Development of a Novel H-Shaped Electrochemical Aptasensor for Detection of Hg2+ Based on Graphene Aerogels–Au Nanoparticles Composite
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Instrumentation
2.3. Fabrication of the GAs-AuNPs Sensing Electrode
2.4. Fabrication of the Hg2+ Aptasensor
2.5. Pretreatment of Real Samples
3. Results and Discussion
3.1. Characterization of Morphology and Composition of GAs-AuNPs
3.2. Electrochemical Characterization of the Hg2+ Aptasensor
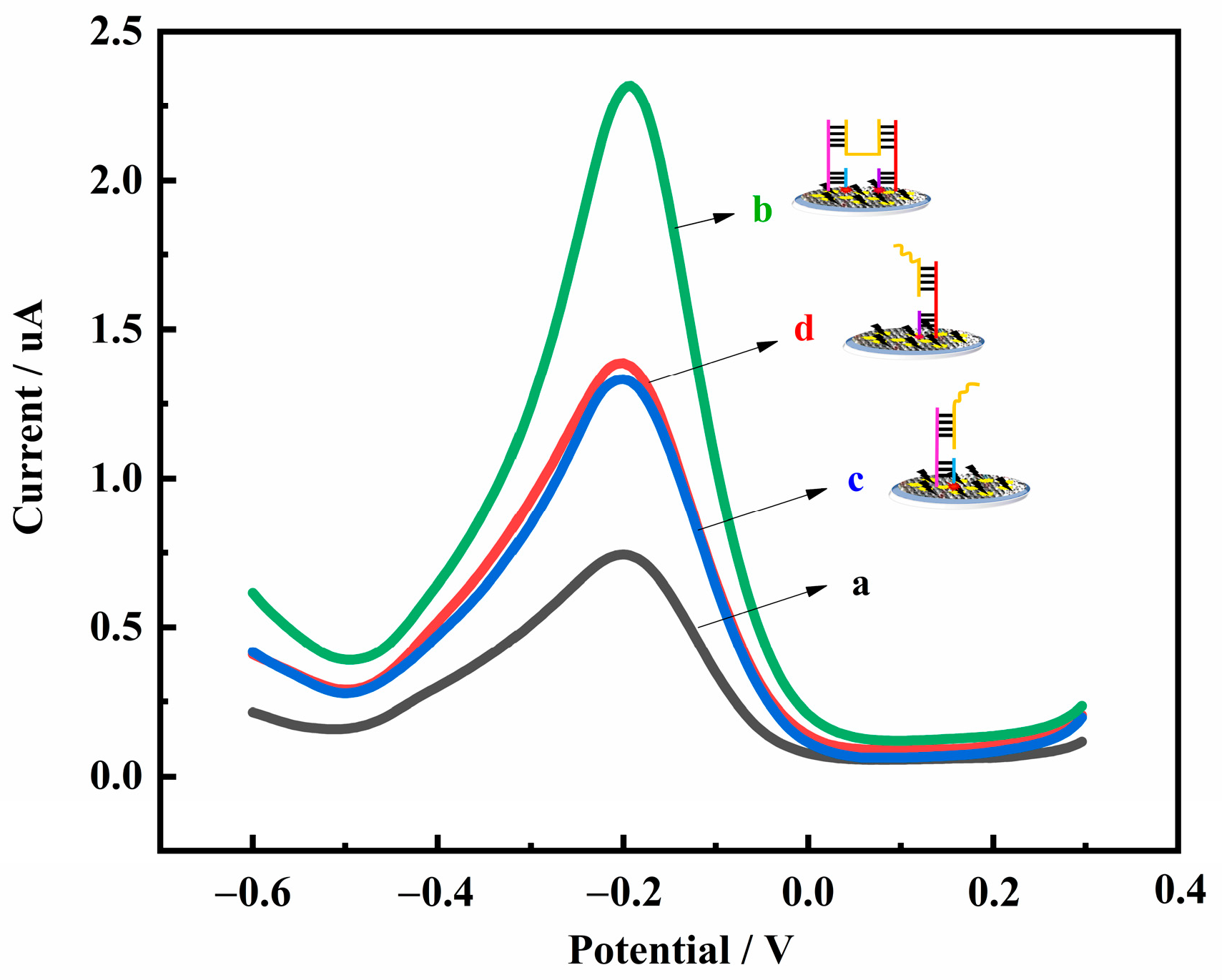
3.3. Signal Amplification Using H-Shaped Structure
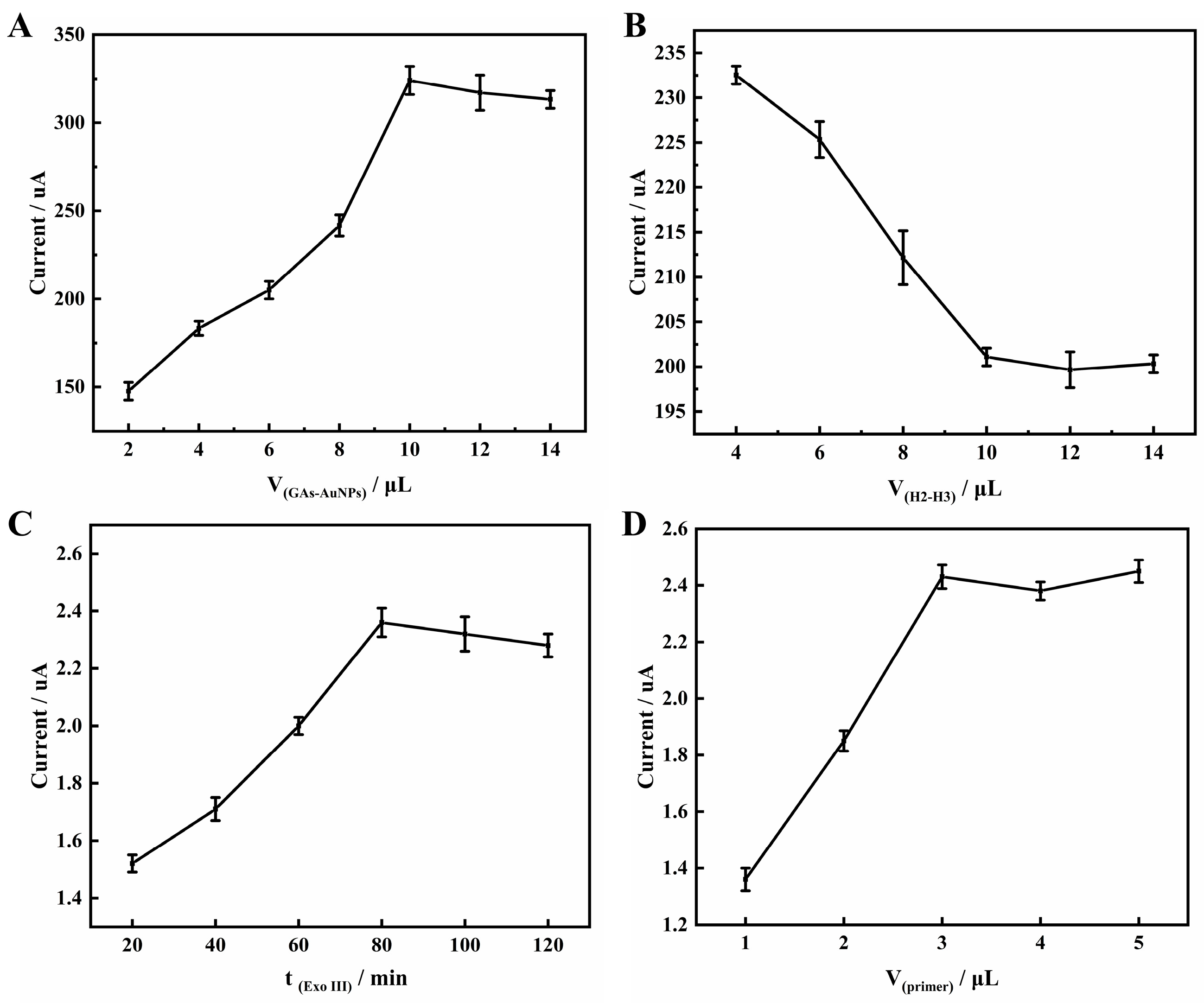
3.4. Optimization of Experimental Conditions
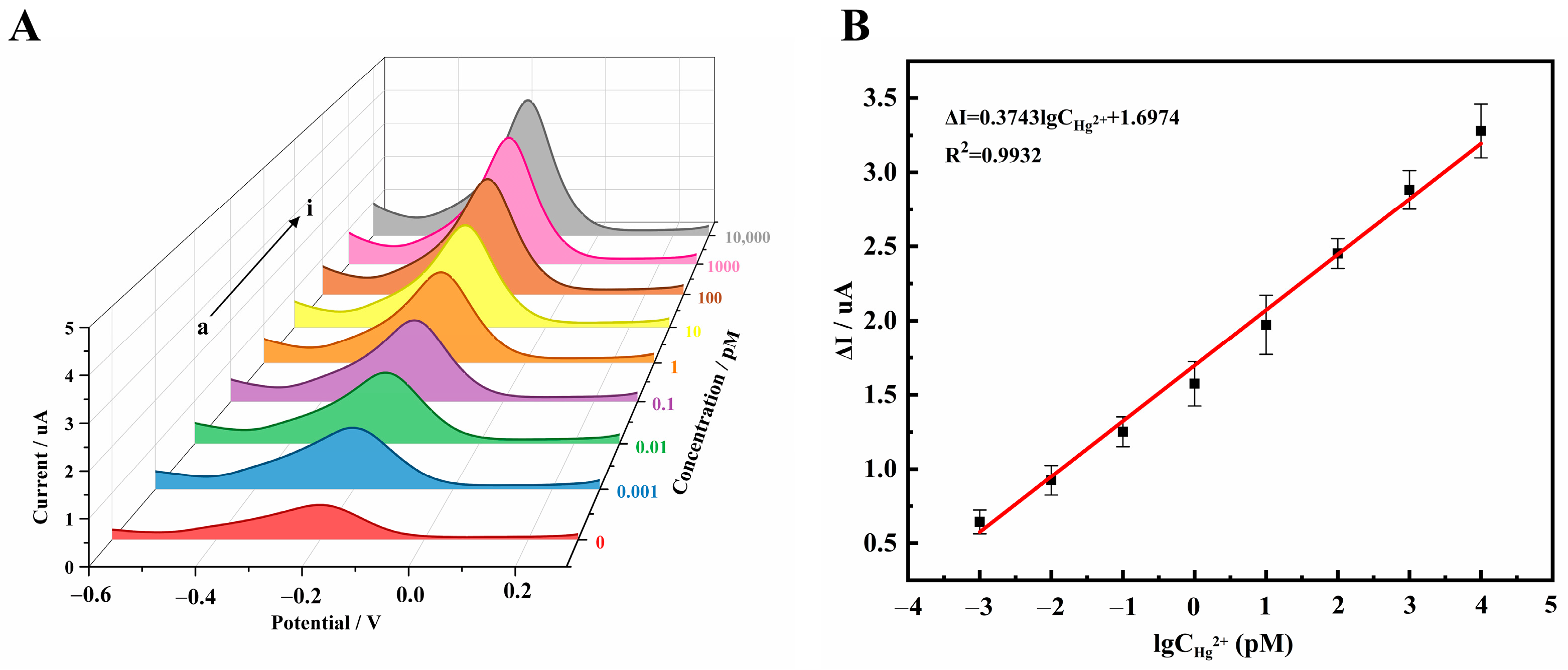
3.5. Detection Performances of Hg2+ Electrochemical Sensor
3.6. Specificity, Reproducibility, and Stability of the Electrochemical Biosensor
3.7. Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef]
- Yin, K.; Lv, M.; Wang, Q.; Wu, Y.; Liao, C.; Zhang, W.; Chen, L. Simultaneous bioremediation and biodetection of mercury ion through surface display of carboxylesterase E2 from Pseudomonas aeruginosa PA1. Water Res. 2016, 103, 383–390. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, W. Simultaneous electrochemical detection of multiple heavy metal ions in milk based on silica-modified magnetic nanoparticles. Food Chem. 2023, 406, 135034. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; Cerveira, C.; Miceli, T.M.; Moraes, D.P.; Mesko, M.F.; Pereira, J.S.F. Evaluation of sample preparation methods for cereal digestion for subsequent As, Cd, Hg and Pb determination by M-based techniques. Food Chem. 2020, 321, 126715. [Google Scholar] [CrossRef]
