Topoisomerase 1 Activity Is Reduced in Response to Thermal Stress in Fruit Flies and in Human HeLa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. DNA Substrates and Oligonucleotides
2.3. Fly Stock and Maintenance
2.4. Thermal Stress of D. melanogaster
2.5. Cell Culture
2.6. Thermal Stress of HeLa Cells
2.7. Preparation of Cell Extract for Molecular Analyses
2.7.1. HeLa
2.7.2. D. melanogaster
2.8. REEAD Assay
2.8.1. Preparation of the Slides
2.8.2. Circularization, Rolling Circle Amplification, and Detection
2.9. Heat Denaturation of Purified Human Topoisomerase 1
2.10. Western Blot
2.11. Quantitative PCR
2.12. Protein Purifications
2.12.1. Human Topoisomerase 1
2.12.2. Phi29 Polymerase
2.13. Statistical Analyses
3. Results and Discussion
3.1. Short-Term Heat Stress Leads to Reduced TOP1 Activity Levels in D. melanogaster
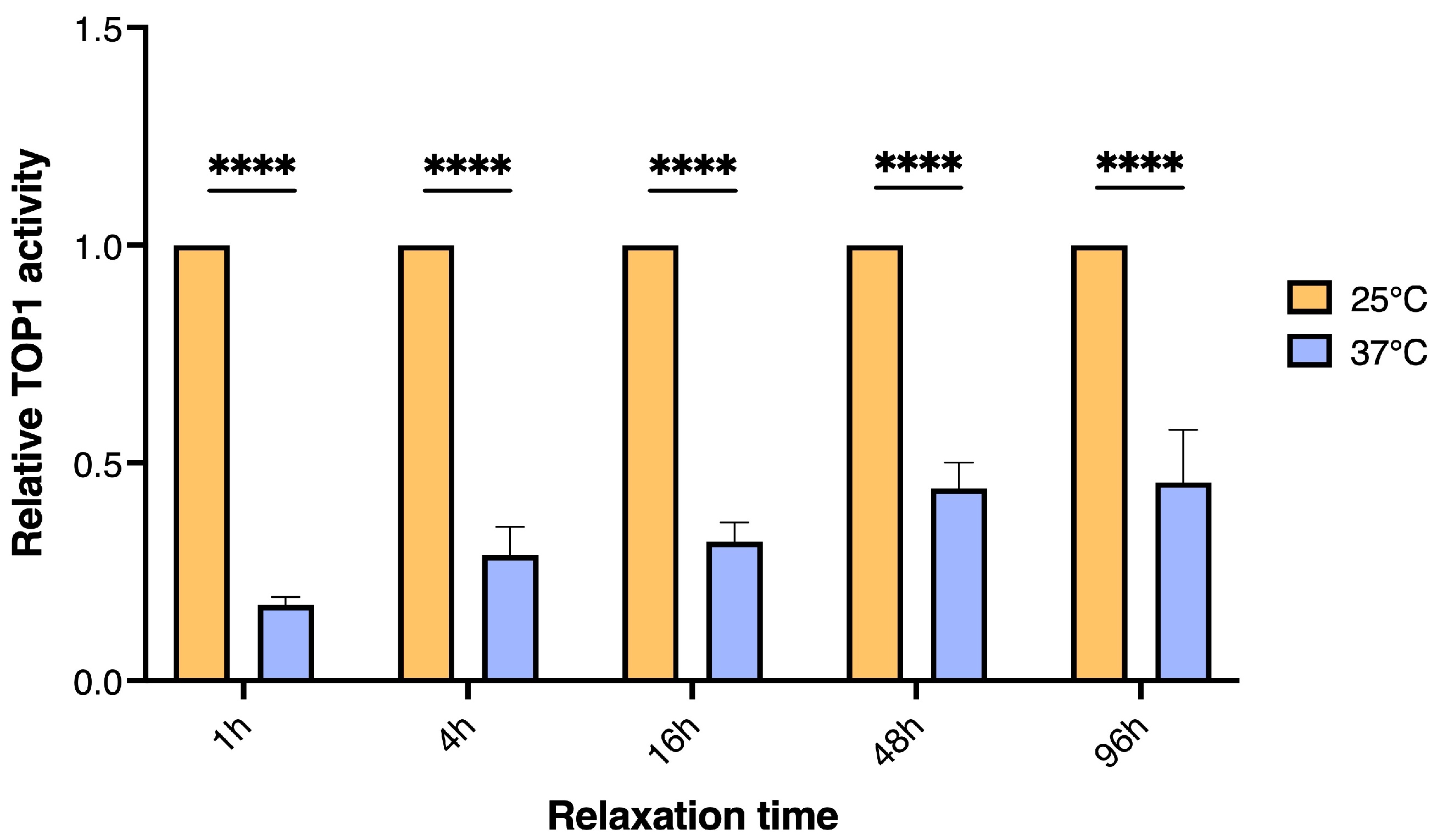
3.2. The Effect of Long-Tern Heat Stress in D. melanogaster on TOP1 Activity
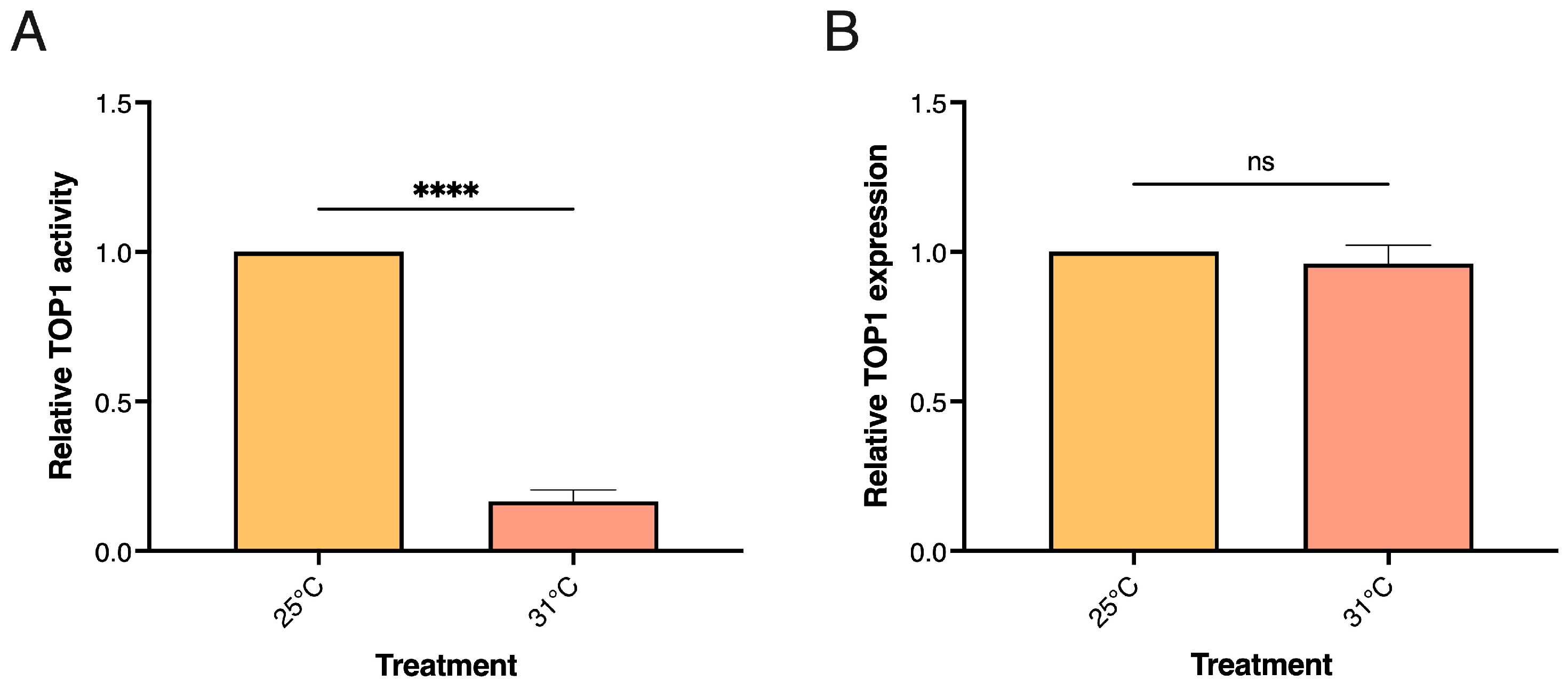
3.3. TOP1 Activity Levels in Human Cells Are Reduced in Response to Short-Term Cold and Heat Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kogler, L.; Müller, V.I.; Chang, A.; Eickhoff, S.B.; Fox, P.T.; Gur, R.C.; Derntl, B. Psychosocial versus physiological stress-Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage 2015, 119, 235–251. [Google Scholar] [CrossRef]
- Martin, L.; Andreassi, E.; Watson, W.; Coon, C. Stress and animal health: Physiological mechanisms and ecological consequences. Nat. Educ. Knowl. 2011, 3, 11. [Google Scholar]
- Padhy, S.K.; Sarkar, S.; Panigrahi, M.; Paul, S. Mental health effects of climate change. Indian J. Occup. Environ. Med. 2015, 19, 3–7. [Google Scholar] [CrossRef]
- Cianconi, P.; Betrò, S.; Janiri, L. The impact of climate change on mental health: A systematic descriptive review. Front. Psychiatry 2020, 11, 74. [Google Scholar] [CrossRef]
- Dadar, M.; Dhama, K.; Iqbal, H.; Munjal, A.; Khandia, R.; Karthik, K.; Sachan, S.; Latheef, S.K.; Samad, H.A.; Joshi, S.K. Molecular signatures of biomarkers in cancer development, diagnosis, and its prognostic accuracy. Curr. Biomark. 2016, 6, 89–96. [Google Scholar]
- Abbas, M.; Habib, M.; Naveed, M.; Karthik, K.; Dhama, K.; Shi, M.; Dingding, C. The relevance of gastric cancer biomarkers in prognosis and pre- and post- chemotherapy in clinical practice. Biomed. Pharmacother. 2017, 95, 1082–1090. [Google Scholar] [CrossRef]
- Prajapati, B.M.; Gupta, J.P.; Pandey, D.P.; Parmar, G.A.; Chaudhari, J.D. Molecular markers for resistance against infectious diseases of economic importance. Vet. World 2017, 10, 112–120. [Google Scholar] [CrossRef]
