Fluorescent-Based Neurotransmitter Sensors: Present and Future Perspectives
Abstract
:1. Introduction
| S.no | NTs | Plasma | Ref. |
|---|---|---|---|
| 1 | Dopamine | 0 to 30 pg/mL | [13] |
| Epinephrine | 0 to 140 pg/mL | [14] | |
| 2 | Nor epinephrine | 70 to 1700 pg/mL | [14] |
| 3 | Serotonin | 50 to 200 ng/mL | [13] |
| 4 | Gamma-amino butyric Acid (GABA) | 30 to 79 pmol/mL | [15] |
| 5 | Acetylcholine (ACh) | 0.20 to 1.31 µmole/L | [13] |
| 6 | Glutamate | 40 to 60 μM | [16] |
2. Principle of Fluorescence-Based Biosensors
2.1. Fluorescence Resonance Energy Transfer (FRET)
2.2. Photon-Induced Electron Transfer (PET)
2.3. Excited-State Intramolecular Proton Transfer (ESIPT)
2.4. Aggregation-Induced Emission (AIE)
2.5. Dual- or Triple-Sensing Mechanisms
3. Neurotransmitters and Their Importance
3.1. Dopamine (DA)
3.2. Epinephrine (EP)
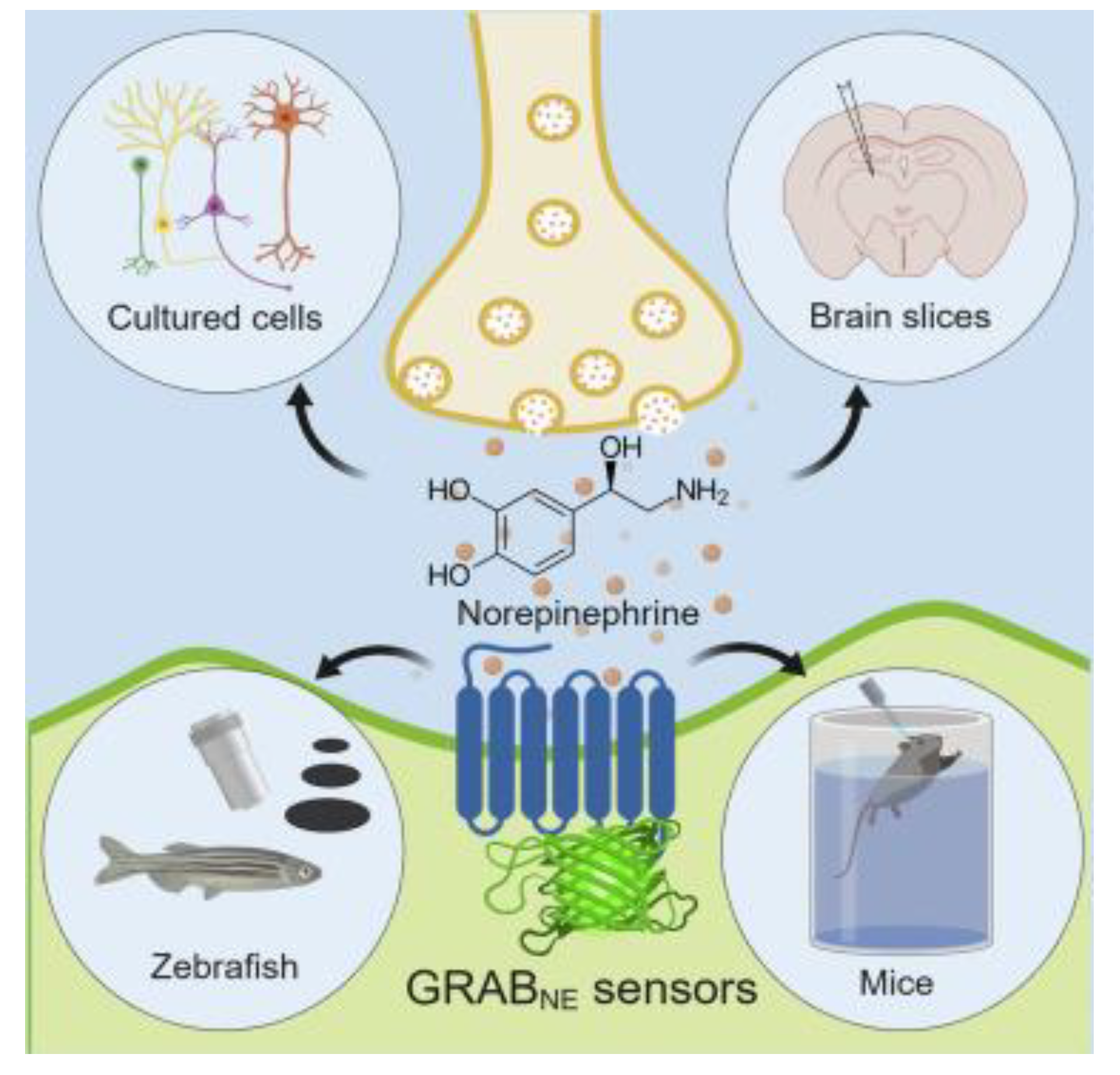
3.3. Norepinephrine (NE)
3.4. Serotonin
3.5. Gamma-Aminobutyric Acid (GABA)
3.6. Acetylcholine (ACh)
3.7. Glutamate
4. Fluorescence-Based Biosensors for NTs
4.1. Nanomaterial-Based Biosensors
4.1.1. Metal Nanoclusters (M-NCs)
| Nano Materials | Target | LOD | Linear Range | Ref. |
|---|---|---|---|---|
| Quantum dots (thioglycolic acid capped) | DA | 2.55 nM | 46.7 nM~0.394 μM | [103] |
| Quantum dots (Ndoped graphene) | DA | 3.3 nM | 10~3000 nM | [104] |
| Quantum dots (InP/ZnS) | DA | 5 nM | 0~100 nM | [105] |
| Quantum dots (Graphene) | DA | 22 nM | 1~40 μM | [106] |
| Quantum dots (Graphene) | DA | 90 nM | 0.25~50 μM | [107] |
| Carbon dots (boronic acid and amino groups) | DA | 0.1 pM | 1 Pm~1 μM | [108] |
| Carbon dots (Sulphur doped) | DA | 47 pM | 0~20 μM | [109] |
| Carbon dots (Nitrogen doped) | DA | 5.54 μM | 3.3~500 μM | [110] |
| N-Quantum dots (Nitrogen doped) | DA | 0.07 µM | 1~200 μM | [111] |
| N-Carbon dots (Glutathione modified) | DA | 1.01 nM | 20 nM~10 μM | [112] |
| Gold NFs | DA | 0.21 nM | 0.8~300 nM | [113] |
| Graphene Oxide | DA | 94 nM | 0.25~20 μM | [45] |
| Quantum dots (CdTe) | EP | 6.8 nM | 10 nM~20 μM | [114] |
| Quantum dots (CuInS2 capped by L-Cys) | EP | 3.6 nM | 1 × 10–8~1 × 10–4 M | [115] |
| Copper NPs | EP | 0.2 μM | 3 × 10–5~5 × 10–7 M | [116] |
| Carbon NPs | EP | 88 nM | 0.1~50 μM | [117] |
| Carbon NTs | EP | 50 nM | 2.3 nM~9.4 μM | [118] |
| Gold NCs | EP | 910 pM | 10~100 μM | [119] |
| Fluorescence dye | EP | 0.14 nM | 1~120 nM | [49] |
| Quantum dots (molecularly imprinted polymers) | NEP | 9 nM | 0.08~20 μM | [120] |
| Quantum dots (3-mercapropionic acid-coated CdTe QDs) | NEP | 2.1 nM | 5 nM~10 μM | [114] |
| Quantum dots (thioglycolic acid functionalized cadmium telluride (CdTe)) | NEP | 0.3 μM | 2.5~20.0 μM | [121] |
| Quantum dots (molecularly imprinted polymer CdTe@ SiO2) | NEP | 8 nM | 0.04~10 μM | [122] |
| Gold NCs | NEP | 49 nM | 0.5~40.0 μM | [123] |
| Carbon NPs | NEP | 91 nM | 0.1~50 μM | [117] |
