Synthetic Receptors for Early Detection and Treatment of Cancer
Abstract
:1. Introduction
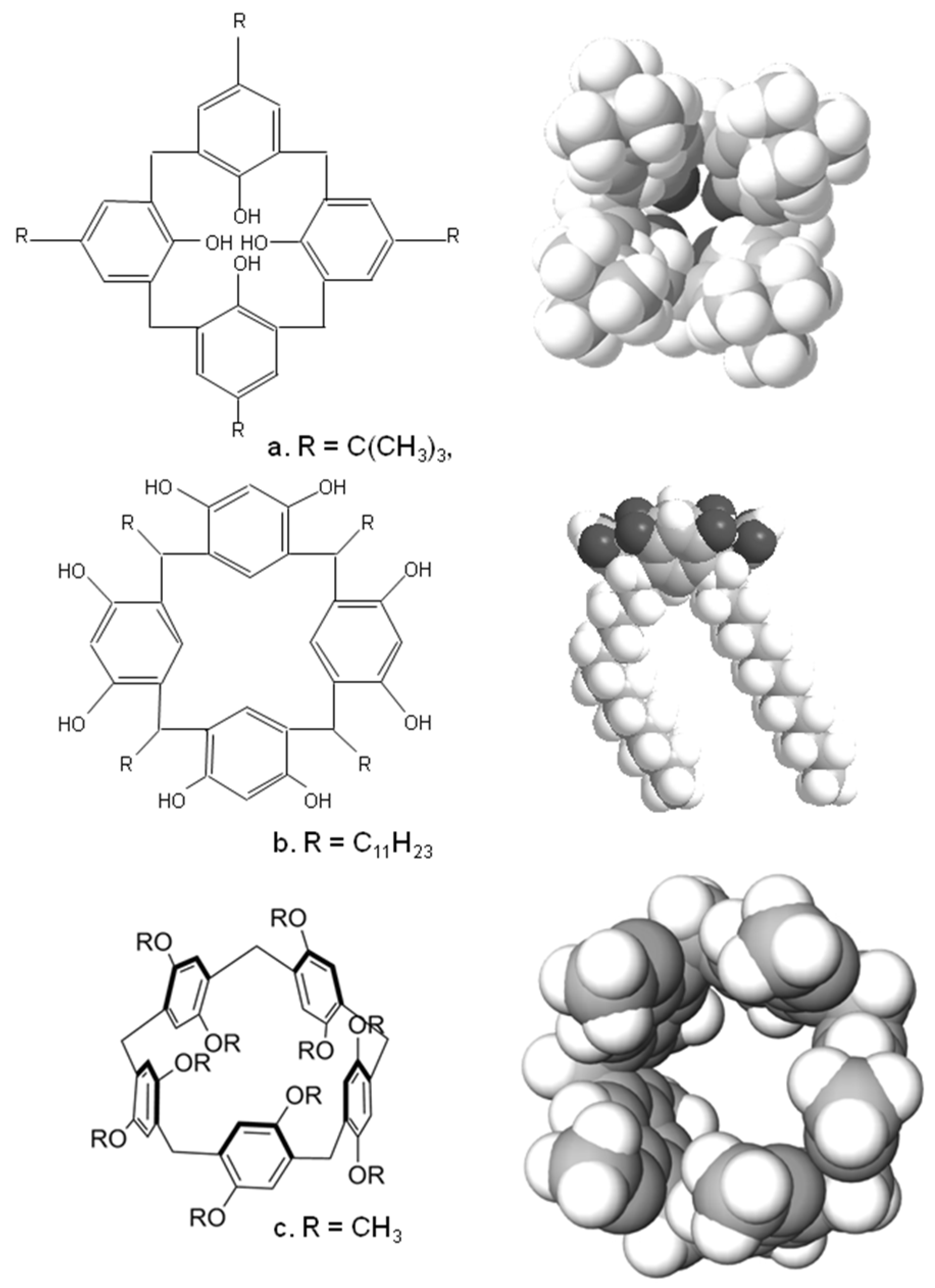
2. Calixarenes, Resorcinarenes and Pillarenes
Sensing Applications


3. Cyclodextrins
3.1. Sensing Applications
3.2. Medical Applications
4. Cucurbiturils
5. Other Recent Work
6. Detection of Carcinogens
7. Applications of Supermolecular Systems
7.1. Photodynamic Therapy
7.2. Imaging
7.3. Other Applications
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 25 November 2022).
