pH Regulator on Digital Microfluidics with Pico-Dosing Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
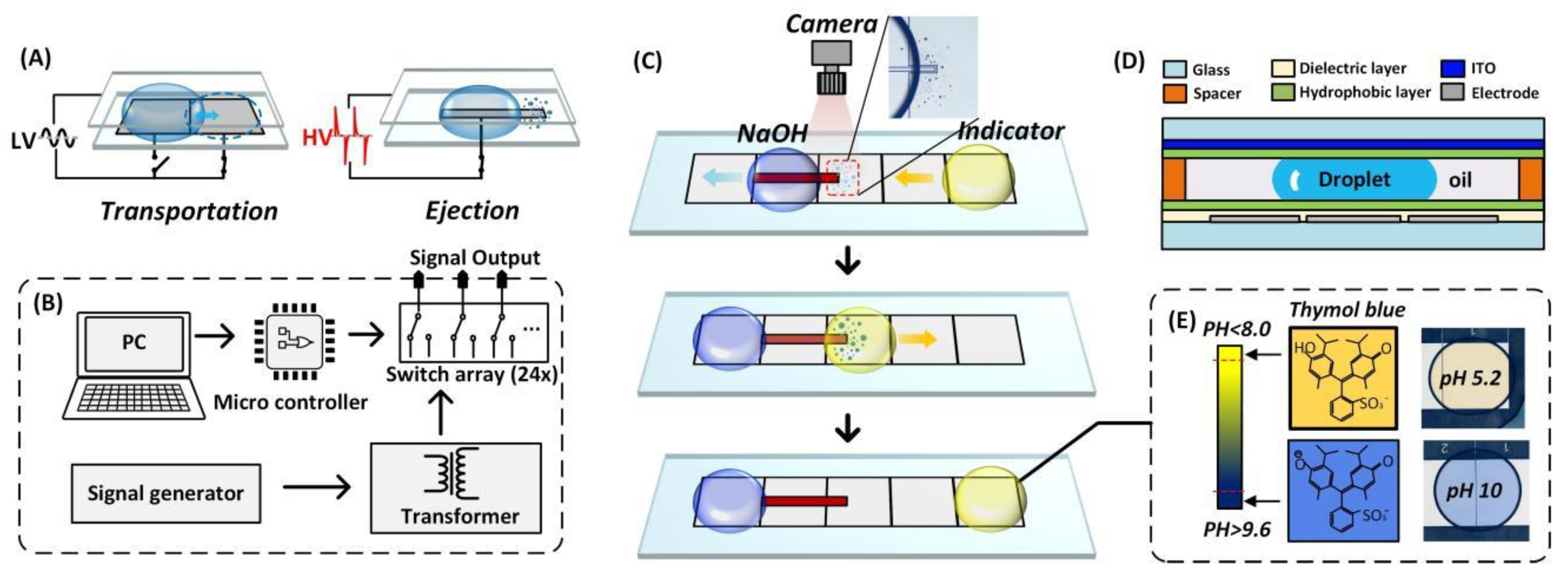
2.2. DMF System Setup
2.3. DMF Chip Fabrication
2.4. Preparation of Standard pH Solutions for Colorimetric Analysis
3. Results
3.1. pH Regulator on DMF
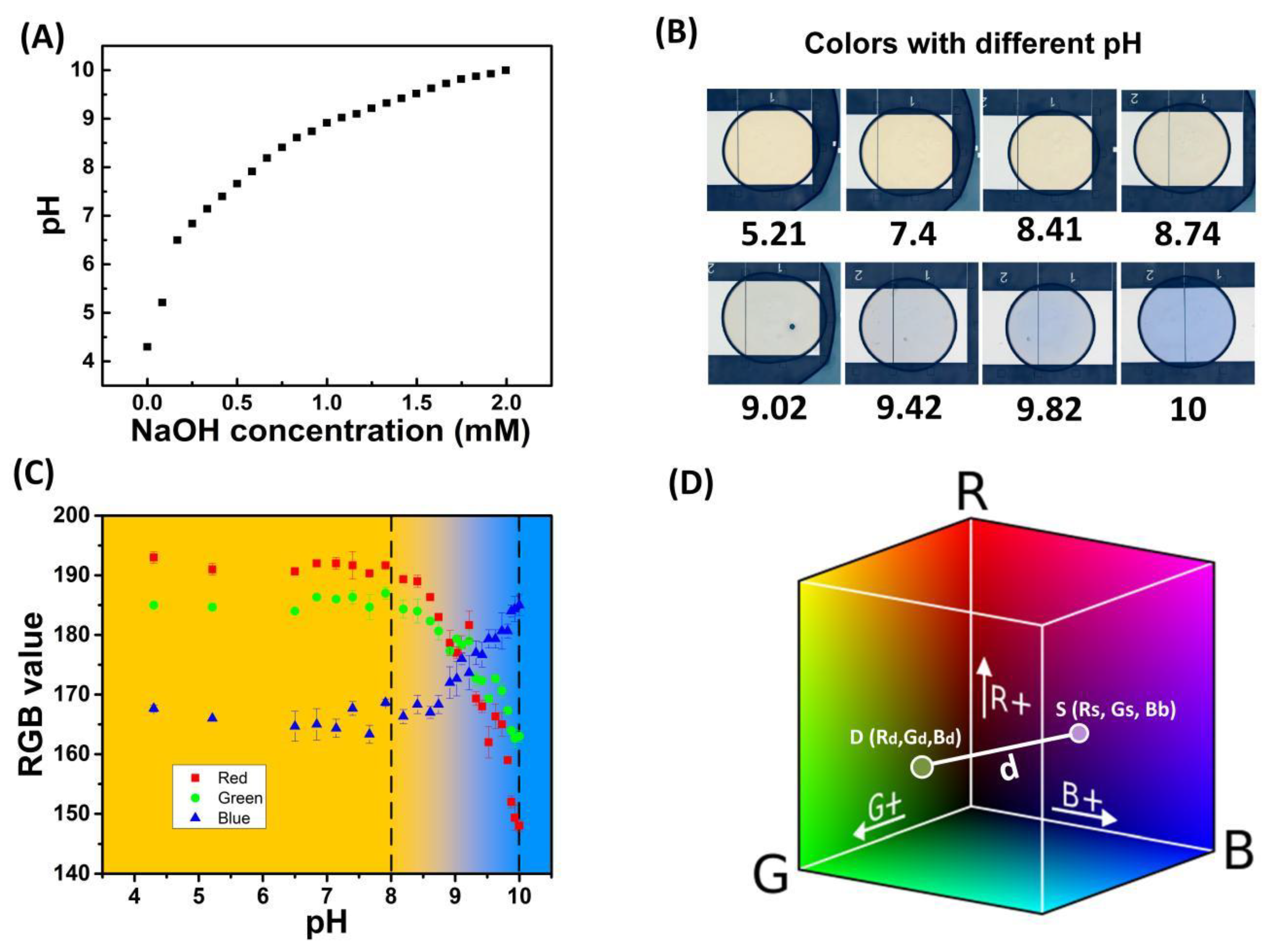
3.2. pH Determination with Colorimetric Analysis
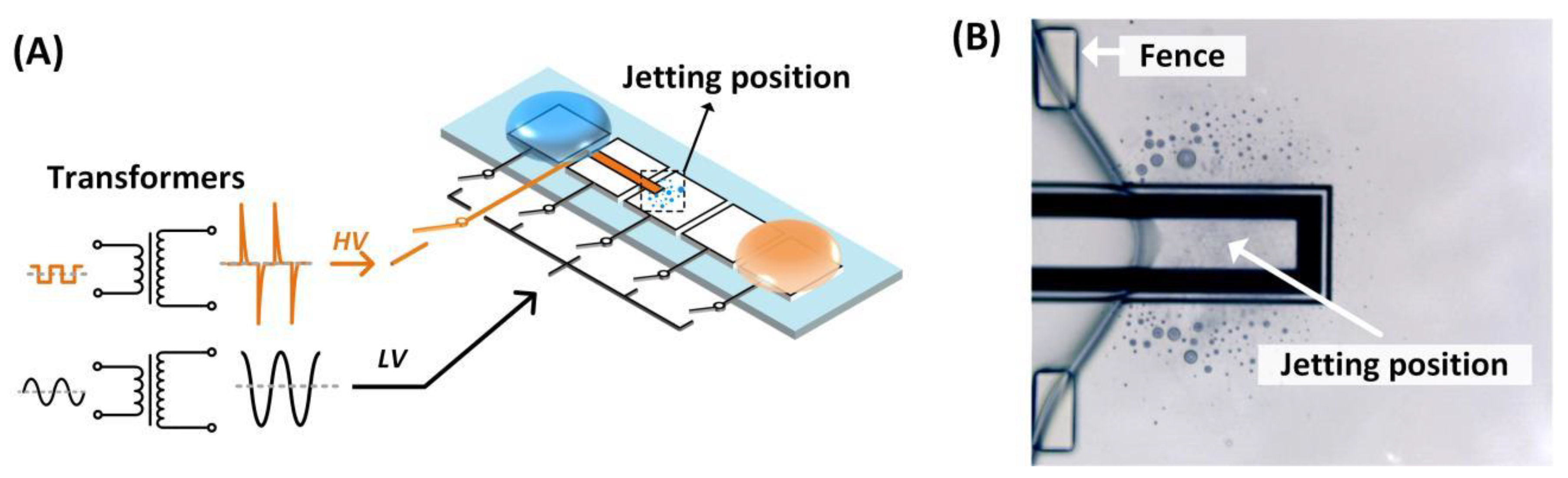
3.3. Image Analysis of Satellite Droplets
3.4. pH Regulation on Chip
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steiner, F.; Lana, M.d.C. Effect of PH on Boron Adsorption in Some Soils of Paraná, Brazil. Chil. J. Agric. Res. 2013, 73, 28–29. [Google Scholar] [CrossRef]
- Andersson, S.; Nilsson, S.I. Influence of PH and Temperature on Microbial Activity, Substrate Availability of Soil-Solution Bacteria and Leaching of Dissolved Organic Carbon in a Mor Humus. Soil Biol. Biochem. 2001, 33, 1181–1191. [Google Scholar] [CrossRef]
- Cao, H.; Chen, R.; Wang, L.; Jiang, L.; Yang, F.; Zheng, S.; Wang, G.; Lin, X. Soil pH, Total Phosphorus, Climate and Distance Are the Major Factors Influencing Microbial Activity at a Regional Spatial Scale. Sci. Rep. 2016, 6, 25815. [Google Scholar] [CrossRef]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. PH Control Mechanisms of Tumor Survival and Growth. J. Cell. Physiol. 2011, 226, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, P.-M.L.; Robitaille, P.A.; Gordon Brown, G.; Brown, G.G. An Analysis of the PH-Dependent Chemical-Shift Behavior of Phosphorus-Containing Metabolites. J. Magn. Reson. 1991, 92, 73–84. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The Chemistry, Physiology and Pathology of PH in Cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, J.; Ma, W.; Zheng, W. PCR Microfluidic Devices for DNA Amplification. Biotechnol. Adv. 2006, 24, 243–284. [Google Scholar] [CrossRef]
- Keng, P.Y.; Chen, S.; Ding, H.; Sadeghi, S.; Shah, G.J.; Dooraghi, A.; Phelps, M.E.; Satyamurthy, N.; Chatziioannou, A.F.; Kim, C.J.; et al. Micro-Chemical Synthesis of Molecular Probes on an Electronic Microfluidic Device. Proc. Natl. Acad. Sci. USA 2012, 109, 690–695. [Google Scholar] [CrossRef]
- Demello, A.J. Control and Detection of Chemical Reactions in Microfluidic Systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef]
- Ng, A.H.C.; Uddayasankar, U.; Wheeler, A.R. Immunoassays in Microfluidic Systems. Anal. Bioanal. Chem. 2010, 397, 991–1007. [Google Scholar] [CrossRef]
- Shamsi, M.H.; Choi, K.; Ng, A.H.C.; Wheeler, A.R. A Digital Microfluidic Electrochemical Immunoassay. Lab Chip 2014, 14, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M.; Tay, S. Microfluidic Cell Culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.; Christen, J.B. Real-Time Feedback Control of PH within Microfluidics Using Integrated Sensing and Actuation. Lab Chip 2014, 14, 1191. [Google Scholar] [CrossRef] [PubMed]
- Läritz, C.; Pagel, L. A Microfluidic PH-Regulation System Based on Printed Circuit Board Technology. Sens. Actuators A Phys. 2000, 84, 230–235. [Google Scholar] [CrossRef]
- Contento, N.M.; Branagan, S.P.; Bohn, P.W. Electrolysis in Nanochannels for in Situ Reagent Generation in Confined Geometries. Lab Chip 2011, 11, 3634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, G.; Yao, S. A Droplet-Based PH Regulator in Microfluidics. Lab Chip 2014, 14, 1917–1922. [Google Scholar] [CrossRef]