| Silver NPs | NEP | 5.59 μM | 8.92 × 10–3~5.66 × 10–5 M | [124] |
| Quantum dots (Mn2+ doped ZnS) | 5-HT | 3.91 nM | 0.28~2.8 μM | [125] |
| Gold NCs | 5-HT | 49 nM | 0.2~50 μM | [126] |
| rGraphene oxide | 5-HT | 55 nM | NA | [127] |
| Quantum dots (NADP+-functionalized) | GABA | 22 nM | NA | [128] |
| Carbon dots (silica functionalized) | GABA | 6.46 μM | 0~90 μM | [129] |
4.1.2. Carbon Dots (CDs)
4.1.3. Graphene QDs (GQDs)
4.2. Enzyme-Based Biosensors
4.3. Aptamer-Based Fluorescence Biosensors
| S. No | Biosensor | Analyte | Fluorescence Probe | Nanomaterials | Sample | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Aptamer | DA | SYBR Green I | - | Mouse brain Tissue | 8 × 10−5 µM | 10−4~10−3 μM | [152] |
| 2 | Aptamer | DA | MoS2 Quantum dots as fluorophore | Quantum dots (Molybdenum disulphide) | Human serum | 45 pM | 0.1~1000 nM | [154] |
| 3 | Aptamer | DA | Ru complex | Quantum dots (RU Cadmium-Tellurim) | Fetal bovine serum | 19 nM | 0.03~0.21 μM | [155] |
| 4 | Aptamer | DA | Carboxy fluorescein (FAM) | Graphene oxide | Human serum | 0.031 nM | 3~1680 nM | [151] |
| 5 | Aptamer | DA | Carboxy fluorescein (FAM) | Graphene oxide | Human plasma and serum | 1.0 nmol/L | - | [156] |
| 6 | Aptamer | DA | 5-carboxy-fluorescein (FAM) | SWCNs | Human serum | 5 μM | 0.02~2.20 mM | [153] |
| 7 | Enzyme | DA | Adenosine monophosphate (AMP) and Cu2+ | Polymer dots (Pdots@AMP-Cu) | Human serum | 4 μM | 10~400 μM | [157] |
| 8 | Enzyme | GABA | GABase enzyme | Carbon dots (corn juice) | Human serum | 95.09 nM. | 0–20 µM | [148] |
4.4. GE Fluorescence Biosensors
5. Conclusion and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Neurotransmitters | NTs |
| High-pressure liquid chromatography | HPLC |
| Mass Spectroscopy | MS |
| Capillary Electrophoresis | CE |
| Nanoparticles | NPs |
| Nanotubes | NT |
| Nanoclusters | NCs |
| Nanorods | NRs |
| Nanocomposites | NC |
| Quantum dots | QDs |
| carbon dots | CDs |
| Förster resonance energy transfer | FRET |
| Genetically encoded | GE |
| Photon-induced electron transfer | PET |
| Intramolecular charge transfer | ICT |
| Excited-state intramolecular proton transfer | ESIPT |
| Silver nanoclusters | Ag NCs |
| Glutathione | GSH |
| Dihydrolipoic acid | DHLA |
| Fluorescence resonance energy transfer–intramolecular charge transfer | FRET-ICT |
| Fluorescence resonance energy transfer–photo-induced electron transfer | FRET-PET |
| Through bond energy transfer–photo-induced electron transfer | TBET-PET |
| Intramolecular charge transfer–photo-induced electron transfer | ICT-PET |
| Excited-state intramolecular proton transfer–aggregated induced emission | ESIPT-AIE |
| Excited-state intramolecular proton transfer –photo-induced electron transfer | ESIPT-PET |
| Excited-state intramolecular proton transfer–fluorescence resonance energy transfer | ESIPT-FRET |
