A Fiber Bragg Grating Sensor Based on Cladding Mode Resonance for Label-Free Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Antibody Immobilization
2.3. GO Deposition and Antibody Immobilization
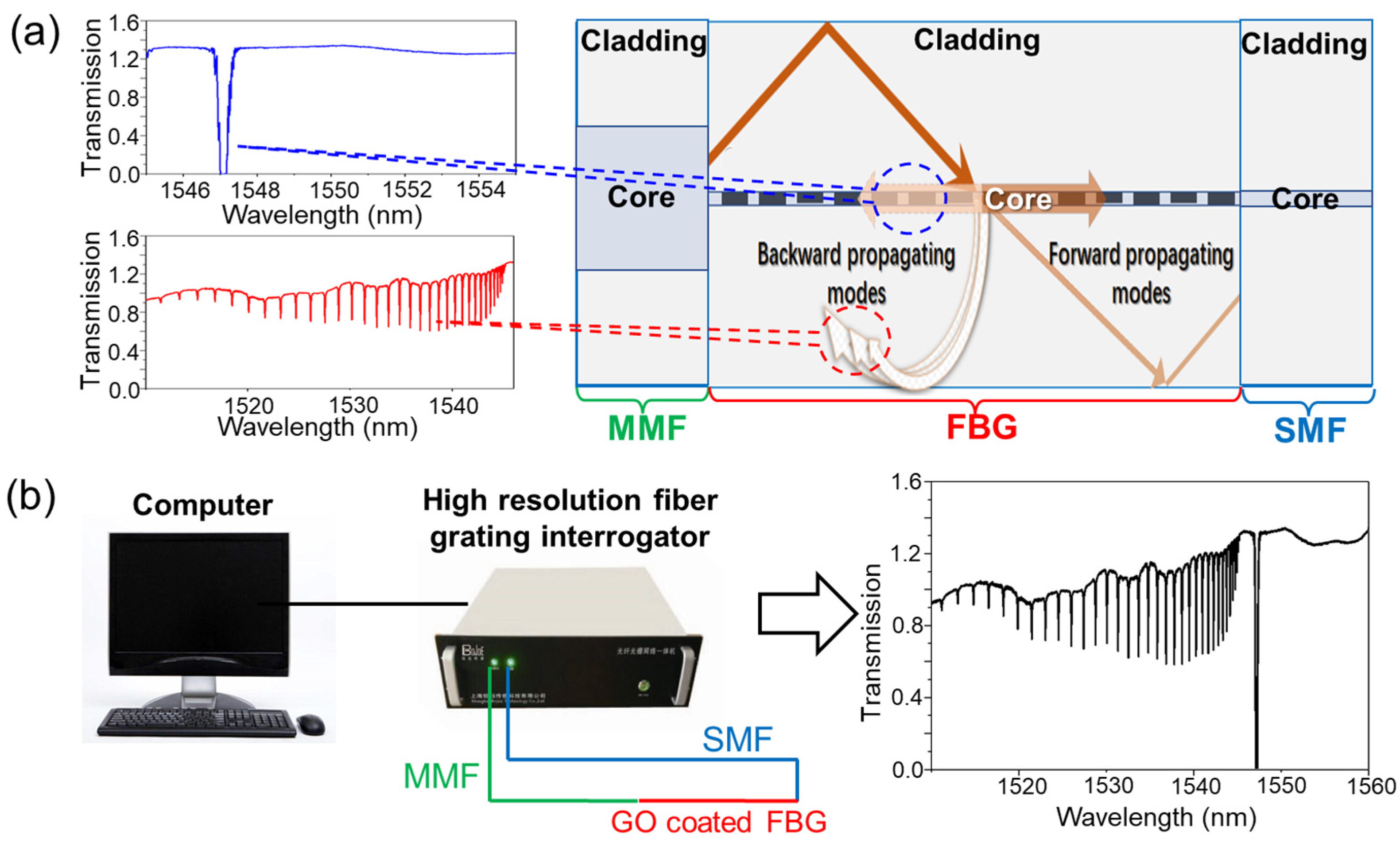
2.4. Sensor Preparation and Interrogation
3. Results and Discussion
3.1. Sensing Characteristics of the Sensing Probe
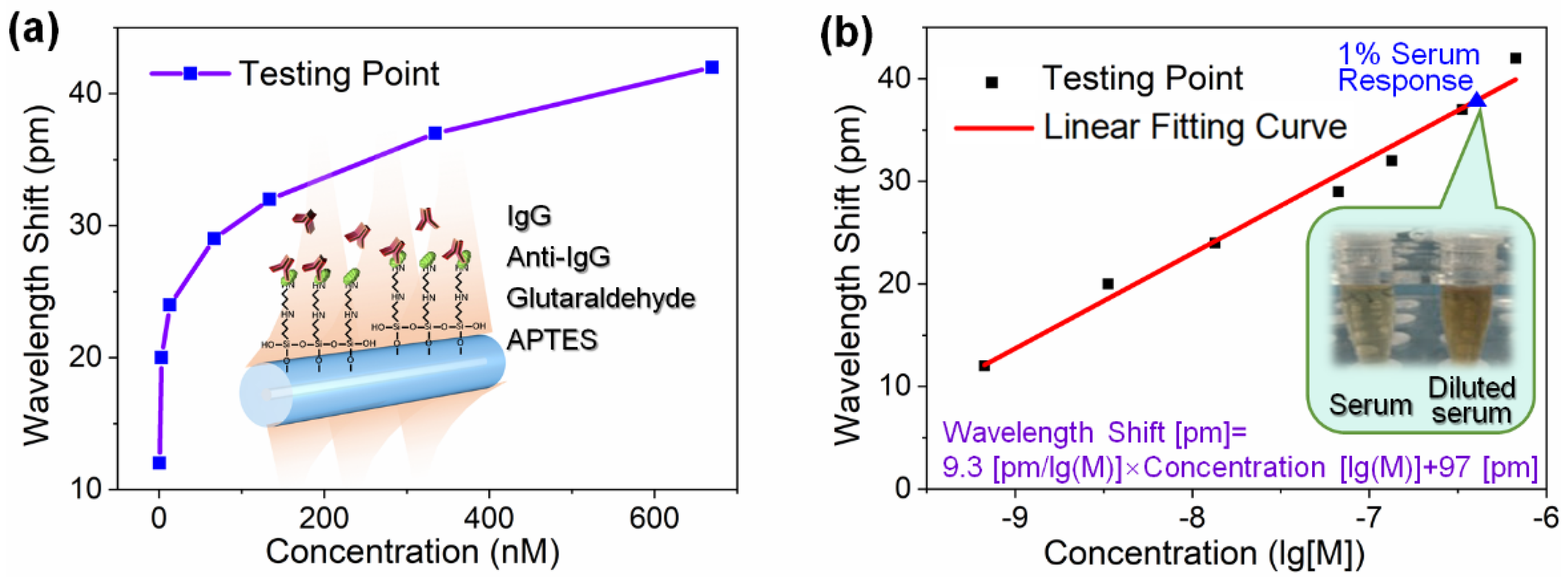
3.2. Protein Detection Using the Sensing System
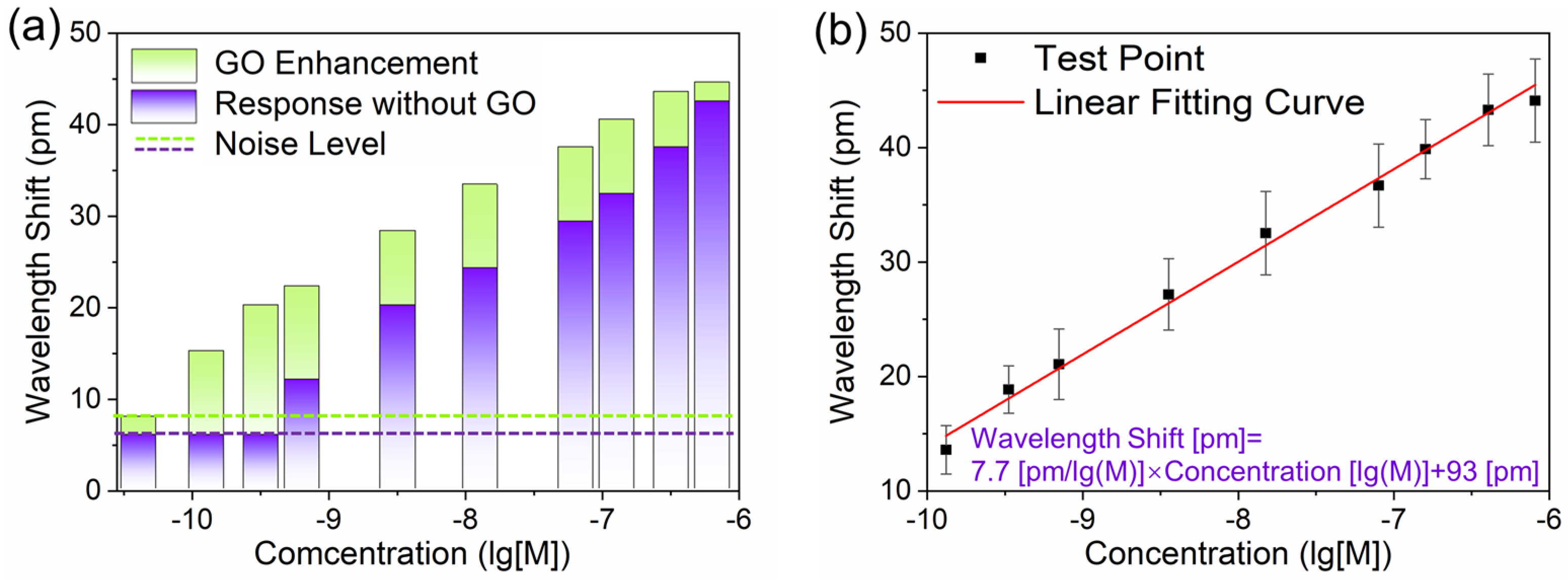
3.3. GO-HRI Layer and Sensitivity Enhancement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Yu, Y.; Li, X.; Zhou, H.; Hong, X.; Geng, Y. Fiber-optic urine specific gravity sensor based on surface plasmon resonance. Sens. Actuators B Chem. 2016, 226, 412–418. [Google Scholar] [CrossRef]
- Yin, M.J.; Gu, B.; An, Q.F.; Yang, C.; Guan, Y.L.; Yong, K.T. Recent development of fiber-optic chemical sensors and biosensors: Mechanisms, materials, micro/nanofabrications and applications. Coord. Chem. Rev. 2018, 376, 348–392. [Google Scholar] [CrossRef]
- Principe, M.; Consales, M.; Micco, A.; Crescitelli, A.; Castaldi, G.; Esposito, E.; La Ferrara, V.; Cutolo, A.; Galdi, V.; Cusano, A. Optical fibre meta-tips. Light Sci. Appl. 2017, 6, e16226. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, P.; Carotenuto, B.; Pisco, M.; Ricciardi, A.; Quero, G.; Consales, M.; Crescitelli, A.; Esposito, E.; Cusano, A. Lab on Fibre Technology for biological sensing applications. Laser Photonics Rev. 2016, 10, 922–961. [Google Scholar] [CrossRef]
- Jiang, N.; Ahmed, R.; Rifat, A.A.; Guo, J.; Yin, Y.; Montelongo, Y.; Butt, H.; Yetisen, A.K. Functionalized flexible soft polymer optical fibres for laser photomedicine. Adv. Opt. Mater. 2018, 6, 1701118. [Google Scholar] [CrossRef]
- Carotenuto, B.; Ricciardi, A.; Micco, A.; Amorizzo, E.; Mercieri, M.; Cutolo, A.; Cusano, A. Smart optical catheters for epidurals. Sensors 2018, 18, 2101. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pandey, A.K.; Kaur, B. A review of advancements (2007–2017) in plasmonics-based optical fiber sensors. Opt. Fiber Technol. 2018, 43, 20–34. [Google Scholar] [CrossRef]