- Selleck, M.J.; Senthil, M.; Wall, N.R. Making Meaningful Clinical Use of Biomarkers. Biomark. Insights 2017, 12, 1177271917715236. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, M.I.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Georgescu, S.R. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis. Markers 2018, 2018, 5823684. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Evolution of the cellular stress proteome: From monophyletic origin to ubiquitous function. J. Exp. Biol. 2003, 206, 3119–3124. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Sejerkilde, M.; Sørensen, J.G.; Loeschcke, V. Effects of cold- and heat hardening on thermal resistance in Drosophila melanogaster. J. Insect Physiol. 2003, 49, 719–726. [Google Scholar] [CrossRef]
- Trautinger, F.; Kindås-Mügge, I.; Knobler, R.M.; Hönigsmann, H. Stress proteins in the cellular response to ultraviolet radiation. J. Photochem. Photobiol. B Biol. 1996, 35, 141–148. [Google Scholar] [CrossRef]
- Kiriyama, M.T.; Oka, M.; Takehana, M.; Kobayashi, S. Expression of a Small Heat Shock Protein 27 (HSP27) in Mouse Skin Tumors Induced by UVB-Irradiation. Biol. Pharm. Bull. 2001, 24, 197–200. [Google Scholar] [CrossRef]
- Werner, I.; Nagel, R. Stress proteins HSP60 and HSP70 in three species of amphipods exposed to cadmium, diazinon, dieldrin and fluoranthene. Environ. Toxicol. Chem. 1997, 16, 2393–2403. [Google Scholar] [CrossRef]
- Aït-Aïssa, S.; Porcher, J.M.; Arrigo, A.P.; Lambré, C. Activation of the hsp70 promoter by environmental inorganic and organic chemicals: Relationships with cytotoxicity and lipophilicity. Toxicology 2000, 145, 147–157. [Google Scholar] [CrossRef]
- Yang, D.; Lu, X.; Zhang, W.; He, F. Biochemical changes in primary culture of skeletal muscle cells following dimethoate exposure. Toxicology 2002, 174, 79–85. [Google Scholar] [CrossRef]
- Nazir, A.; Saxena, D.K.; Kar Chowdhuri, D. Induction of hsp70 in transgenic Drosophila: Biomarker of exposure against phthalimide group of chemicals. Biochim. Biophys. Acta BBA Gen. Subj. 2003, 1621, 218–225. [Google Scholar] [CrossRef]
- Collins, P.L.; Hightower, L.E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J. Virol. 1982, 44, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Polla, B.S. A role for heat shock proteins in inflammation? Immunol. Today 1988, 9, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Deitch, E.A.; Beck, S.C.; Cruz, N.C.; De Maio, A. Induction of heat shock gene expression in colonic epithelial cells after incubation with Escherichia coli or endotoxin. Crit. Care Med. 1995, 23, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martínez, J.; Barbosa, A.; Møller, A.P.; de Lope, F.; Pérez, J.; Rodríguez-Caabeiro, F. Increase in a heat-shock protein from blood cells in response of nestling house martins (Delichon urbica) to parasitism: An experimental approach. Oecologia 1998, 116, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ropp, M.; Courgeon, A.M.; Calvayrac, R.; Best-Belpomme, M. The possible role of the superoxide ion in the induction of heat-shock and specific proteins in aerobic Drosophila cells during return to normoxia after a period of anaerobiosis. Can. J. Biochem. Cell Biol. 1983, 61, 456–461. [Google Scholar] [CrossRef]