- Tangsuwanjinda, S.; Chen, Y.Y.; Lai, C.H.; Jhou, G.T.; Chiang, Y.W.; Cheng, H.M. Microporous oxide-based surface-enhanced Raman scattering film for quadrillionth detection of mercury ion (II). Processes 2021, 9, 794. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Gong, Z.; Wang, X.; Yang, Y.; Wang, Y. Determination of trace elements in samples with high salt content by inductively coupled plasma mass spectrometry after solid-phase preconcentration. Int. J. Mass Spectrom. 2018, 431, 22–26. [Google Scholar] [CrossRef]
- Sorouraddin, S.M.; Farajzadeh, M.A.; Dastoori, H. Development of a dispersive liquid-liquid microextraction method based on a ternary deep eutectic solvent as chelating agent and extraction solvent for preconcentration of heavy metals from milk samples. Talanta 2020, 208, 120485. [Google Scholar] [CrossRef]
- Che, S.; Yin, L.; Chen, M.; Fan, Y.; Xu, A.; Zhou, C.; Fu, H.; She, Y. Real-time monitoring of mercury (II) in water and food samples using a quinoline-based ionic probe. Food Chem. 2023, 407, 135052. [Google Scholar] [CrossRef]
- Khani, H.; Abbasi, S.; Yaraki, M.T.; Tan, Y.N. A naked-eye colorimetric assay for detection of Hg2+ ions in real water samples based on gold nanoparticles-catalyzed clock reaction. J. Mol. Liq. 2022, 345, 118243. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, Y.; Li, Y.; Tang, L.; Yan, X. A new aggregation-induced emission-based fluorescent probe for effective detection of Hg2+ and its multiple applications. Chin. Chem. Lett. 2023, 34, 108237. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Li, N.; Liu, X.; Ma, Y.; Luo, H.; Hou, C.; Huo, D. An ultrasensitive electrochemical sensor based on antimonene simultaneously detect multiple heavy metal ions in food samples. Food Chem. 2023, 421, 136131. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.H.; Park, K.; Kim, S.K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interfac. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zheng, Y.; Xu, Y.; Zheng, Z.; Chen, X.; Liu, W. Versatile aerogels for sensors. Small 2019, 15, 1902826. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.W.; Wang, J. Graphene aerogel-metal-organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Zuo, W.; Chen, F.; Wang, B. 3D MoS2 composition aerogels as chemosensors and adsorbents for colorimetric detection and high-capacity adsorption of Hg2+. ACS Sustain. Chem. Eng. 2016, 4, 3398–3408. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew. Chem. Int. Ed. 2004, 116, 4400–4402. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Jiang, Y.; Jiang, Y.; Shen, J.; Han, E. Structure-switching electrochemical aptasensor for single-step and specific detection of trace mercury in dairy products. J. Agric. Food Chem. 2018, 66, 10106–10112. [Google Scholar] [CrossRef]
- He, W.; Qiao, B.; Li, F.; Pan, L.; Chen, D.; Cao, Y.; Tu, J.; Wang, X.; Lv, C.; Wu, Q. A novel electrochemical biosensor for ultrasensitive Hg2+ detection via a triple signal amplification strategy. Chem. Commun. 2021, 57, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhai, H.; Yin, J.; Guo, Q.; Zhang, Y.; Yang, Q.; Li, F.; Sun, X.; Guo, Y.; Zhang, Y. Dual-target electrochemical DNA sensor for detection of Pb2+ and Hg2+ simultaneously by exonuclease I–assisted recycling signal amplification. Microchim. Acta 2022, 189, 460. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seo, K.D.; Park, H.; Mahmudunnabi, R.G.; Lee, K.H.; Shim, Y.B. Graphene-anchored conductive polymer aerogel composite for the electrocatalytic detection of hydrogen peroxide and bisphenol A. Appl. Surf. Sci. 2022, 604, 154430. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Frick, J.J.; Davey, A.K.; Dods, M.N.; Carraro, C.; Senesky, D.G.; Maboudian, R. Synthesis and characterization of UiO-66-NH2 incorporated graphene aerogel composites and their utilization for absorption of organic liquids. Carbon 2023, 201, 561–567. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xu, A.; Tang, K.; Huang, Y.; Lu, C. In situ reduced and assembled three-dimensional graphene aerogel for efficient dye removal. J. Alloys Compd. 2017, 714, 522–529. [Google Scholar] [CrossRef]
- Trinh, T.T.P.N.X.; Giang, N.T.H.; Thinh, D.B.; Dat, N.M.; Trinh, D.N.; Hai, N.D.; Oanh, D.T.Y.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Hydrothermal synthesis of titanium dioxide/graphene aerogel for photodegradation of methylene blue in aqueous solution. J. Sci. Adv. Mater. Dev. 2022, 7, 100433. [Google Scholar] [CrossRef]
- Tu, T.H.; Cam, P.T.N.; Phong, M.T.; Nam, H.M.; Hieu, N.H. Synthesis and application of graphene oxide aerogel as an adsorbent for removal of dyes from water. Mater. Lett. 2019, 238, 134–137. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Zhao, C.; Pan, E.; Jia, M. Fe3O4 nanoparticle/graphene aerogel composite with enhanced lithium storage performance. Appl. Surf. Sci. 2018, 458, 1035–1042. [Google Scholar] [CrossRef]
- Ren, Z.; Li, H.; Li, J.; Cai, J.; Zhong, L.; Ma, Y.; Pang, Y. Green synthesis of reduced graphene oxide/chitosan/gold nanoparticles composites and their catalytic activity for reduction of 4-nitrophenol. Int. J. Biol. Macromol. 2023, 229, 732–745. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Li, M.; Liu, D. Design fabrication of electrochemical sensor based on Ru(bpy)22+/SMWCNTs/Au/GCE electrode for the selective determination of 5′-guanosine monophosphate. Food Chem. 2023, 418, 135841. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef]