- Gupta, B.D.; Kant, R. Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Li, Z.; Liao, C.; Chen, D.; Song, J.; Jin, W.; Peng, G.D.; Zhu, F.; Wang, Y.; He, J.; Wang, Y. Label-free detection of bovine serum albumin based on an in-fibre MachZehnder interferometric biosensor. Opt. Express 2017, 25, 17105. [Google Scholar] [CrossRef]
- Chen, L.H.; Ang, X.M.; Chan, C.C.; Shaillender, M.; Neu, B.; Wong, W.C.; Zu, P.; Leong, K.C. Layer-By-Layer (Chitosan/Polystyrene Sulfonate) Membrane-Based Fabry-Perot Interferometric Fiber Optic Biosensor. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1457–1464. [Google Scholar] [CrossRef]
- Silva, S.; Pachon, E.G.P.; Franco, M.A.R.; Hayashi, J.G.; Malcata, F.X.; Frazão, O.; Jorge, P.; Cordeiro, C.M.B. Ultrahigh-sensitivity temperature fibre sensor based on multimode interference. Appl. Opt. 2012, 51, 3236–3242. [Google Scholar] [CrossRef] [PubMed]
- Socorro, A.B.; Del Villar, I.; Corres, J.M.; Arregui, F.J.; Matias, I.R. Sensitivity enhancement in a multimode interference-based SMS fibre structure coated with a thin-film: Theoretical and experimental study. Sens. Actuators B Chem. 2014, 190, 363–369. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, S.; Lee, D.; Oh, K. Compact three segmented multimode fibre modal interferometer for high sensitivity refractive-index measurement. Meas. Sci. Technol. 2006, 17, 1129. [Google Scholar] [CrossRef]
- Hu, D.J.J.; Lim, J.L.; Park, M.K.; Kao, L.T.H.; Wang, Y.; Wei, H.; Tong, W. Photonic crystal fibre-based interferometric biosensor for streptavidin and biotin detection. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1293–1297. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.M. A comprehensive review of lossy mode resonance-based fibre optic sensors. Opt. Lasers Eng. 2018, 100, 47–60. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.N.; Yang, M.; Hong, W.; Lu, P. Refractive index sensor based on a microhole in single-mode fiber created by the use of femtosecond laser micromachining. Opt. Lett. 2009, 34, 3328–3330. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.D.; Sun, L.P.; Guo, T.; Guan, B. Label-free thrombin detection using a tapered fiber-optic interferometric aptasensor. J. Light. Technol. 2018, 37, 2756–2761. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, W.; Li, Z.; Chen, G.; Chen, L.; Zhou, J.; Lin, H.; Guan, J.; Fang, W.; Liu, X. High-performance fibre-optic humidity sensor based on a side-polished fibre wavelength selectively coupled with graphene oxide film. Sens. Actuators B Chem. 2018, 255, 57–69. [Google Scholar] [CrossRef]
