Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles
Abstract
:1. Introduction
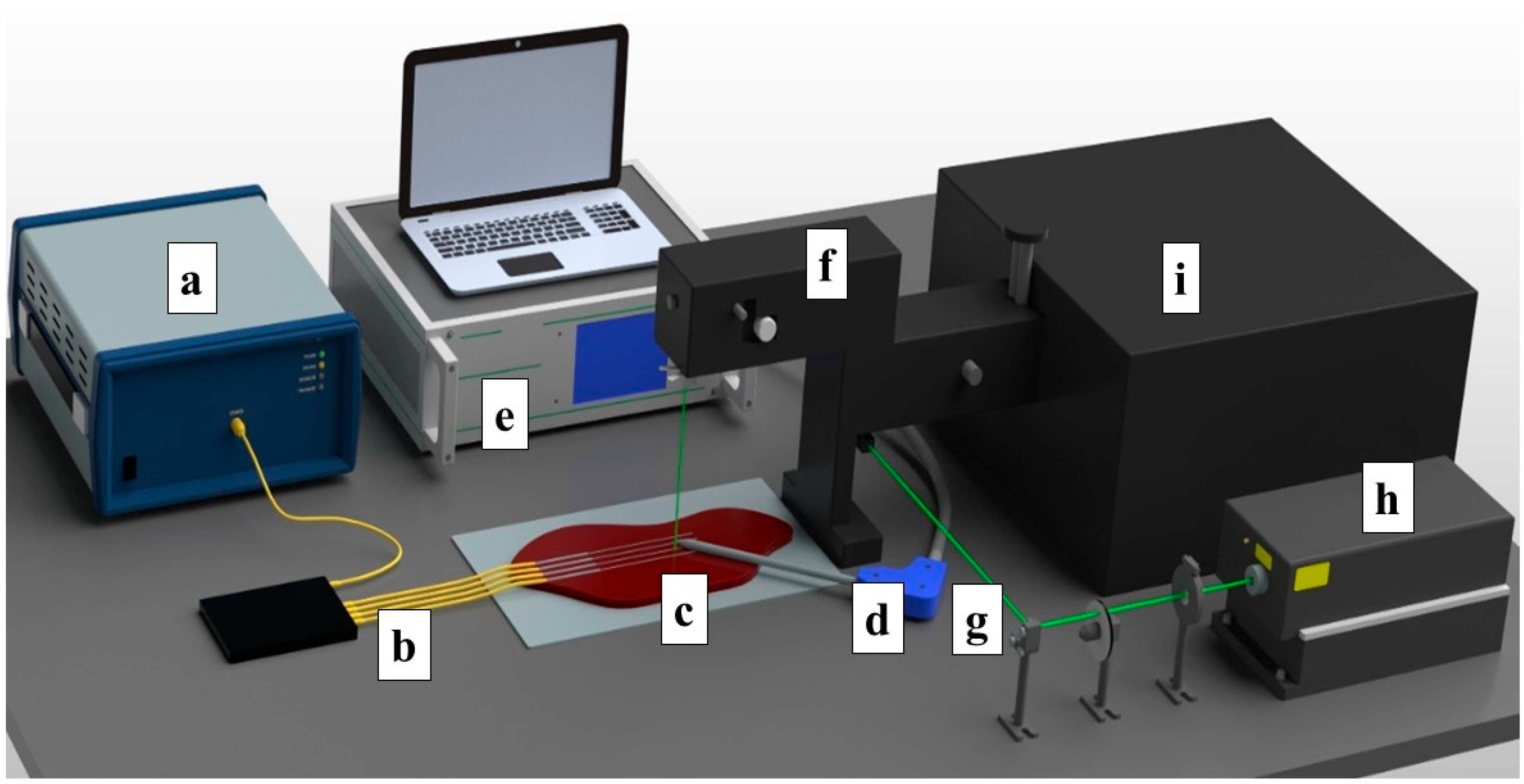
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stauffer, P.R.; Goldberg, S.N. Introduction: Thermal ablation therapy. Int. J. Hyperth. 2004, 20, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, J.I.; Raines, J.K. Radiofrequency ablation and laser ablation in the treatment of varicose veins. Ann. Vasc. Surg. 2006, 20, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Sharma, P.; Overholt, B.F.; Wolfsen, H.C.; Sampliner, R.E. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. Engl. J. Med. 2009, 360, 2277–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arsonval, M.A. Action physiologique des courants alternatifs. CR Soc. Biol. 1891, 43, 283–286. [Google Scholar]
- Rossi, S.; Fornari, F.; Pathies, C.; Buscarini, L. Thermal lesions induced by 480 kHz localized current field in guinea pig and pig liver. Tumori J. 1990, 76, 54–57. [Google Scholar] [CrossRef]
- Obara, K.; Matsumoto, N.; Okamoto, M.; Kobayashi, M.; Ikeda, H.; Takahashi, H.; Katakura, Y.; Matsunaga, K.; Ishii, T.; Okuse, C. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol. Int. 2008, 2, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, P.; Pisa, S. Specific Absorption Rate and Temperature Elevation in a Subject Exposed in the Far-Field of Radio-Frequency Sources Operating in the 10–900-MHz Range. IEEE Trans. Biomed. Eng. 2003, 50, 295–304. [Google Scholar] [CrossRef]
- Shao, Y.L.; Arjun, B.; Leo, H.L.; Chua, K.J. Nano-assisted radiofrequency ablation of clinically extracted irregularly shaped liver tumors. J. Therm. Biol. 2017, 66, 101–113. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A New Era for Cancer Treatment: Gold-Nanoparticle Mediated Thermal Therapies. Phototherm. Ther. 2011, 7, 169–183. [Google Scholar] [CrossRef]
- Guo, M.; Sun, Y.; Zhang, X. Enhanced radiation therapy of gold nanoparticles in liver cancer. Appl. Sci. 2017, 7, 232. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Huang, P.; Marjanovic, M.; Spillman, D.R.; Odintsov, B.M.; Boppart, S.A. Magnetomotive optical coherence elastography (MM-OCE) for thermal therapy dosimetry. In Proceedings of the 3rd Conference on Optical Elastography and Tissue Biomechanics III, San Francisco, CA, USA, 13–15 February 2016. [Google Scholar]
- Attar, M.M.; Haghpanahi, M.; Shahverdi, H.; Imam, A. Thermo-mechanical analysis of soft tissue in local hyperthermia treatment. J. Mech. Sci. Technol. 2016, 30, 1459–1469. [Google Scholar] [CrossRef]
- Ezzat, M.A. The effects of thermal and mechanical material properties on tumorous tissue during hyperthermia treatment. J. Therm. Biol. 2020, 92, 102649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lay, R.J.; Roberts, S.K.; Chauhan, S. Towards real-time finite-strain anisotropic thermo-visco-elastodynamic analysis of soft tissues for thermal ablative therapy. Comput. Methods Programs Biomed. 2021, 198, 105789. [Google Scholar] [CrossRef]
- Grant, S.A.; Zhu, J.; Gootee, J.; Snider, C.L.; Bellrichard, M.; Grant, D.A. Gold Nanoparticle-Collagen Gels for Soft Tissue Augmentation. Tissue Eng. Part A 2018, 24, 1091–1098. [Google Scholar] [CrossRef]