- Gophna, U.; Ron, E.Z. Virulence and the heat shock response. Int. J. Med. Microbiol. 2003, 292, 453–461. [Google Scholar] [CrossRef]
- Rinehart, J.P.; Denlinger, D.L.; Rivers, D.B. Upregulation of transcripts encoding select heat shock proteins in the flesh fly Sarcophaga crassipalpis in response to venom from the ectoparasitoid wasp Nasonia vitripennis. J. Invertebr. Pathol. 2002, 79, 62–63. [Google Scholar] [CrossRef]
- Tissiéres, A.; Mitchell, H.K.; Tracy, U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J. Mol. Biol. 1974, 84, 389–398. [Google Scholar] [CrossRef]
- Jenkins, M.E.; Suzuki, T.C.; Mount, D.W. Evidence that heat and ultraviolet radiation activate a common stress-response program in plants that is altered in the uvh6 mutant of Arabidopsis thaliana. Plant Physiol. 1997, 115, 1351–1358. [Google Scholar] [CrossRef]
- Otsuka, Y.; Takano, T.S.; Yamazaki, T. Genetic variation in the expression of the six hsp genes in the presence of heat shock in Drosophila melanogaster. Genes Genet. Syst. 1997, 72, 19–24. [Google Scholar] [CrossRef]
- Li, Q.-B.; Haskell, D.W.; Guy, C.L. Coordinate and non-coordinate expression of the stress 70 family and other molecular chaperones at high and low temperature in spinach and tomato. Plant Mol. Biol. 1999, 39, 21–34. [Google Scholar] [CrossRef]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.G.; Yoshida, K.M.; Kimura, M.T. Accumulation of Hsp70 mRNA under environmental stresses in diapausing and nondiapausing adults of Drosophila triauraria. J. Insect Physiol. 1998, 44, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Perez-Serrano, J.; Bernadina, W.E.; Rodriguez-Caabeiro, F. Stress Response to Cold in Trichinella Species. Cryobiology 2001, 43, 293–302. [Google Scholar] [CrossRef]
- Fortunato, A.K.; Pontes, W.M.; De Souza, D.M.S.; Prazeres, J.S.F.; Marcucci-Barbosa, L.S.; Santos, J.M.M.; Veira, É.L.M.; Bearzoti, E.; Pinto, K.M.C.; Talvani, A.; et al. Strength Training Session Induces Important Changes on Physiological, Immunological, and Inflammatory Biomarkers. J. Immunol. Res. 2018, 2018, 9675216. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.; Helal, M.A.Y.; Sabek, A.; Shousha, S. Clinical, behavioral and biochemical alterations due to shearing stress in Ossimi sheep. J. Vet. Med. Sci. 2018, 80, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; Humphrey, T.C. Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol. 2001, 11, 426–433. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Macario, A.J.; Lange, M.; Ahring, B.K.; Conway de Macario, E. Stress genes and proteins in the archaea. Microbiol. Mol. Biol. Rev. 1999, 63, 923–967, table of contents. [Google Scholar] [CrossRef]