| Excited-state intramolecular proton transfer–intramolecular charge transfer | ESIPT-ICT |
| Photo-induced electron transfer–intramolecular charge transfer–excited-state intramolecular proton transfer | PET-ICT-ESIPT |
| Photo-induced electron transfer–intramolecular charge transfer–fluorescence resonance energy transfer | PET-ICT-FRET |
| Dopamine | DA |
| Epinephrine | EP |
| Norepinephrine | NP |
| Electrochemical detection | ECD |
| 5-hydroxytryptamine | 5-HT |
| 5-hydroxyindole-3-acetic acid | 5-HI-3-AA |
| Gamma-aminobutyric acid | GABA |
| Evaporative light scattering detector | ELSD |
| Acetylcholine | Ach |
| N-methyl-d-aspartic acid | NMDA |
| α-amino-3hydroxyl-5-methyl-4-isoxazole-propionate | AMPA |
| Metabotropic glutamate receptors | mGluRs |
| Metal nanoclusters | M-NCs |
| Photoluminescence | PL |
| Gold nanoclusters | AuNCs |
| 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride/N-hydroxy-succinimide | EDC/NHS |
| Graphite-like carbon nitride nanosheets | g-C3N4NSs |
| Bovine serum albumin | BSA |
| Gold–silver NCs | Au-AgNCs |
| Copper NCs | CuNCs |
| Carbon dots | CDs |
| Ascorbic acid | AA |
| Uric acid | UA |
| N-doped CDs | NCDs |
| Limit of detection | LOD |
| N- and B-co-doped CQDs | NB-CQDs |
| Graphene QDs | GQDs |
| Nitrogen-doped GQDs | N-GQDs |
| Sulphur-doped GQDs | S-GQDs |
| Nitrogen- and sulphur-doped GQDs | NS-GQDs |
| Nitrogen- and phosphorous-doped GQDs | NP-GQDs |
| CDs/tyrosinase | CDs/TYR |
| Dopaquinone | DQ |
| Cerebrospinal fluid | CSF |
| Molecular beacon aptamers | MBAs |
| Complementary strand DNA | cDNA |
| Single-walled carbon nano horns | SWCNHs |
| G-protein-coupled receptors | GPCRs |
References
- Dinarvand, M.; Elizarova, S.; Daniel, J.; Kruss, S. Imaging of Monoamine Neurotransmitters with Fluorescent Nanoscale Sensors. ChemPlusChem 2020, 85, 1465–1480. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladacenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Shippenberg, T.S.; Thompson, A.C. Overview of microdialysis. Curr. Protoc. Neurosci. 1997, 47, 7.1.1–7.1.22. [Google Scholar] [CrossRef] [PubMed]
- Nováková, D.; Kudláček, K.; Novotný, J.; Nesměrák, K. Improvement of conditions for the determination of neurotransmitters in rat brain tissue by HPLC with fluorimetric detection. Monatshefte Chem. Chem. Mon. 2022, 153, 753–758. [Google Scholar] [CrossRef]
- Olesti, E.; Rodriguez-Morato, J.; Gomez-Gomez, A.; Ramaekers, J.G.; de la Torre, R.; Pozo, O.J. Quantification of endogenous neurotransmitters and related compounds by liquid chromatography coupled to tandem mass spectrometry. Talanta 2019, 192, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Francis, K.A.; Patel, J.; Jha, S.K.; Basu, S. A decoupler-free simple paper microchip capillary electrophoresis device for simultaneous detection of dopamine, epinephrine and serotonin. RSC Adv. 2020, 10, 25487–25495. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kim, E.H.; Flask, C.A.; Clark, H.A. Nanosensors for the Chemical Imaging of Acetylcholine Using Magnetic Resonance Imaging. ACS Nano 2018, 12, 5761–5773. [Google Scholar] [CrossRef]