- Palombo, F.; Winlove, C.; Edginton, R.S.; Green, E.; Stone, N.; Caponi, S.; Madami, M.; Fioretto, D. Biomechanics of fibrous proteins of the extracellular matrix studied by Brillouin scattering. J. R. Soc. Interface 2014, 11, 20140739. [Google Scholar] [CrossRef] [Green Version]
- Hammad, H.; Ma, M.; Hydamaka, A.; Elkhedir, A.; Jin, G. Effect of Freeze and Re-freeze on Chemical Composition of Beef and Poultry Meat at Storage Period 4.5 Months (SP4.5). J. Food Process. Technol. 2019, 10, 791. [Google Scholar]
- DeWall, R.; Varghese, T.; Brace, C. Quantifying Local Stiffness Variations in Radiofrequency Ablations With Dynamic Indentation. IEEE Trans. Biomed. Eng. 2012, 59, 728–735. [Google Scholar] [CrossRef]
- Chan, J.; Omana, D.; Betti, M. Functional and rheological properties of proteins in frozen turkey breast meat with different ultimate pH. Poult. Sci. 2011, 90, 1112–1123. [Google Scholar] [CrossRef]
- Dileep, A.O.; Shamasundar, B.A.; Binsi, P.K.; Badii, F.; Howell, N.K. Effect of Ice Storage on the Physicochemical and Dynamic Viscoelastic Properties of Ribbonfish (Trichiurus spp.) Meat. J. Food Sci. 2005, 70, E537–E545. [Google Scholar] [CrossRef]
- Brunton, N.; Lyng, J.; Zhang, L.; Jacquier, J. The use of dielectric properties and other physical analyses for assessing protein denaturation in beef biceps femoris muscle during cooking from 5 to 85 °C. Meat Sci. 2006, 72, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Sapareto, S.; Dewey, W. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, S.; Wang, C.Y.; Ma, H.Y.; Lin, C.C.; Tsui, P.H. Monitoring Radiofrequency Ablation Using Real-Time Ultrasound Nakagami Imaging Combined with Frequency and Temporal Compounding Techniques. PLoS ONE 2015, 10, e0118030. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Martino, N.; Kwok, S.; Bernstein, L.; Yun, S. Label-free histological imaging of tissues using Brillouin light scattering contrast. Biomed. Opt. Express 2021, 12, 1437. [Google Scholar] [CrossRef]
- Fioretto, D.; Scarponi, F. Dynamics of a Glassy Polymer Studied by Brillouin Light Scattering. Mater. Sci. Eng. A 2009, 521, 243–246. [Google Scholar] [CrossRef]
- Almas, N.; Kurbanova, B.; Zhakiyev, N.; Rakhadilov, B.; Sagdoldina, Z.; Andybayeva, G.; Serik, N.; Alsar, Z.; Utegulov, Z.; Insepov, Z. Mechano-chemical properties of electron beam irradiated polyetheretherketone. Polymers 2022, 14, 3067. [Google Scholar] [CrossRef]
- Speziale, S.; Marquardt, H.; Duffy, T.S. Brillouin Scattering and its Application in Geosciences. Rev. Mineral. Geochem. 2014, 78, 543–603. [Google Scholar] [CrossRef]
- Akilbekova, D.; Ogay, V.; Yakupov, T.; Sarsenova, M.; Umbayev, B.; Nurakhmetov, A.; Tazhin, K.; Yakovlev, V.V.; Utegulov, Z.N. Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones. J. Biomed. Opt. 2018, 23, 097004. [Google Scholar] [CrossRef] [Green Version]
- Rakymzhan, A.; Yakupov, T.; Yelemessova, Z.; Bukasov, R.; Yakovlev, V.V.; Utegulov, Z.N. Time-resolved assessment of drying plants by Brillouin and Raman spectroscopies. J. Raman Spectrosc. 2019, 50, 1881–1889. [Google Scholar] [CrossRef]