- Agur, Z.; Arnon, R.; Schechter, B. Reduction of cytotoxicity to normal tissues by new regimens of cell-cycle phase-specific drugs. Math. Biosci. 1988, 92, 1–15. [Google Scholar] [CrossRef]
- Makin, G. Principles of chemotherapy. Paediatr. Child Health 2014, 24, 161–165. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.; Higson, S.P.J. Macrocycles: Construction, Chemistry and Nanotechnology Applications; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gutsche, C.D. Calixarenes Revisited; Royal Society of Chemistry: Cambridge, UK, 1998. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef]
- Adams, H.; Davis, F.; Stirling, C.J.M. Selective adsorption in gold-thiol monolayers of calix-4-resorcinarenes. J. Chem. Soc. Chem. Commun. 1994, 21, 2527–2629. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, X.; Liu, B.; Qiao, P.; Li, J.; Wang, L. Macrocyclic host molecules with aromatic building blocks: The state of the art and progress. Chem. Commun. 2021, 57, 12379–12405. [Google Scholar] [CrossRef]
- Zheng, Z.; Geng, W.-C.; Gao, J.; Wang, Y.; Suna, H.; Guo, D.S. Ultrasensitive and specific fluorescence detection of a cancer biomarker via nanomolar binding to a guanidinium-modified calixarene. Chem. Sci. 2018, 9, 2087–2091. [Google Scholar] [CrossRef]
- Yu, H.; Geng, W.-C.; Zheng, Z.; Gao, J.; Wang, Y. Facile Fluorescence Monitoring of Gut Microbial Metabolite Trimethylamine N-oxide via Molecular Recognition of Guanidinium-Modified Calixarene. Theranostics 2019, 9, 4624–4632. [Google Scholar] [CrossRef]
- Dionisio, M.; Schnorr, J.M.; Michaelis, V.K.; Griffin, R.G.; Swager, T.M.; Dalcanale, E. Cavitand-functionalized SWCNTs for N-methylammonium detection. J. Am. Chem. Soc. 2012, 134, 6540–6543. [Google Scholar] [CrossRef]
- Retout, M.; Blond, P.; Jabin, I.; Bruylants, G. Ultrastable PEGylated Calixarene-Coated Gold Nanoparticles with a Tunable Bioconjugation Density for Biosensing Applications. Bioconjugate Chem. 2021, 32, 290–300. [Google Scholar] [CrossRef]
- Valenti, G.; Rampazzo, E.; Biavardi, E.; Villani, E.; Fracasso, G.; Marcaccio, M.; Bertani, F.; Ramarli, D.; Dalcanale, E.; Paoluccia, F.; et al. An electrochemiluminescence-supramolecular approach to sarcosine detection for early diagnosis of prostate cancer. Faraday Discuss. 2015, 185, 299–309. [Google Scholar] [CrossRef]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Biochemical sensing with macrocyclic receptors. Chem. Soc. Rev. 2018, 47, 7006–7016. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Granata, G.; Fragassi, G.; Grossi, M.; Sallese, M.; Geraci, C. Design and synthesis of a multivalent fluorescent folate–calix[4]arene conjugate: Cancer cell penetration and intracellular localization. Org. Biomol. Chem. 2015, 13, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, J.; Liang, H.; Li, C.-P. Electrochemical sensor for cancer cell detection using calix[8]arene/polydopamine/phosphorene nanocomposite based on host−guest recognition. Sens. Actuat. B 2020, 317, 128193. [Google Scholar] [CrossRef]
- Yousaf, A.; Hamid, S.A.; Bunnori, N.M.; Ishola, A.A. Applications of calixarenes in cancer chemotherapy: Facts and perspectives. Drug Des. Dev. Ther. 2015, 9, 2831–2838. [Google Scholar] [CrossRef]
- Basilotta, R.; Mannino, D.; Filippone, A.; Casili, G.; Prestifilippo, A.; Colarossi, A.; Raciti, G.; Esposito, E.; Campolo, M. Role of Calixarene in Chemotherapy Delivery Strategies. Molecules 2021, 26, 3963. [Google Scholar] [CrossRef] [PubMed]
- Isik, A.; Oguz, M.; Kocak, A.; Yilmaz, M. Calixarenes: Recent progress in supramolecular chemistry for application in cancer therapy. J. Incl. Phenom. Macrocyc. Chem. 2022, 102, 439–449. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, X.; Li, Q.; Zhang, Z.; Liu, Y. Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment. Molecules 2023, 28, 1241. [Google Scholar] [CrossRef]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [CrossRef]
- Cherenok, S.; Vovk, A.; Muravyova, I.; Shivanyuk, A.; Kukhar, V.; Lipkowski, J.; Kalchenko, V. Calix [4] arene α-aminophosphonic acids: Asymmetric synthesis and enantioselective inhibition of an alkaline phosphatase. Org. Lett. 2006, 8, 549–552. [Google Scholar] [CrossRef]
- Baggetto, L.G.; Coleman, W.A.; Lazar, A.N.; Magnard, S.; Michaud, M.H. Calixarene Derivatives as Anticancer Agent. US Patent 20100056482 A1, 4 March 2010. [Google Scholar]
- Hulíková, K.; Grobárová, V.; Křivohla, V.R.; Fišerová, A. Antitumor activity of N-acetyl-D-glucosamine-substituted glycoconjugates and combined therapy with keyhole limpet hemocyanin in B16F10 mouse melanoma model. Folia Microbiol. 2010, 55, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Viola, S.; Merlo, S.; Consoli, G.M.; Drago, F.; Geraci, C.; Sortino, M.A. Modulation of C6 glioma cell proliferation by ureido-calix [8] arenes. Pharmacology 2010, 86, 182–188. [Google Scholar] [CrossRef]
- Nasuhi Pur, F.; Dilmaghani, K.A. Calixplatin: Novel potential anticancer agent based on the platinum complex with functionalized calixarene. J. Coord. Chem. 2014, 67, 440–448. [Google Scholar] [CrossRef]
- Addepalli, Y.; Yang, X.; Zhou, M.; Reddy, D.P.; Zhang, S.-L.; Wang, Z.; He, Y. Synthesis and anticancer activity evaluation of novel azacalix [2] arene [2] pyrimidines. Eur. J. Med. Chem. 2018, 151, 214–225. [Google Scholar] [CrossRef]
- Santos, D.; Medeiros-Silva, J.; Cegonho, S.; Alves, E.; Ramilo-Gomes, F.; Santos, A.O.; Silvestre, S.; Cruz, C. Cell proliferation effects of calix[4] arene derivatives. Tetrahedron 2015, 71, 7593–7599. [Google Scholar] [CrossRef]