- Yadav, T.K.; Narayanaswamy, R.; Abu Bakar, M.H.; Kamil, Y.M.; Mahdi, M.A. Single mode tapered fibre-optic interferometer based refractive index sensor and its application to protein sensing. Opt. Express 2014, 22, 22802–22807. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Dukenbayev, K.; Shaimerdenova, M.; Bekniyazov, I.; Ayupova, T.; Sypabekova, M.; Molardi, C.; Tosi, D. Etched fiber Bragg grating biosensor functionalized with aptamers for detection of thrombin. Sensors 2018, 18, 4298. [Google Scholar] [CrossRef]
- Del Villar, I. Ultrahigh-sensitivity sensors based on thin-film coated long period gratings with reduced diameter, in transition mode and near the dispersion turning point. Opt. Express 2015, 23, 8389. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Albert, J.; Caucheteur, C.; Wattiez, R. Cytokeratins biosensing using tilted fiber gratings. Biosensors 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Zhang, Y.F.; Zhang, M.X.; Leung, L.M.G.; Liu, L.Q. Application of FBG sensors for geotechnical health monitoring, a review of sensor design, implementation methods and packaging techniques. Sens. Actuators A Phys. 2016, 244, 184–197. [Google Scholar] [CrossRef]
- Campanella, C.E.; Cuccovillo, A.; Campanella, C.; Yurt, A.; Passaro, V. Fibre Bragg grating based strain sensors: Review of technology and applications. Sensors 2018, 18, 3115. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.O.; Meltz, G. Fiber Bragg grating technology fundamentals and overview. J. Light. Technol. 1997, 15, 1263–1276. [Google Scholar] [CrossRef]
- Othonos, A. Fiber bragg gratings. Rev. Sci. Instrum. 1997, 68, 4309–4341. [Google Scholar] [CrossRef]
- Fonseca, J.P.T.D.; Gondek, J.T.; Possetti, G.R.C.; Muller, M.; Luís, J.; Kamikawachi, R.C. Optical sensor based on etched fiber Bragg gratings for assessment of biodiesel quality. In Proceedings of the 2011 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC 2011), Natal, Brazil, 29 October–1 November 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 458–460. [Google Scholar]