- Gaipov, A.; Utegulov, Z.; Bukasov, R.; Turebekov, D.; Tarlykov, P.; Markhametova, Z.; Nurekeyev, Z.; Kunushpayeva, Z.; Sultangaziyev, A. Development and validation of hybrid Brillouin-Raman spectroscopy for non-contact assessment of mechano-chemical properties of urine proteins as biomarkers of kidney diseases. BMC Nephrol. 2020, 21, 229. [Google Scholar] [CrossRef] [PubMed]
- Aitekenov, S.; Abdirova, P.; Yussupova, L.; Sultangaziyev, A.; Gaipov, A.; Utegulov, Z.; Bukasov, R. Raman, Infrared and Brillouin spectroscopies of biofluids for medical diagnostics and for detection of biomarkers. Crit. Rev. Anal. Chem. 2022, 1–30. [Google Scholar]
- Antonacci, G.; Braakman, S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 2016, 6, 37217. [Google Scholar] [CrossRef] [PubMed]
- Elsayad, K.; Werner, S.; Gallemi, M.; Kong, J.; Guajardo, E.; Zhang, L.; Jaillais, Y.; Greb, T.; Belkhadir, Y. Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission–Brillouin imaging. Sci. Signal. 2016, 9, rs5. [Google Scholar] [CrossRef]
- Troyanova-Wood, M.; Meng, Z.; Yakovlev, V.V. Differentiating melanoma and healthy tissues based on elasticity-specific Brillouin microspectroscopy. Biomed. Opt. Express 2019, 10, 1774–1781. [Google Scholar] [CrossRef]
- Harley, R.; James, D.; Miller, A.; White, J.W. Phonons and the elastic moduli of collagen and muscle. Nature 1977, 267, 285–287. [Google Scholar] [CrossRef]
- Cusack, S.; Miller, A. Determination of the Elastic Constants of Collagen by Brillouin Light Scattering. J. Mol. Biol. 1979, 135, 39–51. [Google Scholar] [CrossRef]
- Berovic, N.; Thomas, N.; Thornhill, R.A.; Vaughan, J.M. Observation of Brillouin scattering from single muscle fibres. Eur. Biophys. J. 1989, 17, 69–74. [Google Scholar] [CrossRef]
- Yoshihara, A.; Miyazaki, A.; Maeda, T.; Fukushima, M.; Abe, T. Brillouin Light Scattering from Thin Albumen of Chicken Egg. J. Phys. Soc. Jpn. 2010, 79, 125001. [Google Scholar] [CrossRef]
- Beisenova, A.; Issatayeva, A.; Sovetov, S.; Korganbayev, S.; Jebuldina, M.; Ashikbayeva, Z.; Blanc, W.; Schena, E.; Sales, S.; Moraldi, C.; et al. Multi-fiber distributed thermal profiling of minimally invasive thermal ablation with scattering-level multiplexing in MgO-doped fibers. Biomed. Opt. Express 2019, 10, 1282–1296. [Google Scholar] [CrossRef]
- Martines-Arano, H.; Palacios-Barreto, S.; Castillo-Cruz, J.; Meda-Campana, J.A.; García-Pérez, B.E.; Torres-Torres, C. Fractional photodamage triggered by chaotic attractors in human lung epithelial cancer cells. Int. J. Therm. Sci. 2022, 181, 107734. [Google Scholar] [CrossRef]
- Coker, Z.; Troyanova-Wood, M.; Traverso, A.J.; Yakupov, T.; Utegulov, Z.; Yakovlev, V.V. Assessing performance of modern Brillouin spectrometers. Opt. Express 2018, 26, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Ashikbayeva, Z.; Aitkulov, A.; Jelbuldina, M.; Issatayeva, A.; Beisenova, A.; Molardi, C.; Saccomandi, P.; Blanc, W.; Inglezakis, V.; Tosi, D. Distributed 2D temperature sensing during nanoparticles assisted laser ablation by means of high-scattering fiber sensors. Sci. Rep. 2020, 10, 12593. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J. Colloidal Gold: Part II Colour, Coagulation, Adhesion, Alloying and Catalytic Properties. Gold Bull. 1985, 18, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Bouchard, R.; Sokolov, K. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed. Opt. Express 2019, 10, 3472–3483. [Google Scholar] [CrossRef] [PubMed]