- Binnie, C.; Prior, P. Electroencephalography. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1308–1319. [Google Scholar] [CrossRef]
- Chauhan, N.; Soni, S.; Agrawal, P.; Balhara, Y.P.S.; Jain, U. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process Biochem. 2020, 91, 241–259. [Google Scholar] [CrossRef]
- Arumugasamy, S.K.; Chellasamy, G.; Gopi, S.; Govindaraju, S.; Yun, K. Current advances in the detection of neurotransmitters by nanomaterials: An update. TrAC Trends Anal. Chem. 2020, 123, 115766. [Google Scholar] [CrossRef]
- Leopold, A.V.; Shcherbakova, D.M.; Verkhusha, V.V. Fluorescent biosensors for neurotransmission and neuromodulation: Engineering and applications. Front. Cell. Neurosci. 2019, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Lee, J.-S. Recent advances in optical detection of dopamine using nanomaterials. Microchim. Acta 2017, 184, 1239–1266. [Google Scholar] [CrossRef]
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis and Management by Laboratory Methods; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 9780323755085. [Google Scholar]
- Young, W.F. Adrenal medulla, catecholamines, and pheochromocytoma. In Goldman’s Cecil Medicine, 24th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 1470–1475. [Google Scholar] [CrossRef]
- Schmidt, D.; Löscher, W. Plasma and cerebrospinal fluid gamma-aminobutyric acid in neurological disorders. J. Neurol. Neurosurg. Psychiatry 1982, 45, 931–935. [Google Scholar] [CrossRef]
- Bai, W.; Zhu, W.-L.; Ning, Y.-L.; Li, P.; Zhao, Y.; Yang, N.; Chen, X.; Jiang, Y.-L.; Yang, W.-Q.; Jiang, D.-P. Dramatic increases in blood glutamate concentrations are closely related to traumatic brain injury-induced acute lung injury. Sci. Rep. 2017, 7, 5380. [Google Scholar] [CrossRef]
- Kakkar, S.; Gupta, P.; Kumar, N.; Kant, K. Progress in Fluorescence Biosensing and Food Safety towards Point-of-Detection (PoD) System. Biosensors 2023, 13, 249. [Google Scholar] [CrossRef]
- Li, G.Y.; Han, K.L. The sensing mechanism studies of the fluorescent probes with electronically excited state calculations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1351. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Mukherjee, P.; Saikia, T.; Pal, K.; Sahu, S.K. A design of fluorescence-based sensor for the detection of dopamine via FRET as well as live cell imaging. Microchem. J. 2020, 159, 105590. [Google Scholar] [CrossRef]
- Niu, H.; Liu, J.; O’Connor, H.M.; Gunnlaugsson, T.; James, T.D.; Zhang, H. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. A novel nitrogen-doped carbon quantum dots as effective fluorescent probes for detecting dopamine. J. Photochem. Photobiol. A Chem. 2020, 391, 112374. [Google Scholar] [CrossRef]
