Non-Invasive Electroretinogram Recording with Simultaneous Optogenetics to Dissect Retinal Ganglion Cells Electrophysiological Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. AAV Packaging
2.3. Intravitreal AAV Injection
2.4. ERG Recording
2.5. ERG Featured Signals Dissection
2.6. Immunostaining
2.7. Data Analysis
3. Results and Discussion
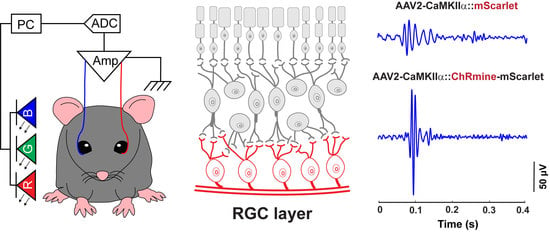
3.1. A Custom-Designed ERG Recording System Associated with Optical Stimulation and Algorithmic Signal Feature Classification
3.2. ERG Responses under Different Color Light Stimulation
3.3. Sensitizing Mouse RGCs to Express ChRmine for Non-Invasive Optogenetics
3.4. ERG Responses of RGCs from Optogenetic Stimulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, H.A. Open-angle glaucoma. N. Engl. J. Med. 1993, 328, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic Nerve Damage in Human Glaucoma: II. The Site of Injury and Susceptibility to Damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.J.; Medeiros, F.A.; Zangwill, L.M.; Weinreb, R.N. Strategies to improve early diagnosis in glaucoma. Prog. Brain Res. 2015, 221, 103–133. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, R. Gradual Painless Visual Loss: Glaucoma. Clin. Geriatr. Med. 1999, 15, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sample, P.A.; Zangwill, L.M.; Schuman, J.S. Diagnostic Tools for Glaucoma Detection and Management. Surv. Ophthalmol. 2008, 53, S17–S32. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, G.J.; Caprioli, J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am. J. Ophthalmol. 2004, 137, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.; Hee, M.R.; Arya, A.V.; Pedut-Kloizman, T.; Puliafito, C.A.; Fujimoto, J.G.; Swanson, E.A. Optical coherence tomography: A new tool for glaucoma diagnosis. Curr. Opin. Ophthalmol. 1995, 6, 89–95. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Tatham, A.J. Structure versus Function in Glaucoma: The Debate That Doesn’t Need to Be. Ophthalmology 2016, 123, 1170–1172. [Google Scholar] [CrossRef] [Green Version]
- Wilsey, L.J.; Fortune, B. Electroretinography in glaucoma diagnosis. Curr. Opin. Ophthalmol. 2016, 27, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Fazio, D.T.; Heckenlively, J.R.; Martin, D.A.; Christensen, R.E. The electroretinogram in advanced open-angle glaucoma. Doc. Ophthalmol. 1986, 63, 45–54. [Google Scholar] [CrossRef]
- Perlman, I. The electroretinogram: ERG by IDO Perlman. In Webvision: The Organization of the Retina and Visual System; Webvision: Online, 2015. [Google Scholar]
- Brown, K.T.; Wiesel, T.N. Localization of origins of electroretinogram components by intraretinal recording in the intact cat eye. J. Physiol. 1961, 158, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Poloschek, C.M. Electrophysiology and glaucoma: Current status and future challenges. Cell Tissue Res. 2013, 353, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The Development and Application of Optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Picaud, S.; Sahel, J.A.; Roska, B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012, 19, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, J. Optogenetics Restores Partial Vision in a Patient with Blindness. JAMA 2021, 326, 124. [Google Scholar] [CrossRef]
- Chen, R.; Gore, F.; Nguyen, Q.-A.; Ramakrishnan, C.; Patel, S.; Kim, S.H.; Raffiee, M.; Kim, Y.S.; Hsueh, B.; Krook-Magnusson, E.; et al. Deep brain optogenetics without intracranial surgery. Nat. Biotechnol. 2020, 39, 161–164. [Google Scholar] [CrossRef]
- Gong, X.; Mendoza-Halliday, D.; Ting, J.T.; Kaiser, T.; Sun, X.; Bastos, A.M.; Wimmer, R.D.; Guo, B.; Chen, Q.; Zhou, Y. An ultra-sensitive step-function opsin for minimally invasive optogenetic stimulation in mice and macaques. Neuron 2020, 107, 38–51.e8. [Google Scholar] [CrossRef]
- Gauvain, G.; Akolkar, H.; Chaffiol, A.; Arcizet, F.; Khoei, M.A.; Desrosiers, M.; Jaillard, C.; Caplette, R.; Marre, O.; Bertin, S.; et al. Optogenetic therapy: High spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 2021, 4, 125. [Google Scholar] [CrossRef]
- Jacobs, G.H. The distribution and nature of colour vision among the mammals. Biol. Rev. 1993, 68, 413–471. [Google Scholar] [CrossRef]
- Kishi, K.E.; Kim, Y.S.; Fukuda, M.; Inoue, M.; Kusakizako, T.; Wang, P.Y.; Ramakrishnan, C.; Byrne, E.F.; Thadhani, E.; Paggi, J.M.; et al. Structural basis for channel conduction in the pump-like channelrhodopsin ChRmine. Cell 2022, 185, 672–689.e23. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.C.; Choi, V.W.; Samulski, R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006, 1, 1412–1428. [Google Scholar] [CrossRef]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef]
- Robson, J.G.; Frishman, L.J. The rod-driven a-wave of the dark-adapted mammalian electroretinogram. Prog. Retin. Eye Res. 2013, 39, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, J.; Wright, T.; Westall, C.A. Rod a-wave analysis using high intensity flashes adds information on rod system function in 25% of clinical ERG recordings. Vis. Res. 2008, 48, 1920–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, S. Rods, cones, and the chemical basis of vision. Physiol. Rev. 1937, 17, 239–290. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Cheng, Y.; Zhou, S.; Wang, Q.; Monavarfeshani, A.; Gao, K.; Jiang, W.; Kawaguchi, R.; Wang, Q.; Tang, M.; et al. Core transcription programs controlling injury-induced neurodegeneration of retinal ganglion cells. Neuron 2022, 110, 2607–2624.e8. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, E.; Glynn, C.; Wang, Q.; Rao, S. Non-Invasive Electroretinogram Recording with Simultaneous Optogenetics to Dissect Retinal Ganglion Cells Electrophysiological Dynamics. Biosensors 2023, 13, 42. https://doi.org/10.3390/bios13010042
Hong E, Glynn C, Wang Q, Rao S. Non-Invasive Electroretinogram Recording with Simultaneous Optogenetics to Dissect Retinal Ganglion Cells Electrophysiological Dynamics. Biosensors. 2023; 13(1):42. https://doi.org/10.3390/bios13010042
Chicago/Turabian StyleHong, Eunji, Christopher Glynn, Qianbin Wang, and Siyuan Rao. 2023. "Non-Invasive Electroretinogram Recording with Simultaneous Optogenetics to Dissect Retinal Ganglion Cells Electrophysiological Dynamics" Biosensors 13, no. 1: 42. https://doi.org/10.3390/bios13010042
APA StyleHong, E., Glynn, C., Wang, Q., & Rao, S. (2023). Non-Invasive Electroretinogram Recording with Simultaneous Optogenetics to Dissect Retinal Ganglion Cells Electrophysiological Dynamics. Biosensors, 13(1), 42. https://doi.org/10.3390/bios13010042