- Li, M.; Zhe, T.; Li, R.; Bai, F.; Jia, P.; Xu, Z.; Wang, X.; Bu, T.; Wu, H.; Wang, L. ZIF-derived Co nanoparticles embedded into N-doped carbon nanotube composites for highly efficient electrochemical detection of nitrofurantoin in food. Food Chem. 2023, 418, 135948. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Zhang, F.; Pei, L.; Cui, Z.; Shen, J.; Ye, M. Ni, Co and Mn doped SnS2-graphene aerogels for supercapacitors. J. Alloys Compd. 2018, 767, 583–591. [Google Scholar] [CrossRef]
- Ma, X.; Chen, D.; Tu, X.; Gao, F.; Xie, Y.; Dai, R.; Lu, L.; Wang, X.; Qu, F.; Yu, Y.; et al. Ratiometric electrochemical sensor for sensitive detection of sunset yellow based on three-dimensional polyethyleneimine functionalized reduced graphene oxide aerogels@ Au nanoparticles/SH-β-cyclodextrin. Nanotechnology 2019, 30, 475503. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, G.; Cui, F.; Qiu, Q.; Chen, X.; Huang, H. Double determination of long noncoding RNAs from lung cancer via multi-amplified electrochemical genosensor at sub-femtomole level. Biosens. Bioelectron. 2018, 113, 116–123. [Google Scholar] [CrossRef]
- Peng, G.; Li, X.; Cui, F.; Qiu, Q.; Chen, X.; Huang, H. Aflatoxin B1 electrochemical aptasensor based on tetrahedral DNA nanostructures functionalized three dimensionally ordered macroporous MoS2-AuNPs film. ACS Appl. Mater. Interfaces 2018, 10, 17551–17559. [Google Scholar] [CrossRef]
- Peng, G.; Yu, Y.; Chen, X.; Huang, H. Highly sensitive amperometric α-ketoglutarate biosensor based on reduced graphene oxide-gold nanocomposites. Int. J. Anal. Chem. 2020, 2020, 4901761. [Google Scholar] [CrossRef]
- GB 5009.268-2016; Determination of Multiple Elements in Foods. National Standard for Food Safety. Chinese Standard GB/T: Beijing, China, 2016.