- An, L.; Han, L.-L.; Zheng, Y.-G.; Peng, X.-N.; Xue, Y.-S.; Gu, X.-K.; Sun, J.; Yan, C.-G. Synthesis, X-ray crystal structure and anti-tumor activity of calix[n]arene polyhydroxyamine derivatives. Eur. J. Med. Chem. 2016, 123, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Oguz, M.; Gul, A.; Karakurt, S.; Yilmaz, M. Synthesis and evaluation of the antitumor activity of Calix[4]arene L-proline derivatives. Bioorg. Chem. 2020, 94, 103207. [Google Scholar] [CrossRef] [PubMed]
- Oguz, M.; Gul, A.; Karakurt, S.; Yilmaz, M. Synthesis of new picolylamine bearing calix [8] arene derivatives as antiproliferative agents for colorectal carcinoma. ChemistrySelect 2020, 5, 12250–12254. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bayrac, A.T.; Bayrakci, M. Evaluation of anticancer activities of novel facile synthesized calix[n]arene sulfonamide analogs. Appl. Biochem. Biotechnol. 2020, 190, 1484–1497. [Google Scholar] [CrossRef]
- Pelizzaro-Rocha, K.-J.; de Jesus, M.B.; Ruela-de-Sousa, R.R.; Nakamura, C.V.; Reis, F.S.; de Fátima, A.; Ferreira-Halder, C.V. Calix[6]arene bypasses human pancreatic cancer aggressiveness: Downregulation of receptor tyrosine kinases and induction of cell death by reticulum stress and autophagy. Biochim. Biophys. Acta 2013, 1833, 2856–2865. [Google Scholar] [CrossRef]
- Rocha-Brito, K.J.P.; Fonseca, E.M.B.; Oliveira, B.G.D.; de Fatima, A.; Ferreira-Halder, C.V. Calix[6]arene diminishes receptor tyrosine kinase lifespan in pancreatic cancer cells and inhibits their migration and invasion efficiency. Bioorg. Chem. 2020, 100, 103381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, D.A.; Baldini, L.; Ennis, E.; Jain, R.; Carie, A.; Sebti, S.M.; Hamilton, A.D. Structure-activity studies on a library of potent calix[4]arene-based PDGF antagonists that inhibit PDGF-stimulated PDGFR tyrosine phosphorylation. Org. Biomol. Chem. 2006, 4, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.; Chen, X.; Hellebrekers, D.M.; Hoye, T.R.; Griffioen, A.W.; Mayo, K.H. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J. Natl. Cancer Inst. 2006, 98, 932–936. [Google Scholar] [CrossRef]
- Dings, R.P.; Levine, J.I.; Brown, S.G.; Astorgues-Xerri, L.; MacDonald, J.R.; Hoye, T.R.; Raymond, E.; Mayo, K.H. Polycationic calixarene PTX013, a potent cytotoxic agent against tumors and drug resistant cancer. Investig. New Drugs. 2013, 31, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.M.; Miller, M.C.; Nesmelova, I.; Astorgues-Xerri, L.; Kumar, N.; Serova, M.; Chen, X.; Raymond, E.; Hoye, T.R.; Mayo, K.H. Antitumor agent calixarene 0118 targets human galectin-1 as an allosteric inhibitor of carbohydrate binding. J. Med. Chem. 2012, 55, 5121–5129. [Google Scholar] [CrossRef]
- Läppchen, T.; Dings, R.P.; Rossin, R.; Simon, J.F.; Visser, T.J.; Bakker, M.; Walhe, P.; van Mourik, T.; Donato, K.; van Beijnum, J.R.; et al. Novel analogs of antitumor agent calixarene 0118: Synthesis, cytotoxicity, click labeling with 2-[F-18] fluoroethylazide, and in vivo evaluation. Eur. J. Med. Chem. 2015, 89, 279–295. [Google Scholar] [CrossRef]
- An, L.; Wang, C.; Zheng, Y.G.; Liu, J.D.; Huang, T.H. Design, synthesis and evaluation of calix[4]arene-based carbonyl amide derivatives with antitumor activities. Eur. J. Med. Chem. 2021, 210, 112984. [Google Scholar] [CrossRef]
- Oguz, M. Synthesis and anticancer activity of new p-tertbutylcalix[4]arenes integrated with trifluoromethyl aniline groups against several cell lines. Tetrahedron 2022, 116, 132816. [Google Scholar] [CrossRef]
- Arpaci, P.U.; Ozcan, F.; Gok, E.; Ozcan, F.; Ertul, S. Improved anticancer efficacy of p-tert-butylcalix[4]arene through surface modifications. In Proceedings of the IEEE 7th International Conference Nanomaterials: Application & Properties (NAP), Odessa, Ukraine, 14 December 2017. [Google Scholar] [CrossRef]
- Kamada, R.; Yoshino, W.; Nomura, T.; Chuman, Y.; Imagawa, T.; Suzuki, T.; Sakaguchi, K. Enhancement of transcriptional activity of mutant p53 tumor suppressor protein through stabilization of tetramer formation by calix [6] arene derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 4412–4415. [Google Scholar] [CrossRef]
- Geraci, C.; Consoli, G.M.; Galante, E.; Bousquet, E.; Pappalardo, M.; Spadaro, A. Calix [4] arene decorated with four Tn antigen glycomimetic units and P3CS immunoadjuvant: Synthesis, characterization, and anticancer immunological evaluation. Bioconjugate Chem. 2008, 19, 751–758. [Google Scholar] [CrossRef]
- Geraci, C.; CConsoli, G.M.; Granata, G.; Galante, E.; Palmigianom, A.; Pappalardo, M.S.; Di Puma, S.; Spadaro, A. First self-adjuvant multicomponent potential vaccine candidates by tethering of four or eight MUC1 antigenic immunodominant PDTRP units on a calixarene platform: Synthesis and biological evaluation. Bioconjugate Chem. 2013, 24, 1710–1720. [Google Scholar] [CrossRef]
- Xu, L.; Chai, J.; Wang, Y.; Zhao, X.; Guo, D.-S.; Shi, L.; Zhang, Z.; Liu, Y. Calixarene-integrated nano-drug delivery system for tumor-targeted delivery and tracking of anti-cancer drugs in vivo. Nano Res. 2022, 15, 7295–7303. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhao, X.; Xu, L.; Zheng, Y.; Li, H.-B.; Guo, D.-S.; Shi, L.; Liu, Y. Calixarene-modified albumin for stoichiometric delivery of multiple drugs in combination-chemotherapy. Theranostics 2022, 12, 3747–3757. [Google Scholar] [CrossRef]
- Yue, Y.-X.; Zhang, Z.; Wang, Z.-H.; Ma, R.; Chen, M.-M.; Ding, F.; Li, H.-B.; Li, J.-J.; Shi, L.; Liu, Y.; et al. Promoting Tumor Accumulation of Anticancer Drugs by Hierarchical Carrying of Exogenous and Endogenous Vehicles. Small Struct. 2022, 3, 2200067. [Google Scholar] [CrossRef]