- Weller, A. Innermolekularer protonenübergang im angeregten zustand. Z. Elektrochem. Berichte Bunsenges. Phys. Chem. 1956, 60, 1144–1147. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, T.; Ma, Y.; Sun, J.; Zheng, G.; Ma, T.; Yang, X.; Song, Z.; Lv, Y.; Zhang, J. Monitoring acetylcholinesterase level changes under oxidative stress through ESIPT-ICT-based near-infrared fluorescent probe. Sens. Actuators B Chem. 2023, 380, 133392. [Google Scholar] [CrossRef]
- Mao, L.; Liu, Y.; Yang, S.; Li, Y.; Zhang, X.; Wei, Y. Recent advances and progress of fluorescent bio-/chemosensors based on aggregation-induced emission molecules. Dye. Pigment. 2019, 162, 611–623. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, M.; Xu, D.; Mao, L.; Huang, H.; Gao, P.; Deng, F.; Zhang, X.; Wei, Y. Fabrication of amphiphilic fluorescent nanoparticles with an AIE feature via a one-pot clickable mercaptoacetic acid locking imine reaction: Synthesis, self-assembly and bioimaging. Polym. Chem. 2016, 7, 4559–4566. [Google Scholar] [CrossRef]
- Yuan, Y.; Kwok, R.T.; Feng, G.; Liang, J.; Geng, J.; Tang, B.Z.; Liu, B. Rational design of fluorescent light-up probes based on an AIE luminogen for targeted intracellular thiol imaging. Chem. Commun. 2014, 50, 295–297. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.; Zhang, G.; Tang, Y.; Wang, S.; Zhu, D. Fluorescence turn-on detection of DNA and label-free fluorescence nuclease assay based on the aggregation-induced emission of silole. Anal. Chem. 2008, 80, 6443–6448. [Google Scholar] [CrossRef]
- Gu, B.; Wu, W.; Xu, G.; Feng, G.; Yin, F.; Chong, P.H.J.; Qu, J.; Yong, K.T.; Liu, B. Precise two-photon photodynamic therapy using an efficient photosensitizer with aggregation-induced emission characteristics. Adv. Mater. 2017, 29, 1701076. [Google Scholar] [CrossRef]
- Das, D.; Maity, A.; Shyamal, M.; Maity, S.; Mudi, N.; Misra, A. Aggregation induced emission of 9-Anthraldehyde microstructures and its selective sensing behavior towards picric acid. J. Mol. Liq. 2018, 261, 446–455. [Google Scholar] [CrossRef]
- Zhu, Z.; Qian, J.; Zhao, X.; Qin, W.; Hu, R.; Zhang, H.; Li, D.; Xu, Z.; Tang, B.Z.; He, S. Stable and size-tunable aggregation-induced emission nanoparticles encapsulated with nanographene oxide and applications in three-photon fluorescence bioimaging. ACS Nano 2016, 10, 588–597. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Z.; Tebyetekerwa, M.; Zhang, J.; Wang, Y.; Gao, T.; Wang, J.; Ding, Y.; Xie, Y. Preparation of silica/polymer nanocomposites with aggregation-induced emission properties as fluorescent responsive coatings. Prog. Org. Coat. 2019, 127, 8–15. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, L.; Zhang, X.Y.; Wang, X.H.; Zhou, J.; Sun, Z.; Li, N.B.; Luo, H.Q. Ratiometric fluorescence detection of dopamine based on effect of ligand on the emission of Ag nanoclusters and aggregation-induced emission enhancement. Sens. Actuators B Chem. 2020, 310, 127858. [Google Scholar] [CrossRef]