- Sametova, A.; Kurmashev, S.; Ashikbayeva, Z.; Amantayeva, A.; Blanc, W.; Atabaev, T.S.; Tosi, D. Fiber-Optic Distributed Sensing Network for Thermal Mapping of Gold Nanoparticles-Mediated Radiofrequency Ablation. Biosensors 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Sapin-De Brosses, E.; Gennisson, J.; Pernot, M.; Fink, M.; Tanter, M. Temperature dependence of the shear modulus of soft tissues assessed by ultrasound. Phys. Med. Biol. 2010, 55, 1701–1718. [Google Scholar] [CrossRef]
- Pervin, F.; Chena, W.; Weerasooriya, T. Dynamic compressive response of bovine liver tissues. J. Mech. Behav. Biomed. Mater. 2011, 4, 76–84. [Google Scholar] [CrossRef]
- Liu, Z.; Bilston, L. On the Viscoelastic Character of Liver Tissue: Experiments and Modelling of the Linear Behaviour. Biorheology 2000, 37, 191–201. [Google Scholar]
- Bianchi, L.; Cavarzan, F.; Ciampitti, L.; Cremonesi, M.; Grilli, F.; Saccomandi, P. Thermophysical and mechanical properties of biological tissues as a function of temperature: A systematic literature review. Int. J. Hyperth. 2022, 39, 297–340. [Google Scholar] [CrossRef]
- Tornberg, E. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Lin, S.; Chang, Y.; Chen, M.; Hung, S. Determining the critical effective temperature and heat dispersal pattern in monopolar radiofrequency ablation using temperature-time integration. Exp. Ther. Med. 2016, 11, 763–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, M.Z.; Daniels, M.J.; Varghese, T. Investigation of temperature-dependent viscoelastic properties of thermal lesions in ex vivo animal liver tissue. J. Biomech. 2009, 42, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wex, C.; Arndt, S.; Brandstadter, K.; Hermann, L.; Bruns, C. Biomechanical Characterization of Material Properties of Porcine Liver After Thermal Treatment. Soft Mater. 2014, 12, 411–419. [Google Scholar] [CrossRef]
- Li, H.; Flé, G.; Bhatt, M.; Qu, Z.; Ghazavi, S.; Yazdani, L.; Bosio, G.; Rafati, I.; Cloutier, G. Viscoelasticity Imaging of Biological Tissues and Single Cells Using Shear Wave Propagation. Front. Phys. 2021, 9, 666192. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbanova, B.; Ashikbayeva, Z.; Amantayeva, A.; Sametova, A.; Blanc, W.; Gaipov, A.; Tosi, D.; Utegulov, Z. Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles. Biosensors 2023, 13, 8. https://doi.org/10.3390/bios13010008
Kurbanova B, Ashikbayeva Z, Amantayeva A, Sametova A, Blanc W, Gaipov A, Tosi D, Utegulov Z. Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles. Biosensors. 2023; 13(1):8. https://doi.org/10.3390/bios13010008
Chicago/Turabian StyleKurbanova, Bayan, Zhannat Ashikbayeva, Aida Amantayeva, Akbota Sametova, Wilfried Blanc, Abduzhappar Gaipov, Daniele Tosi, and Zhandos Utegulov. 2023. "Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles" Biosensors 13, no. 1: 8. https://doi.org/10.3390/bios13010008
APA StyleKurbanova, B., Ashikbayeva, Z., Amantayeva, A., Sametova, A., Blanc, W., Gaipov, A., Tosi, D., & Utegulov, Z. (2023). Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles. Biosensors, 13(1), 8. https://doi.org/10.3390/bios13010008