- Song, N.; Lou, X.-Y.; Ma, L.; Gao, H.; Yang, Y.-W. Supramolecular nanotheranostics based on pillarenes. Theranostics 2019, 9, 3075–3093. [Google Scholar] [CrossRef]
- Zheng, D.-D.; Fu, D.-Y.; Wu, Y.; Sun, Y.-L.; Tan, L.-L.; Zhou, T.; Ma, S.-Q.; Zha, X.; Yang, Y.-W. Efficient inhibition of human papillomavirus 16 L1 pentamer formation by a carboxylatopillarene and a p-sulfonatocalixarene. Chem. Commun. 2014, 50, 3201–3203. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, W.; Zheng, Y.; Yao, C.; Peng, L.; Zhang, D.; Hu, X.-Y.; Wang, L. GSH-Responsive supramolecular nanoparticles constructed by b-D-galactose-modified pillar [5] arene and camptothecin prodrug for targeted anticancer drug delivery. Chem. Commun. 2017, 53, 8596. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. Supramolecular Drug Delivery System from Macrocycle-Based Self Assembled Amphiphiles for Effective Tumor Therapy. Appl. Mater. Interfac. 2021, 13, 53564–53573. [Google Scholar] [CrossRef]
- Qiu, N.; Li, X.; Liu, J. Application of cyclodextrins in cancer treatment. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 229–246. [Google Scholar] [CrossRef]
- Zhang, D.; Ly, P.; Zhou, C.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef]
- Karthic, A.; Roy, A.; Lakkakula, J.; Alghamdi, S.; Shakoori, A.; Babalghith, A.O.; Bin Emran, T.; Sharma, R.; Lima, C.M.G.; Kim, B.; et al. Cyclodextrin nanoparticles for diagnosis and potential cancer therapy: A systematic review. Front. Cell Dev. Biol. 2022, 10, 984311. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, D.N.; Niculescu, A.-G.; Bolocan, A.; Andronic, O.; Grumezescu, A.M.; Bîrlă, R. An Updated Overview of Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, J.; Wu, C.; Jiang, H.; Zhou, Y.; Zuo, J.; Wang, X. Highly sensitive identification of cancer cells by combining the new tetrathiafulvalene derivative with a β-cyclodextrin/multi-walled carbon nanotubes modified GCE. Analyst 2010, 135, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Li, L.; Wang, B.; Zhi, J.; Xiang, Y.; Li, G. Dynamic electrochemical control of cell capture-and-release based on redox-controlled host-guest interactions. Anal. Chem. 2016, 88, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; You, M.; Zhang, F.; Wang, Q.; He, P. A sensitive electrochemical aptasensing platform based on exonuclease recycling amplification and host-guest recognition for detection of breast cancer biomarker HER2. Sens. Actuat. B Chem. 2018, 258, 796802. [Google Scholar] [CrossRef]
- Nag, S.; Duarte, L.; Bertrand, E.; Celton, V.; Castro, M.; Choudhary, V.; Guegan, P.; Feller, J.F. Ultrasensitive QRS made by supramolecular assembly of functionalized cyclodextrins and graphene for the detection of lung cancer VOC biomarkers. J. Mater. Chem. B 2014, 2, 6571–6579. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Qiao, X.; Wang, H.; Sun, Z.; Qi, Y.; Hong, C. An electrochemical immunosensor for simultaneous point-of-care cancer markers based on the host–guest inclusion of β-cyclodextrin–graphene oxide. J. Mater. Chem. B 2016, 4, 990–996. [Google Scholar] [CrossRef]
- Mortezazadeh, T.; Gholibegloo, E.; Alam, N.R.; Dehghani, S.; Haghgoo, S.; Ghanaati, H.; Khoobi, M. Gadolinium (III) oxide nanoparticles coated with folic acid-functionalized poly(β-cyclodextrin-co-pentetic acid) as a biocompatible targeted nano-contrast agent for cancer diagnostic: In vitro and in vivo studies. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 487–500. [Google Scholar] [CrossRef]
- Shen, H.; Liu, E.; Xu, S.; Tang, W.; Sun, J.; Gao, Z.; Gong, J. Modular assembly of drug and monodisperse SPIONs for superior magnetic and T2-imaging performance. Bioconjugate Chem. 2020, 32, 182–191. [Google Scholar] [CrossRef]
- Sembo-Backonly, B.S.; Estour, F.; Gouhier, G. Cyclodextrins: Promising scaffolds for MRI contrast agents. RSC Adv. 2021, 11, 29762. [Google Scholar] [CrossRef] [PubMed]
- Carmona, T.; Marcelo, G.; Rinaldi, L.; Martina, K.; Cravotto, G.; Mendicuti, F. Soluble cyanine dye/b-cyclodextrin derivatives: Potential carriers for drug delivery and optical imaging. Dye. Pigment. 2015, 114, 204–214. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Zhang, Y.; Tai, F.; Wang, Y.; Dong, Q.; Nie, Y.; Zhao, Q.; Wong, W.-Y. Achieving highly water-soluble and luminescent gold nanoclusters modified by β–cyclodextrin as multifunctional nanoprobe for biological applications. Dye. Pigment. 2018, 157, 359–368. [Google Scholar] [CrossRef]
- Barlas, F.B.; Aydindogan, E.; Arslan, M.; Timur, S.; Yagci, Y. Gold nanoparticle conjugated poly(p-phenylene-β-cyclodextrin)-graft-poly(ethylene glycol) for theranostic applications. J. Appl. Polym. Sci. 2019, 136, 47250–47257. [Google Scholar] [CrossRef]
- Deng, P.; Sun, J.; Chen, J.; Zou, X.; Liao, L. Fast responsive photo#switchable dual-color fluorescent cyclodextrin nanogels for cancer cell imaging. Carbohydr. Polym. 2019, 210, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kun, Q.; Lin, Y.; Peng, H.; Cheng, L.; Cui, H.F.; Hong, N.; Fan, H. A “signal#on” switch electrochemiluminescence biosensor for the detection of tumor cells. J. Electroanal. Chem. 2018, 808, 101–106. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Stevens, D.A. Itraconazole in cyclodextrin solution. Pharmacotherapy 1999, 19, 603–611. [Google Scholar] [CrossRef]
- Committee for Human Medicinal Products. Background Review for Cyclodextrins Used as Excipients; European Medicines Agency: Amsterdam The Netherlands, 2014; pp. 1–17.