- He, L.; Dong, B.; Liu, Y.; Lin, W. Fluorescent chemosensors manipulated by dual/triple interplaying sensing mechanisms. Chem. Soc. Rev. 2016, 45, 6449–6461. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, S.; Liu, Z.; Shi, Y.; Yue, W.; Yi, C. Rapid determination of dopamine in human plasma using a gold nanoparticle-based dual-mode sensing system. Mater. Sci. Eng. C 2016, 61, 207–213. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Conrad, B. The Role of Dopamine as a Neurotransmitter in the Human Brain. Sci. Cent. 2018. [Google Scholar]
- Napier, T.C.; Kirby, A.; Persons, A.L. The role of dopamine pharmacotherapy and addiction-like behaviors in Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109942. [Google Scholar]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and Dopamine Receptors in Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef]
- Battal, D.; Aktas Sukuroglu, A.; Alkas, F.B.; Unlusayin, I. A Rapid, Precise, and Sensitive LC-MS/MS Method for the Quantitative Determination of Urinary Dopamine Levels via a Simple Liquid-liquid Extraction Technique. Turk. J. Pharm. Sci. 2021, 18, 761–769. [Google Scholar] [CrossRef]
- Shinohara, H.; Wang, F. Real-time detection of dopamine released from a nerve model cell by an enzyme-catalyzed luminescence method and its application to drug assessment. Anal. Sci. 2007, 23, 81–84. [Google Scholar] [CrossRef]
- Lee, H.C.; Chen, T.H.; Tseng, W.L.; Lin, C.H. Novel core etching technique of gold nanoparticles for colorimetric dopamine detection. Analyst 2012, 137, 5352–5357. [Google Scholar] [CrossRef]
- Chen, J.L.; Yan, X.P.; Meng, K.; Wang, S.F. Graphene oxide based photoinduced charge transfer label-free near-infrared fluorescent biosensor for dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Shen, Y.; Zhang, J.; Zhu, J.J. Electrogenerated chemiluminescence of Au nanoclusters for the detection of dopamine. Anal. Chem. 2011, 83, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Nelson, G.W.; Abda, J.; Foord, J.S. Novel Modifications to Carbon-Based Electrodes to Improve the Electrochemical Detection of Dopamine. ACS Appl. Mater. Interfaces 2016, 8, 28338–28348. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.h.; Mittal, J.; Yan, D.; Eshraghi, A.A. Neurotransmitters: The critical modulators regulating gut–brain axis. J. Cell Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Malecha, K.; Swist, A.; Cabaj, J. Fluorescence Sensing Platforms for Epinephrine Detection Based on Low Temperature Cofired Ceramics. Sensors 2020, 20, 1429. [Google Scholar] [CrossRef]