- Shivananju, B.N.; Renilkumar, M.; Prashanth, G.R.; Asokan, S.; Varma, M.M. Detection limit of etched fiber Bragg grating sensors. J. Light. Technol. 2013, 31, 2441–2447. [Google Scholar] [CrossRef]
- Albert, J.; Shao, L.Y.; Caucheteur, C. Tilted fibre Bragg grating sensors. Laser Photonics Rev. 2013, 7, 83–108. [Google Scholar] [CrossRef]
- Chan, C.F.; Chen, C.; Jafari, A.; Laronche, A.; Thomson, D.J.; Albert, J. Optical Fibre Refractometer Using Narrowband Cladding-Mode Resonance Shifts. Appl. Opt. 2007, 46, 1142–1149. [Google Scholar] [CrossRef]
- Guo, T.; Liu, F.; Liang, X.; Qiu, X.; Huang, Y.; Xie, C.; Xu, P.; Mao, W.; Guan, B.O.; Albert, J. Highly sensitive detection of urinary protein variations using tilted fibre grating sensors with plasmonic nanocoatings. Biosens. Bioelectron. 2016, 78, 221–228. [Google Scholar] [CrossRef]
- Carrasquilla, C.; Xiao, Y.; Xu, C.Q.; Li, Y.; Brennan, J.D. Enhancing sensitivity and selectivity of long-period grating sensors using structure-switching aptamers bound to gold-doped macroporous silica coatings. Anal. Chem. 2011, 83, 7984–7991. [Google Scholar] [CrossRef] [PubMed]
- Anemogiannis, E.; Glytsis, E.N.; Gaylord, T.K. Transmission characteristics of longperiod fibre gratings having arbitrary azimuthal/radial refractive index variations. J. Light. Technol. 2003, 21, 218–227. [Google Scholar] [CrossRef]
- Cusano, A.; Iadicicco, A.; Pilla, P.; Contessa, L.; Campopiano, S.; Cutolo, A.; Giordano, M. Mode transition in high refractive index coated long period gratings. Opt. Express 2006, 14, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Villar, I.D.; Achaerandio, M.; Matías, I.R.; Arregui, F.J. Deposition of overlays by electrostatic self-assembly in long-period fibre gratings. Opt. Lett. 2005, 30, 720. [Google Scholar] [CrossRef]
- Nemova, G.; Kashyap, R. Fiber-Bragg-grating-assisted surface plasmon-polariton sensor. Opt. Lett. 2006, 31, 2118–2120. [Google Scholar] [CrossRef]