- Onodera, R.; Motoyama, K.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Involvement of cholesterol depletion from lipid rafts in apoptosis induced by methyl-β-cyclodextrin. Int. J. Pharm. 2013, 452, 116–123. [Google Scholar] [CrossRef]
- Motoyama, K.; Kameyama, K.; Onodera, R.; Araki, N.; Hirayama, F.; Uekama, K.; Arima, H. Involvement of PI3K-Akt-Bad pathway in apoptosis induced by 2,6-di-O-methyl-betacyclodextrin, not 2,6-di-O-methyl-alpha-cyclodextrin, through cholesterol depletion from lipid rafts on plasma membranes in cells. Eur. J. Pharm. Sci. 2009, 38, 249–261. [Google Scholar] [CrossRef]
- Grosse, P.Y.; Bressolle, F.; Pinguet, F. Antiproliferative effect of methyl-beta-cyclodextrin in vitro and in human tumour xenografted athymic nude mice. Br. J. Cancer 1998, 78, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Onodera, R.; Motoyama, K.; Arima, H. Design and evaluation of folate-appended methyl-beta-cyclodextrin as a new antitumor agent. J. Incl. Phenom. Macrocycl. Chem. 2013, 70, 321–326. [Google Scholar] [CrossRef]
- Onodera, R.; Motoyama, K.; Okamatsu, A.; Higashi, T.; Arima, H. Potential use of folate-appended methyl-β-cyclodextrin as an anticancer agent. Sci. Rep. 2013, 3, 1104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Miyagawa, M.; Lou, X.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. Methyl-β-cyclodextrin potentiates the BITC-induced anti-cancer effect through modulation of the Akt phosphorylation in human colorectal cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 158–2167. [Google Scholar] [CrossRef]
- Mohammad, N.; Malvi, P.; Meena, A.S.; Singh, S.V.; Chaube, B.; Vannuruswamy, G.; Kulkarni, M.J.; Bhat, M.K. Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol. Cancer 2014, 123, 204. [Google Scholar] [CrossRef]
- Motoyama, K.; Onodera, R.; Tanaka, N.; Kameyama, K.; Higashi, T.; Kariya, N.; Okada, S.; Arima, H. Evaluation of Antitumor Effects of Folate-Conjugated Methyl-β-cyclodextrin in Melanoma. Biol. Pharm. Bull. 2015, 38, 374–379. [Google Scholar] [CrossRef]
- Saito, S.; Koya, Y.; Kajiyama, H.; Yamashita, M.; Kikkawa, F.; Nawa, A. Folate-appended cyclodextrin carrier targets ovarian cancer cells expressing the proton-coupled folate transporter. Cancer Sci. 2022, 111, 1794–1804. [Google Scholar] [CrossRef]
- Onodera, R.; Sakai, A.; Tokuda, A.; Higashi, T.; Motoyama, K. The effect of folate-appended methyl-β-cyclodextrin increases on survival rates in a peritoneal dissemination mouse models of human ovarian cancer. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 143–149. [Google Scholar] [CrossRef]
- Frömming, K.H.; Szejtli, J. Pharmacokinetics and toxicology of cyclodextrins. In Cyclodextrins in Pharmacy; Frömming, K.H., Szejtli, J., Eds.; Springer: Budapest, Hungary, 1996; pp. 33–45. [Google Scholar] [CrossRef]
- Yokoo, M.; Kubota, Y.; Motoyama, K.; Higashi, T.; Taniyoshi, M.; Tokumaru, H.; Nishiyama, R.; Tabe, Y.; Mochinaga, S.; Sato, A.; et al. 2-Hydroxypropyl-β-cyclodextrin acts as a novel anticanceragent. PLoS ONE 2015, 10, e0141946. [Google Scholar] [CrossRef]
- Cougnoux, A.; Pergande, M.R.; Serna-Perez, F.; Cologna, S.M. Investigation of 2-Hydroxypropyl-β-Cyclodextrin Treatment in a Neuronal-Like Cell Model of Niemann–Pick Type C Using Quantitative Proteomics. J. Am. Soc. Mass Spectrom. 2023, 34, 668–675. [Google Scholar] [CrossRef]
- Saha, S.T.; Abdulla, N.; Zininga, T.; Shonhai, A.; Wadee, R.; Kaur, M. 2-Hydroxypropyl-β-cyclodextrin (HPβCD) as a Potential Therapeutic Agent for Breast Cancer. Cancers 2023, 15, 2828. [Google Scholar] [CrossRef] [PubMed]
- Hoshiko, T.; Kubota, Y.; Onodera, R.; Higashi, T.; Yokoo, M.; Motoyama, K.; Kimura, S. Folic Acid-Appended Hydroxypropyl-β-Cyclodextrin Exhibits Potent Antitumor Activity in Chronic Myeloid Leukemia Cells via Autophagic Cell Death. Cancers 2021, 13, 5413. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, C.Y.; Wei, H. Application of Cyclodextrin for Cancer Immunotherapy. Molecules 2023, 28, 5610. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liang, M.; Ye, M.; Xu, J.; Liu, H.; Zhang, X.; Sun, W.; Xue, P.; Kang, Y.; Xu, Z. Reduction-triggered polycyclodextrin supramolecular nanocage induces immunogenic cell death for improved chemotherapy. Carbohydr. Polym. 2023, 301, 120365. [Google Scholar] [CrossRef]
- Sinn, S.; Biedermann, F. Chemical Sensors Based on Cucurbit[n]uril Macrocycles. Isr. J. Chem. 2018, 58, 357–412. [Google Scholar] [CrossRef]
- Tan, L.L.; Wei, M.; Shang, L.; Yang, Y.-W. Cucurbiturils-Mediated Noble Metal Nanoparticles forApplications in Sensing, SERS, Theranostics, and Catalysis. Adv. Funct. Mater. 2021, 31, 2007277. [Google Scholar] [CrossRef]
- Das, D.; Assaf, K.I.; Nau, W.M. Applications of Cucurbiturils in Medicinal Chemistry and Chemical Biology. Front. Chem. 2019, 7, 619–641. [Google Scholar] [CrossRef]