- Guo, N.; Xu, G.; Zhang, Q.; Song, P.; Xia, L. AgNPs functionalized with dithizone for the detection of Hg2+ based on surface-enhanced Raman scattering spectroscopy. Plasmonics 2022, 17, 1419–1426. [Google Scholar] [CrossRef]
- Liu, K.; Xia, C.; Guo, Y.; Yu, H.; Xie, Y.; Yao, W. Polyethylenimine-functionalized nitrogen and sulfur co-doped carbon dots as effective fluorescent probes for detection of Hg2+ ions. Spectrochim. Acta A 2023, 292, 122395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, D.; Chen, C.; Chen, M.; Li, Z.; Wu, Y.; Zhu, S.; Peng, G. A hydrostable and bromine-functionalized manganese-organic framework with luminescence sensing of Hg2+ and antiferromagnetic properties. J. Solid State Chem. 2019, 269, 257–263. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Pan, Z.; Zheng, T.; Song, Y.; Zhang, J.; Xiao, Z. Highly selective and sensitive detection of Hg2+ by a novel fluorescent probe with dual recognition sites. Spectrochim. Acta A 2023, 291, 122379. [Google Scholar] [CrossRef]
- Zhong, Y.Q.; Ning, T.J.; Cheng, L.; Xiong, W.; Wei, G.B.; Liao, F.S.; Ma, G.Q.; Hong, N.; Cui, H.F.; Fan, H. An electrochemical Hg2+ sensor based on signal amplification strategy of target recycling. Talanta 2021, 223, 121709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, P.; Hu, Z.; Liang, Y.; Han, H.; Yang, M.; Luo, X.; Hou, C.; Huo, D. Amino-functionalized multilayer Ti3C2Tx enabled electrochemical sensor for simultaneous determination of Cd2+ and Pb2+ in food samples. Food Chem. 2023, 402, 134269. [Google Scholar] [CrossRef] [PubMed]
- Karaman, C. Orange peel derived-nitrogen and sulfur Co-doped carbon dots: A nano-booster for enhancing ORR electrocatalytic performance of 3D graphene networks. Electroanalysis 2021, 33, 1356–1369. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimi-Maleh, H. In situ synthesis of label-free electrochemical aptasensor-based sandwich-like AuNPs/PPy/Ti3C2Tx for ultrasensitive detection of lead ions as hazardous pollutants in environmental fluids. Chemosphere 2023, 324, 138302. [Google Scholar] [CrossRef] [PubMed]



| Methods | Linear Range | LOD | References |
|---|---|---|---|
| SERS | 0.01 μM–0.1 mM | 9.83 nM | [38] |
| Fluorescence | 0–500 nM | 9.2 nM | [39] |
| Luminescence | 0–0.03 M | 48 μM | [40] |
| Fluorescence | 0–40 μM | 0.12 μM | [41] |
| Electrochemical | 1 fM–10 nM | 0.19 fM | [20] |
| Electrochemical | 0.2–2000 pM | 0.12 pM | [42] |
| Electrochemical | 1 fM–10 nM | 0.16 fM | This work |
| Sample | No. | Added | Proposed | Recovery | RSD |
|---|---|---|---|---|---|
| (nM) | Aptasensor (nM) | (%) | (n = 3, %) | ||
| 1 | 0.3 | 0.292 | 97.3 | 3.37 | |
| milk | 2 | 1.0 | 0.978 | 97.8 | 2.68 |
| 3 | 2.5 | 2.633 | 105.3 | 1.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, G.; Guo, M.; Liu, Y.; Yang, H.; Wen, Z.; Chen, X. Development of a Novel H-Shaped Electrochemical Aptasensor for Detection of Hg2+ Based on Graphene Aerogels–Au Nanoparticles Composite. Biosensors 2023, 13, 932. https://doi.org/10.3390/bios13100932
Peng G, Guo M, Liu Y, Yang H, Wen Z, Chen X. Development of a Novel H-Shaped Electrochemical Aptasensor for Detection of Hg2+ Based on Graphene Aerogels–Au Nanoparticles Composite. Biosensors. 2023; 13(10):932. https://doi.org/10.3390/bios13100932
Chicago/Turabian StylePeng, Gang, Mengxue Guo, Yuting Liu, Han Yang, Zuorui Wen, and Xiaojun Chen. 2023. "Development of a Novel H-Shaped Electrochemical Aptasensor for Detection of Hg2+ Based on Graphene Aerogels–Au Nanoparticles Composite" Biosensors 13, no. 10: 932. https://doi.org/10.3390/bios13100932
APA StylePeng, G., Guo, M., Liu, Y., Yang, H., Wen, Z., & Chen, X. (2023). Development of a Novel H-Shaped Electrochemical Aptasensor for Detection of Hg2+ Based on Graphene Aerogels–Au Nanoparticles Composite. Biosensors, 13(10), 932. https://doi.org/10.3390/bios13100932