- Hecker, M.; Völker, U. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 2001, 44, 35–91. [Google Scholar] [CrossRef] [PubMed]
- Ewert, A.; Chang, Y. Levels of Nature and Stress Response. Behav. Sci. 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Skoluda, N.; Strahler, J. Biomarkers of stress in behavioural medicine. Curr. Opin. Psychiatry 2013, 26, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied. Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Ivković, N.; Božović, Đ.; Račić, M.; Popović-Grubač, D.; Davidović, B. Biomarkers of stress in saliva. Acta Fac. Medicae Naissensis 2015, 32, 91–99. [Google Scholar] [CrossRef]
- Velichko, A.K.; Petrova, N.V.; Razin, S.V.; Kantidze, O.L. Mechanism of heat stress-induced cellular senescence elucidates the exclusive vulnerability of early S-phase cells to mild genotoxic stress. Nucleic Acids Res. 2015, 43, 6309–6320. [Google Scholar] [CrossRef]
- Leppard, J.B.; Champoux, J.J. Human DNA topoisomerase I: Relaxation, roles, and damage control. Chromosoma 2005, 114, 75–85. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Ciavarra, R.P.; Goldman, C.; Wen, K.K.; Tedeschi, B.; Castora, F.J. Heat stress induces hsc70/nuclear topoisomerase I complex formation in vivo: Evidence for hsc70-mediated, ATP-independent reactivation in vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 1751–1755. [Google Scholar] [CrossRef]
- Ciavarra, R.P.; Duvall, W.; Castora, F.J. Induction of thermotolerance in T cells protects nuclear DNA topoisomerase I from heat stress. Biochem. Biophys. Res. Commun. 1992, 186, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Stougaard, M.; Lohmann, J.S.; Mancino, A.; Celik, S.; Andersen, F.F.; Koch, J.; Knudsen, B.R. Single-molecule detection of human topoisomerase I cleavage-ligation activity. ACS Nano 2009, 3, 223–233. [Google Scholar] [CrossRef]
- Indiana University Bloomington; Bloomington Drosophila Stock Center. Drosophila Genetic Reference Panel Strains. Available online: https://bdsc.indiana.edu/stocks/wt/dgrp.html (accessed on 18 September 2023).
- Kjeldsen, E.; Tordrup, D.; Hübner, G.M.; Knudsen, B.R.; Andersen, F.F. Topoisomerase I deficiency results in chromosomal alterations in cervical cancer cells. Anticancer Res. 2010, 30, 3257–3265. [Google Scholar]
- Knudsen, B.R.; Straub, T.; Boege, F. Separation and functional analysis of eukaryotic DNA topoisomerases by chromatography and electrophoresis. J. Chromatogr. B Biomed. Appl. 1996, 684, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lisby, M.; Krogh, B.O.; Boege, F.; Westergaard, O.; Knudsen, B.R. Camptothecins inhibit the utilization of hydrogen peroxide in the ligation step of topoisomerase I catalysis. Biochemistry 1998, 37, 10815–10827. [Google Scholar] [CrossRef]