- Chernikova, E.Y.; Berdnikova, D.V. Cucurbiturils in nucleic acids research. Chem. Commun. 2020, 56, 15360–15376. [Google Scholar] [CrossRef]
- Bockus, A.T.; Smith, L.C.; Grice, A.G.; Ali, O.A.; Young, C.C.; Mobley, W.; Leek, A.; Roberts, J.L.; Vinciguerra, B.; Isaacs, L.; et al. Cucurbit [7] uril-Tetramethylrhodamine Conjugate for Direct Sensing and Cellular Imaging. J. Am. Chem. Soc. 2016, 138, 16549–16552. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Lei, Q.; Zhu, J.-Y.; Wang, W.-J.; Cheng, Q.; Gao, F.; Sun, Y.-X.; Zhang, X.-Z. Cucurbit[8]uril Regulated Activatable Supramolecular Photosensitizer for Targeted Cancer Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 22892–22899. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Sheng, Y.; Liu, L.; Wu, H.-C. Enhanced Sensitivity in Nanopore Sensing of Cancer Biomarkers in Human Blood via Click Chemistry. Small 2019, 15, 1804078. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, T.; Zhang, S.; Song, P.; Guo, B.; Zhao, Y.; Wu, H.-C. Simultaneous Quantification of Multiple Cancer Biomarkers in Blood Samples through DNA-Assisted Nanopore Sensing. Angew. Chem. 2018, 57, 11882–11887. [Google Scholar] [CrossRef]
- Yang, S.; You, M.; Yang, L.; Zhang, F.; Wang, Q.; He, P. A recyclable electrochemical sensing platform for breast cancer diagnosis based on homogeneous DNA hybridization and host-guest interaction between cucurbit [7] uril and ferrocene-nanosphere with signal amplification. J. Electroanal. Chem. 2016, 783, 161–166. [Google Scholar] [CrossRef]
- Chang, Y.; Zhuo, Y.; Chai, Y.; Yuan, R. A novel host-guest recognition-assisted electrochemical release: Its reusable sensing application based on DNA cross configuration-fueled target cycling and SDR amplification. Anal. Chem. 2017, 89, 8266. [Google Scholar] [CrossRef]
- Jiang, T.; Qu, G.; Wang, J.; Ma, X.; Tian, H. Cucurbiturils brighten Au nanoclusters in water. Chem. Sci. 2020, 11, 3531–3537. [Google Scholar] [CrossRef]
- Le, N.D.B.; Tonga, G.Y.; Mout, R.; Kim, S.T.; Wille, M.E.; Rana, S.; Dunphy, K.A.; Jerry, D.J.; Yazdani, M.; Ramanathan, R.; et al. Cancer Cell Discrimination Using Host−Guest “Doubled” Arrays. J. Am. Chem. Soc. 2017, 139, 8008–8012. [Google Scholar] [CrossRef]
- Zhao, J.; Tanga, Y.; Cao, Y.; Chen, T.; Chen, X.; Mao, X.; Yin, Y.; Chen, G. Amplified electrochemical detection of surface biomarker in breast cancer stem cell using self-assembled supramolecular nanocomposites. Electrochim. Acta 2018, 283, 1072–1078. [Google Scholar] [CrossRef]
- Xu, P.; Feng, Q.; Yang, X.; Liu, S.; Xu, C.; Huang, L.; Chen, M.; Liang, F.; Cheng, Y. Near Infrared Light Triggered Cucurbit[7]uril-Stabilized Gold Nanostars as a Supramolecular Nanoplatform for Combination Treatment of Cancer. Bioconjugate Chem. 2018, 29, 2855–2866. [Google Scholar] [CrossRef]
- Barman, S.; Das, G.; Gupta, V.; Mondal, S.; Jana, B.; Bhunia, D.; Khan, J.; Mukherjee, D.; Ghosh, S. Dual-Arm Nanocapsule Targets Neuropilin-1 Receptor and Microtubule: A Potential Nanomedicine Platform. Mol. Pharm. 2019, 16, 2522–2531. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Yang, Y.; Zhang, Y.-H.; Liu, Y. Polysaccharide Nanoparticles for Efficient siRNA Targeting in Cancer Cells by Supramolecular pKa Shift. Sci. Rep. 2016, 6, 28848. [Google Scholar] [CrossRef]
- Liang, Y.; Fang, R.; Rao, Q. An Insight into the Medicinal Chemistry Perspective of Macrocyclic Derivatives with Antitumor Activity: A Systematic Review. Molecules 2022, 27, 2837. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wu, X.; Papageorgiou, A.C.; Yang, Y.; Wang, X.; Qi, D.; Li, J. Dynamic covalent macrocycles co-delivering genes and drugs against drug-resistant cancer. Cell Rep. Phys. Sci. 2022, 3, 101150. [Google Scholar] [CrossRef]
- Kumar, S.; Chawla, S.; Zou, M.C. Calixarenes based materials for gas sensing applications: A review. J. Incl. Phenom. Macrocycl. Chem. 2017, 88, 129–158. [Google Scholar] [CrossRef]
- Sharma, K.; Cragg, P.J. Calixarene based chemical sensors. Chem. Sens. 2011, 1, 1–18. [Google Scholar]
- Davis, F.; Higson, S.P.J.; Oliveira, O.N., Jr.; Shimizu, F.M. Calixarene-Based Gas Sensors. In Functional Nanomaterials; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Hu, J.-L.; Zhou, T.; Zhang, Y.-F.; Wang, Z.; Luo, D.-M.; Cao, Z. Detection of trace formaldehyde gas based on quartz crystal microbalance sensor in living environment. Adv. Mater. Res. 2011, 233–235, 720–723. [Google Scholar] [CrossRef]
- Cao, Z.; Murayama, K.; Aoki, K. Thickness-shear-mode acoustic wave sensor for acetone vapour coated with C-ethylcalix [4] resorcinarene and C–H π interactions as a molecular recognition mechanism. Anal. Chim. Acta 2001, 448, 47–59. [Google Scholar] [CrossRef]
- Kalchenko, V.I.; Koshets, I.A.; Matsas, E.P.; Kopylov, O.N.; Solovyov, A.; Kazantseva, Z.I.; Shirshov, Y.M. Calixarene based QCM sensors array and its response to volatile organic vapours. Mater. Sci. 2002, 20, 73–88. [Google Scholar]