- Lu, Y.C.; Huang, W.P.; Jian, S.S. Full vector complex coupled mode theory for tilted fiber atings. Opt. Express 2010, 18, 713–726. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.H.; Byun, J.Y.; Kim, J.H.; Kim, M.G. An optical fibre-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, K.; Zhang, L.; Bennion, I. Dual-peak long-period fibre gratings with enhanced refractive index sensitivity by finely tailored mode dispersion that uses the light cladding etching technique. Appl. Opt. 2007, 46, 451–455. [Google Scholar] [CrossRef]
- Jia, P.; Yang, J. A plasmonic optical fibre patterned by template transfer as a high performance flexible nanoprobe for real-time biosensing. Nanoscale 2014, 6, 8836–8843. [Google Scholar] [CrossRef]
- Cardona-Maya, Y.; Socorro, A.B.; Del Villar, I.; Cruz, J.L.; Corres, J.M.; Botero-Cadavid, J.F. Label-free wavelength and phase detection based SMS fibre immunosensors optimized with cladding etching. Sens. Actuators B Chem. 2018, 265, 10–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Qian, S.; Liu, Z.; Wang, Q.; Gu, Y.; Wu, Z.; Jing, Z.; Sun, C.; Peng, W. A novel fibre optic surface plasmon resonance biosensors with special boronic acid derivative to detect glycoprotein. Sensors 2017, 17, 2259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Min, D.H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Levy, Y.; Vansteenkiste, N.; Aspect, A.; Seifert, W.; Leipold, D.; Mlynek, J. Resonant enhancement of evanescent waves with a thin dielectric waveguide. Opt. Commun. 1994, 104, 234–240. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface plasmon resonance biosensor based on smart phone platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.Y.; Wang, B.T. Highly sensitive SPR biosensor based on graphene oxide and staphylococcal protein a co-modified TFBG for human IgG detection. IEEE T. Instrum. Meas. 2018, 68, 3350–3357. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Yu, Q.; Peng, W. Microcapillary-based integrated LSPR device for refractive index detection and biosensing. J. Light. Technol. 2020, 38, 2485–2492. [Google Scholar] [CrossRef]
- Juste-Dolz, A.; Delgado-Pinar, M.; Avella-Oliver, M.; Fernández, E.; Pastor, D.; Andrés, M.V.; Maquieira, Á. BIO Bragg gratings on microfibers for label-free biosensing. Biosens. Bioelectron. 2021, 176, 112916. [Google Scholar] [CrossRef]