- Kirkpatrick, D.; Yang, J.; Trehy, M. Determination of the enantiomeric purity of epinephrine by HPLC with circular dichroism detection. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Britz-Mckibbin, P.; Wong, J.; Chen, D.D. Analysis of epinephrine from fifteen different dental anesthetic formulations by capillary electrophoresis. J. Chromatogr. A 1999, 853, 535–540. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Sulka, G.D. Fabrication of highly ordered nanoporous thin Au films and their application for electrochemical determination of epinephrine. Sens. Actuators B Chem. 2016, 222, 270–279. [Google Scholar] [CrossRef]
- Azargoshasb, T.; Parvizi, R.; Navid, H.A.; Parsanasab, G.-M.; Heidari, H. Versatile selective absorption-based optical fiber toward epinephrine detection. Sens. Actuators B Chem. 2022, 372, 132551. [Google Scholar] [CrossRef]
- Grella, S.L.; Gomes, S.M.; Lackie, R.E.; Renda, B.; Marrone, D.F. Norepinephrine as a spatial memory reset signal. Behav. Pharmacol. 2021, 32, 531–548. [Google Scholar] [CrossRef]
- Berridge, C.W.; Schmeichel, B.E.; Espana, R.A. Noradrenergic modulation of wakefulness/arousal. Sleep. Med. Rev. 2012, 16, 187–197. [Google Scholar] [CrossRef] [PubMed]
- McBurney-Lin, J.; Lu, J.; Zuo, Y.; Yang, H. Locus coeruleus-norepinephrine modulation of sensory processing and perception: A focused review. Neurosci. Biobehav. Rev. 2019, 105, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of brain microdialysis. Curr. Protoc. Neurosci. 2009, 47, 7.1.1–7.1.28. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Van der Schyf, C.J. Role of serotonin in Alzheimer’s disease: A new therapeutic target? CNS Drugs 2011, 25, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Lal, G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics 2021, 11, 5296. [Google Scholar] [CrossRef]
- Ewang-Emukowhate, M.; Nair, D.; Caplin, M. The role of 5-hydroxyindoleacetic acid in neuroendocrine tumors: The journey so far. Int. J. Endocr. Oncol. 2019, 6, IJE17. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, X.; Li, H.; Chen, Z.; Guan, Q.; Cheng, L. Simple and reliable serotonin assay in human serum by LC-MS/MS method coupled with one step protein precipitation for clinical testing in patients with carcinoid tumors. J. Chromatogr. B 2020, 1158, 122395. [Google Scholar] [CrossRef]
- Wallingford, R.A.; Ewing, A.G. Separation of serotonin from catechols by capillary zone electrophoresis with electrochemical detection. Anal. Chem. 1989, 61, 98–100. [Google Scholar] [CrossRef]
- Chauveau, J.; Fert, V.; Morel, A.; Delaage, M. Rapid and specific enzyme immunoassay of serotonin. Clin. Chem. 1991, 37, 1178–1184. [Google Scholar] [CrossRef]
- Bogdanski, D.F.; Pletscher, A.; Brodie, B.B.; Udenfriend, S. Identification and assay of serotonin in brain. J. Pharmacol. Exp. Ther. 1956, 117, 82–88. [Google Scholar]