- Fouillet, Y.; Jary, D.; Brachet, A.G.; Berthier, J.; Blervaque, R.; Davous, L.; Roux, J.M.; Achard, J.L.; Peponnet, C. EWOD Digital Microfluidics for Lab on a Chip. In Proceedings of the ASME 4th International Conference on Nanochannels, Microchannels, and Minichannels, Parts A and B, ASMEDC, 1 January 2006; pp. 1255–1264. [Google Scholar]
- Gong, J.; Kim, C.-J. All-Electronic Droplet Generation on-Chip with Real-Time Feedback Control for EWOD Digital Microfluidics. Lab Chip 2008, 8, 898. [Google Scholar] [CrossRef]
- Jain, V.; Raj, T.P.; Deshmukh, R.; Patrikar, R. Design, Fabrication and Characterization of Low Cost Printed Circuit Board Based EWOD Device for Digital Microfluidics Applications. Microsyst. Technol. 2017, 23, 389–397. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, Z.; Kim, C.-J.; Ju, Y.S. EWOD (Electrowetting on Dielectric) Digital Microfluidics Powered by Finger Actuation. Lab Chip 2014, 14, 1117. [Google Scholar] [CrossRef]
- Nelson, W.C.; Kim, C.-J. Droplet Actuation by Electrowetting-on-Dielectric (EWOD): A Review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- Li, H.; Shen, R.; Dong, C.; Chen, T.; Jia, Y.; Mak, P.-I.; Martins, R.P. Turning on/off Satellite Droplet Ejection for Flexible Sample Delivery on Digital Microfluidics. Lab Chip 2020, 20, 3709–3719. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular PH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Zhai, J.; Li, H.; Wong, A.H.-H.; Dong, C.; Yi, S.; Jia, Y.; Mak, P.