- Wang, B.T.; Wang, Q. An interferometric optical fiber biosensor with high sensitivity for IgG/anti-IgG immunosensing. Opt. Commun. 2018, 426, 388–394. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Guo, J.; Liu, W.; Liu, L.; Meng, Y.; Yu, X. Temperature-Insensitive Label-Free Sensors for Human IgG Based on S-Tapered Optical Fiber Sensors. IEEE Access 2021, 9, 116286–116293. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Biswas, P.; Trono, C.; Jana, S.; Bandyopadhyay, S.; Basumallick, N.; Giannetti, A.; Tombelli, S.; Bera, S.; Mallick, A.; et al. Sol–gel-based titania–silica thin film overlay for long period fiber grating-based biosensors. Anal. Chem. 2015, 87, 12024–12031. [Google Scholar] [CrossRef]





| Sensor | Molecule | LOD | Ref. |
|---|---|---|---|
| SPR image sensor | IgG | 47.4 nM | [45] |
| TFBG-SPR sensor based on GO | IgG | 0.5 μg/mL (3.35 nM) | [46] |
| Microcapillary-based LSPR sensor | IgG | 3 nM | [47] |
| Bragg grating sensor based on tapered microfiber | IgG | 0.1 μg/mL (667 pM) | [48] |
| FBG sensor based on cladding mode resonance | IgG | 360 pM | This work |
| MZI sensor with large core-offset fusion splice | IgG | 47 ng/mL (313 pM) | [49] |
| MZI sensor with S-tapered optical fiber | IgG | 28 ng/mL (186 pM) | [50] |
| LPG sensor based on titania–silica thin film overlay | IgG | 53 pM | [51] |
| FBG sensor based on cladding mode resonance with GO-coating | IgG | 32 pM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, C.; Wang, J.; Li, N.; Song, Y.; Wu, H.; Liu, Y. A Fiber Bragg Grating Sensor Based on Cladding Mode Resonance for Label-Free Biosensing. Biosensors 2023, 13, 97. https://doi.org/10.3390/bios13010097
Chen S, Zhang C, Wang J, Li N, Song Y, Wu H, Liu Y. A Fiber Bragg Grating Sensor Based on Cladding Mode Resonance for Label-Free Biosensing. Biosensors. 2023; 13(1):97. https://doi.org/10.3390/bios13010097
Chicago/Turabian StyleChen, Shimeng, Chao Zhang, Jiahui Wang, Na Li, Yongxin Song, Haojun Wu, and Yun Liu. 2023. "A Fiber Bragg Grating Sensor Based on Cladding Mode Resonance for Label-Free Biosensing" Biosensors 13, no. 1: 97. https://doi.org/10.3390/bios13010097
APA StyleChen, S., Zhang, C., Wang, J., Li, N., Song, Y., Wu, H., & Liu, Y. (2023). A Fiber Bragg Grating Sensor Based on Cladding Mode Resonance for Label-Free Biosensing. Biosensors, 13(1), 97. https://doi.org/10.3390/bios13010097