- Tesauro, C.; Simonsen, A.K.; Andersen, M.B.; Petersen, K.W.; Kristoffersen, E.L.; Algreen, L.; Hansen, N.Y.; Andersen, A.B.; Jakobsen, A.K.; Stougaard, M.; et al. Topoisomerase I activity and sensitivity to camptothecin in breast cancer-derived cells: A comparative study. BMC Cancer 2019, 19, 1158. [Google Scholar] [CrossRef]
- Roy, A.; Tesauro, C.; Frøhlich, R.; Hede, M.S.; Nielsen, M.J.; Kjeldsen, E.; Bonven, B.; Stougaard, M.; Gromova, I.; Knudsen, B.R. Decreased camptothecin sensitivity of the stem-cell-like fraction of Caco2 cells correlates with an altered phosphorylation pattern of topoisomerase I. PLoS ONE 2014, 9, e99628. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Gjerset, R.A. Protein kinase CK2 is a central regulator of topoisomerase I hyperphosphorylation and camptothecin sensitivity in cancer cell lines. Biochemistry 2011, 50, 704–714. [Google Scholar] [CrossRef]
- Lee, J.H.; Mosher, E.P.; Lee, Y.-S.; Bumpus, N.N.; Berger, J.M. Control of topoisomerase II activity and chemotherapeutic inhibition by TCA cycle metabolites. Cell Chem. Biol. 2022, 29, 476–489.e476. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Lee, C.; Haghighi, A.; Banères, J.L.; Parello, J.; Gjerset, R.A. Serine phosphorylation-dependent coregulation of topoisomerase I by the p14ARF tumor suppressor. Biochemistry 2007, 46, 14325–14334. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, K.; Li, P.; Gjerset, R.A. The p14ARF alternate reading frame protein enhances DNA binding of topoisomerase I by interacting with the serine 506-phosphorylated core domain. PLoS ONE 2013, 8, e58835. [Google Scholar] [CrossRef] [PubMed]
- Karayan, L.; Riou, J.-F.; Séité, P.; Migeon, J.; Cantereau, A.; Larsen, C.-J. Human ARF protein interacts with Topoisomerase I and stimulates its activity. Oncogene 2001, 20, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.B.; Bæk, I.; Loeschcke, V.; Givskov Sørensen, J. A functional study of the role of Turandot genes in Drosophila melanogaster: An emerging candidate mechanism for inducible heat tolerance. J. Insect Physiol. 2022, 143, 104456. [Google Scholar] [CrossRef]
- Loeschcke, V.; Sørensen, J.G. Acclimation, heat shock and hardening—A response from evolutionary biology. J. Therm. Biol. 2005, 30, 255–257. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sørensen, J.G.; Loeschcke, V. Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J. Therm. Biol. 2003, 28, 175–216. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Feist, G.W.; Varga, Z.M.; Westerfield, M.; Kent, M.L.; Schreck, C.B. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 2009, 297, 157–162. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Sørensen, P.; Kruhøffer, M.; Pedersen, K.S.; Loeschcke, V. Genome-wide analysis on inbreeding effects on gene expression in Drosophila melanogaster. Genetics 2005, 171, 157–167. [Google Scholar] [CrossRef]