-I.; Deng, C.-X.; Martins, R.P. A Digital Microfluidic System with 3D Microstructures for Single-Cell Culture. Microsyst. Nanoeng. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Gao, J.; Chen, T.; Dong, C.; Li, H.; Wen, Y.-Z.; Lun, Z.-R.; Jia, Y.; Mak, P.-I.; Martins, R.P. LampPort: A Handheld Digital Microfluidic Device for Loop-Mediated Isothermal Amplification (LAMP). Biomed. Microdevices 2019, 21, 9. [Google Scholar] [CrossRef]
- Vallet, M.; Vallade, M.; Berge, B. Limiting Phenomena for the Spreading of Water on Polymer Films by Electrowetting. Eur. Phys. J. B 1999, 11, 583–591. [Google Scholar] [CrossRef]


| Input (Vp-p) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Output_sine (Vp-p) | 36.5 | 91.9 | 154.7 | 216.0 | 278.0 | 344.7 | 413.3 | 486.7 | 558.7 | 634.7 |
| Output_square (Vp-p) | 115.3 | 246.8 | 371.8 | 504.0 | 632.3 | 751 | 886.8 | 1015.0 | 117.5 | 1240.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Peng, T.; Zhong, Y.; Liu, M.; Mak, P.-I.; Martins, R.P.; Wang, P.; Jia, Y. pH Regulator on Digital Microfluidics with Pico-Dosing Technique. Biosensors 2023, 13, 951. https://doi.org/10.3390/bios13110951
Li H, Peng T, Zhong Y, Liu M, Mak P-I, Martins RP, Wang P, Jia Y. pH Regulator on Digital Microfluidics with Pico-Dosing Technique. Biosensors. 2023; 13(11):951. https://doi.org/10.3390/bios13110951
Chicago/Turabian StyleLi, Haoran, Tao Peng, Yunlong Zhong, Meiqing Liu, Pui-In Mak, Rui P. Martins, Ping Wang, and Yanwei Jia. 2023. "pH Regulator on Digital Microfluidics with Pico-Dosing Technique" Biosensors 13, no. 11: 951. https://doi.org/10.3390/bios13110951