- Temel, F.; Tabakci, M. Calix[4]arene coated QCM sensors for detection of VOC emissions: Methylene chloride sensing studies. Talanta 2016, 153, 221–227. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Weiss, T.; Thoden van Velzen, E.U.; Engbersen, J.F.J.; Reinhoudt, D.N.; Gopel, W. Molecular Recognition by Self-Assembled Monolayers of Cavitand Receptors. Science 1994, 265, 1413–1415. [Google Scholar] [CrossRef]
- Ozmen, M.; Ozbek, Z.; Bayrakci, M.; Ertul, S.; Ersoz, M.; Capan, R. Preparation and gas sensing properties of Langmuir-Blodgett thin films of calix[n]arenes: Investigation of cavity effect. Sens. Actuator B 2014, 195, 156–164. [Google Scholar] [CrossRef]
- Ozmen, M.; Ozbek, Z.; Buyukcelebi, S.; Bayrakci, M.; Ertul, S.; Ersoz, M.; Capan, R. Fabrication of Langmuir–Blodgett thin films of calix[4]arenes and their gas sensing properties: Investigation of upper rim para substituent effect. Sens. Actuator B 2014, 190, 502–511. [Google Scholar] [CrossRef]
- Şen, S.; Davis, F.; Çapan, R.; Özbek, Z.; Özel, M.E.; Stanciu, G.A. A macrocyclic tetra-undecyl calix[4]resorcinarene thin film receptor for chemical vapour sensor applications. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 237–247. [Google Scholar] [CrossRef]
- Hromadka, J.; James, S.; Davis, F.; Tatam, R.P.; Crump, D.; Korposh, S. Detection of the volatile organic compounds emitted from paints using optical fibre long period grating modified with the mesoporous nanoscale coating. Proc. SPIE 2015, 9634, 96344K1-4. [Google Scholar] [CrossRef]
- Najarzadekan, H.; Kamboh, M.A.; Sereshti, H.; Ahmad, I.; Hassan, I.; Sridewi, N.; Shahabuddinm, S.; Nodeh, H.R. Headspace Extraction of Chlorobenzenes from Water Using Electrospun Nanofibers Fabricated with Calix[4]arene-Doped Polyurethane–Polysulfone. Polymers 2022, 14, 3760. [Google Scholar] [CrossRef]
- Fenyvesi, E.; Jicsinszky, L. Cyclodextrin-containing sensors to provide an early warning of contamination November. Land Contam. Reclam. 2009, 17, 405–412. [Google Scholar] [CrossRef]
- Thomas, J.D.R. Membrane Systems for Piezoelectric and Electrochemical Sensing in Environmental Chemistry. Int. J. Environ. Anal. Chem. 1990, 38, 157–169. [Google Scholar] [CrossRef]
- Fourmentin, S.; Surpateanu, G.G.; Blach, P.; Landy, D.; Decock, P.; Surpateanu, G. Experimental and theoretical study on the inclusion capability of a fluorescent indolizine beta-cyclodextrin sensor towards volatile and semi-volatile organic guest. J. Inclus. Phenom. Macrocycl. Chem. 2006, 55, 263–269. [Google Scholar] [CrossRef]
- Alarie, J.P.; Vo-Dinh, T. A fiber-optic cyclodextrin-based sensor. Talanta 1991, 38, 529–534. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, H.; Wei, Y.; Lin, J.-M. A reversible fluorescence sensor based on insoluble beta-cyclodextrin polymer for direct determination of bisphenol A (BPA). Sens. Actuators B Chem. 2006, B114, 565–572. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, H.; Zhao, L.; Lin, J.-M. Selective determination of bisphenol A (BPA) in water by a reversible fluorescence sensor using pyrene/dimethyl beta-cyclodextrin complex. Anal. Chim. Acta 2006, 556, 313–318. [Google Scholar] [CrossRef]
- Wang, J.; Ueno, A. Naphthol-modified β-cyclodextrins as fluorescent sensors for detecting contaminants in drinking water. Macromol. Rapid Commun. 2000, 21, 887–890. [Google Scholar] [CrossRef]
- Ferancová, A.; Korgová, E.; Labuda, J.; Zima, J.; Barek, J. Cyclodextrin modified carbon paste based electrodes as sensors for the determination of carcinogenic polycyclic aromatic amines. Electroanalysis 2002, 14, 1668–1673. [Google Scholar] [CrossRef]
- Ferancová, A.; Buckova, M.; Korgova, E.; Korbut, O.; Gruendler, P.; Waernmark, I.; Stepan, R.; Barek, J.; Zima, J.; Labuda, J. Association interaction and voltammetric determination of 1-aminopyrene and 1-hydroxypyrene at cyclodextrin and DNA-based electrochemical sensors. Bioelectrochem 2005, 67, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Takeuchi, T.; Ogata, M.; Takanohashi, A.; Mikuni, K.; Nakanishi, K.; Imata, I. Detection of environmental chemicals by SPR assay using branched cyclodextrin as sensor ligand. J. Inclus. Phenom. Macrocycl. Chem. 2007, 57, 339–342. [Google Scholar] [CrossRef]
- Si, S.H.; Fung, S.; Zhu, D.R. Improvement of piezoelectric crystal sensor for the detection of organic vapors using nanocrystalline TiO2 films. Sens. Actuators B Chem. 2005, B108, 165–171. [Google Scholar] [CrossRef]
- Duarte, L.; Nag, S.; Castro, M.; Zaborova, E.; Ménand, M.; Sollogoub, M.; Bennevault, V.; Feller, J.; Guégan, P. Chemical Sensors Based on New Polyamides Biobased on (Z) Octadec-9-Enedioic Acid and β-Cyclodextrin. Macromol. Chem. Phys. 2016, 217, 1620–1628. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef]
- Collyer, S.D.; Bradbury, S.D.; Davis, F.; Hatfield, J.V.; Lucke, A.J.; Stirling, C.J.M.; Higson, S.P.J. Calix [4] resorcinarenetetrathiol Modified Gold Electrodes; Applications to the Adsorption and Electrochemical Determination of N-Nitrosamines. Electroanalysis 2004, 16, 324–327. [Google Scholar] [CrossRef]
