer-based chromatographic strip for rapid visual detection of paraquat residue in agricultural products. Sens. Actuators B Chem. 2022, 368, 132237. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Mao, M.; Peng, C.; Wang, Z. Accelerated CRISPR/Cas12a-based small molecule detection using bivalent aptamer. Biosens. Bioelectron. 2022, 217, 114725. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.Y.; Yang, D.; Cui, Z.J.; He, H.Y.; Mei, S.L.; Lu, H.X.; Fu, Z.Z.; Wang, L.; Zhang, W.L.; Guo, R.Q. Recent Advances in Metal Chalcogenide Quantum Dots: From Material Design to Biomedical Applications. Adv. Funct. Mater. 2022, 32, 23. [Google Scholar] [CrossRef]
- Qian, S.; Han, Y.; Xu, F.; Feng, D.; Yang, X.; Wu, X.; Hao, L.; Yuan, M. A fast, sensitive, low-cost electrochemical paper-based chip for real-time simultaneous detection of cadmium () and lead () via aptamer. Talanta 2022, 247, 123548. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Mai, V.P.; Wu, R.Y.; Yeh, K.L.; Yang, R.J. A Microfluidic Aptamer-Based Sensor for Detection of Mercury(II) and Lead(II) Ions in Water. Micromachines 2021, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.; Momtaz, S.; Vakhshiteh, F.; Maghsoudi, A.S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Biosensors and their applications in detection of organophosphorus pesticides in the environment. Arch Toxicol. 2017, 91, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Salek Maghsoudi, A.; Hassani, S.; Rezaei Akmal, M.; Ganjali, M.R.; Mirnia, K.; Norouzi, P.; Abdollahi, M. An Electrochemical Aptasensor Platform Based on Flower-Like Gold Microstructure-Modified Screen-Printed Carbon Electrode for Detection of Serpin A12 as a Type 2 Diabetes Biomarker. Int. J. Nanomed. 2020, 15, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Raston, N.H.A.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, S.; Wang, S.; Liu, J.H.; Dong, Y.Y. Development of Lateral Flow Immunochromatographic Strips for Micropollutant Screening Using Colorants of Aptamer-Functionalized Nanogold Particles, Part II: Experimental Verification with Aflatoxin B1 and Chloramphenicol. J. AOAC Int. 2018, 101, 1408–1414. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Kaur, B.; Chorti, P. From Point-of-Care Testing to eHealth Diagnostic Devices (eDiagnostics). ACS Cent. Sci. 2018, 4, 1600–1616. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, M.; Ma, T.; Li, W.; Zhang, H. Review on the Selection of Aptamers and Application in Paper-Based Sensors. Biosensors 2023, 13, 39. https://doi.org/10.3390/bios13010039
Wang K, Wang M, Ma T, Li W, Zhang H. Review on the Selection of Aptamers and Application in Paper-Based Sensors. Biosensors. 2023; 13(1):39. https://doi.org/10.3390/bios13010039
Chicago/Turabian StyleWang, Kaifei, Minglu Wang, Teng Ma, Wenyu Li, and Hongyan Zhang. 2023. "Review on the Selection of Aptamers and Application in Paper-Based Sensors" Biosensors 13, no. 1: 39. https://doi.org/10.3390/bios13010039
APA StyleWang, K., Wang, M., Ma, T., Li, W., & Zhang, H. (2023). Review on the Selection of Aptamers and Application in Paper-Based Sensors. Biosensors, 13(1), 39. https://doi.org/10.3390/bios13010039