- Keller, J.G.; Stougaard, M.; Knudsen, B.R. Chapter Three-Enzymatic activity in single cells. In Methods in Enzymology; Allbritton, N.L., Kovarik, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 628, pp. 43–57. [Google Scholar]
- Keller, J.G.; Mizielinski, K.; Petersen, K.V.; Stougaard, M.; Knudsen, B.R.; Tesauro, C. Simple and Fast Rolling Circle Amplification-Based Detection of Topoisomerase 1 Activity in Crude Biological Samples. JoVE 2022, 190, e64484. [Google Scholar] [CrossRef]
- Bijlsma, R.; Loeschcke, V. Environmental stress, adaptation and evolution: An overview. J. Evol. Biol. 2005, 18, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Li, Y.; Bowser, R. RBM45 associates with nuclear stress bodies and forms nuclear inclusions during chronic cellular stress and in neurodegenerative diseases. Acta Neuropathol. Commun. 2020, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, L.; Ieraci, A.; Amadio, P.; Zarà, M.; Barbieri, S.S. Impact of Acute and Chronic Stress on Thrombosis in Healthy Individuals and Cardiovascular Disease Patients. Int. J. Mol. Sci. 2020, 21, 7818. [Google Scholar] [CrossRef]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.H.; Saleem, Y.; Hazrat, H. Physiological biomarkers of chronic stress: A systematic review. Int. J. Health Sci. 2021, 15, 46–59. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juul-Kristensen, T.; Keller, J.G.; Borg, K.N.; Hansen, N.Y.; Foldager, A.; Ladegaard, R.; Ho, Y.-P.; Loeschcke, V.; Knudsen, B.R. Topoisomerase 1 Activity Is Reduced in Response to Thermal Stress in Fruit Flies and in Human HeLa Cells. Biosensors 2023, 13, 950. https://doi.org/10.3390/bios13110950
Juul-Kristensen T, Keller JG, Borg KN, Hansen NY, Foldager A, Ladegaard R, Ho Y-P, Loeschcke V, Knudsen BR. Topoisomerase 1 Activity Is Reduced in Response to Thermal Stress in Fruit Flies and in Human HeLa Cells. Biosensors. 2023; 13(11):950. https://doi.org/10.3390/bios13110950
Chicago/Turabian StyleJuul-Kristensen, Trine, Josephine Geertsen Keller, Kathrine Nygaard Borg, Noriko Y. Hansen, Amalie Foldager, Rasmus Ladegaard, Yi-Ping Ho, Volker Loeschcke, and Birgitta R. Knudsen. 2023. "Topoisomerase 1 Activity Is Reduced in Response to Thermal Stress in Fruit Flies and in Human HeLa Cells" Biosensors 13, no. 11: 950. https://doi.org/10.3390/bios13110950
APA StyleJuul-Kristensen, T., Keller, J. G., Borg, K. N., Hansen, N. Y., Foldager, A., Ladegaard, R., Ho, Y.-P., Loeschcke, V., & Knudsen, B. R. (2023). Topoisomerase 1 Activity Is Reduced in Response to Thermal Stress in Fruit Flies and in Human HeLa Cells. Biosensors, 13(11), 950. https://doi.org/10.3390/bios13110950