- Lu, R.-Q.; Yuan, W.; Croy, R.G.; Essigmann, J.M.; Swager, T.M. Metallocalix[4]arene Polymers for Gravimetric Detection of N-Nitrosodialkylamines. J. Am. Chem. Soc. 2021, 143, 19809–19815. [Google Scholar] [CrossRef]
- Minami, T.; Esipenko, N.A.; Zhang, B.; Kozelkova, M.E.; Isaacs, L.; Nishiyabu, R.; Kubo, Y.; Anzenbacher, P. Supramolecular Sensor for Cancer-Associated Nitrosamines. J. Am. Chem. Soc. 2012, 134, 20021–20024. [Google Scholar] [CrossRef]
- Akceylana, A.; Bahadirb, M.; Yılmaza, M. Removal efficiency of a calix [4] arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J. Hazard. Mater. 2009, 162, 960–966. [Google Scholar] [CrossRef]
- Erdemir, S.; Bahadir, M.; Yilmaz, M. Extraction of carcinogenic aromatic amines from aqueous solution using calix [n] arene derivatives as carrier. J. Hazard. Mater. 2009, 168, 1170–1176. [Google Scholar] [CrossRef]
- Arslan, M.; Sayin, S.; Yilmaz, M. Removal of Carcinogenic Azo Dyes from Water by New Cyclodextrin-Immobilized Iron Oxide Magnetic Nanoparticles. Water Air Soil. Poll. 2013, 224, 1527. [Google Scholar] [CrossRef]
- Serio, N.; Chanthalyma, C.; Prignano, L.; Levine, M. Cyclodextrin-Enhanced Extraction and Energy Transfer of Carcinogens in Complex Oil Environments. ACS Appl. Mater. Interfaces 2013, 5, 11951011957. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Miles, M.S.; Meyer, B.M.; Wonga, R.L.; Overton, E.B. Assessment of cyclodextrin-enhanced extraction of crude oil from contaminated porous media. J. Environ. Monit. 2012, 14, 2164–2169. [Google Scholar] [CrossRef]
- BenMihoub, A.; Larue, K.; Moussaron, A.; Youssef, Z.; Colombeau, L.; Baros, F.; Frochot, C.; Vanderesse, R.; Acherar, S. Use of Cyclodextrins in Anticancer Photodynamic Therapy Treatment. Molecules 2018, 23, 1936. [Google Scholar] [CrossRef]
- Fedorova, O.; Fedorov, Y. Cyclodextrins as Supramolecular Hosts for Dye Molecules. In Cyclodextrins—Core Concepts and New Frontiers; Rashid, A., Ed.; Publisher Intechopen: London, UK, 2023. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-T.; Li, Y.; Duan, X.; Wang, X.; Qi, C.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Substitution Activated Precise Phototheranostics through Supramolecular Assembly of AIEgen and Calixarene. J. Am. Chem. Soc. 2020, 142, 15966–15974. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, B.Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Cui, Z.; Wang, P.; Chen, S.; Yang, G.; Zhang, W. Mitochondria-targeting and ROS-sensitive smart nanoscale supramolecular organic framework for combinational amplified photodynamic therapy and chemotherapy. Acta Biomater. 2021, 130, 447–459. [Google Scholar] [CrossRef]
- Kepes, Z.; Hajdu, I.; Fenyvesi, F.; Trencsenyi, G. Insights into recent preclinical studies on labelled cyclodextrin-based imaging probes: Towards a novel oncological era. Int. J. Pharm. 2023, 640, 122978. [Google Scholar] [CrossRef]
- Benkovics, G.; Malanga, M.; Fenyvesi, E. The ‘Visualized’ macrocycles: Chemistry and application of fluorophore tagged cyclodextrins. Int. J. Pharm. 2017, 531, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Jayawant, M.; Raut, R.; Lakkakula, J.; Roy, A.; Alghamdi, S.; Qusty, N.F.; Sharma, R.; Verma, D.; Khandaker, M.U.; et al. Cyclodextrin nanoparticles in targeted cancer theranostics. Front. Pharmacol. 2023, 14, 1218867. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q. Bioresponsive and multifunctional cyclodextrin-based non-viral nanocomplexes in cancer therapy: Building foundations for gene and drug delivery, immunotherapy and bioimaging. Environ. Res. 2023, 234, 116507. [Google Scholar] [CrossRef]
- Cheng, H.-B.; Zhang, Y.-M.; Liu, Y.; Yoon, J. Turn-On Supramolecular Host-Guest Nanosystems as Theranostics for Cancer. Chem 2019, 5, 553–574. [Google Scholar] [CrossRef]



| Cucurbituril | Cavity Width (nm) | Portal Width (nm) | Cavity Volume (nm3) |
|---|---|---|---|
| CB5 | 0.44 | 0.24 | 0.082 |
| CB6 | 0.58 | 0.39 | 0.164 |
| CB7 | 0.73 | 0.54 | 0.279 |
| CB8 | 0.88 | 0.69 | 0.479 |
| CB10 | 1.13–1.24 | 0.95–1.06 | 0.870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, F.; Higson, S.P.J. Synthetic Receptors for Early Detection and Treatment of Cancer. Biosensors 2023, 13, 953. https://doi.org/10.3390/bios13110953
Davis F, Higson SPJ. Synthetic Receptors for Early Detection and Treatment of Cancer. Biosensors. 2023; 13(11):953. https://doi.org/10.3390/bios13110953
Chicago/Turabian StyleDavis, Frank, and Séamus P. J. Higson. 2023. "Synthetic Receptors for Early Detection and Treatment of Cancer" Biosensors 13, no. 11: 953. https://doi.org/10.3390/bios13110953
APA StyleDavis, F., & Higson, S. P. J. (2023). Synthetic Receptors for Early Detection and Treatment of Cancer. Biosensors, 13(11), 953. https://doi.org/10.3390/bios13110953