- Snyder, S.H.; Axelrod, J.; Zweig, M. A sensitive and specific fluorescence assay for tissue serotonin. Biochem. Pharmacol. 1965, 14, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Hickman, A.B.; Klein, D.C.; Dyda, F. Melatonin biosynthesis: The structure of serotonin N-acetyltransferase at 2.5 Å resolution suggests a catalytic mechanism. Mol. Cell 1999, 3, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yin, C.; Teramoto, A.; Sakuma, Y.; Kato, M. Sexually dimorphic modulation of GABAA receptor currents by melatonin in rat gonadotropin-releasing hormone neurons. J. Physiol. Sci. 2008, 58, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Liao, J.; Cai, Y.; Fan, M.; Li, C.; Zhang, X.; Li, H.; Chen, Y.; Pan, J. Impairment of GABA inhibition in insomnia disorders: Evidence from the peripheral blood system. Front. Psychiatry 2023, 14, 1134434. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, Z.-H.; Liu, C.; Li, G.-Y.; Qiao, X.-Z.; Gan, Y.-J.; Zhang, C.-C.; Deng, Y.-C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef]
- Hantsoo, L.; Epperson, C.N. Allopregnanolone in premenstrual dysphoric disorder (PMDD): Evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol. Stress. 2020, 12, 100213. [Google Scholar] [CrossRef]
- Adkins, C.E.; Pillai, G.V.; Kerby, J.; Bonnert, T.P.; Haldon, C.; McKernan, R.M.; Gonzalez, J.E.; Oades, K.; Whiting, P.J.; Simpson, P.B. α4β3δ GABAAReceptors Characterized by Fluorescence Resonance Energy Transfer-derived Measurements of Membrane Potential. J. Biol. Chem. 2001, 276, 38934–38939. [Google Scholar] [CrossRef]
- Pencheva, D.; Teneva, D.; Denev, P. Validation of HPLC Method for Analysis of Gamma-Aminobutyric and Glutamic Acids in Plant Foods and Medicinal Plants. Molecules 2022, 28, 84. [Google Scholar] [CrossRef]
- Meeploy, M.; Deewatthanawong, R. Determination of γ-aminobutyric acid (GABA) in rambutan fruit cv. Rongrian by HPLC-ELSD and separation of GABA from rambutan fruit using Dowex 50W-X8 column. J. Chromatogr. Sci. 2016, 54, 445–452. [Google Scholar] [CrossRef]
- Westerink, B.H.; de Vries, J.B. On the origin of extracellular GABA collected by brain microdialysis and assayed by a simplified on-line method. Naunyn Schmiedebergs Arch. Pharmacol. 1989, 339, 603–607. [Google Scholar] [CrossRef]
- Arrubla, J.; Tse, D.H.; Amkreutz, C.; Neuner, I.; Shah, N.J. GABA concentration in posterior cingulate cortex predicts putamen response during resting state fMRI. PLoS ONE 2014, 9, e106609. [Google Scholar] [CrossRef] [PubMed]
- Singer, N.K.; Sánchez-Murcia, P.A.; Ernst, M.; González, L. Unravelling the Turn-On Fluorescence Mechanism of a Fluorescein-Based Probe in GABAA Receptors. Angew. Chem. 2022, 134, e202205198. [Google Scholar] [CrossRef]
- Simpson, B.; Rich, M.M.; Voss, A.A.; Talmadge, R.J. Acetylcholine receptor subunit expression in Huntington’s disease mouse muscle. Biochem. Biophys. Rep. 2021, 28, 101182. [Google Scholar] [CrossRef] [PubMed]
- Amare, A.T.; Thalamuthu, A.; Schubert, K.O.; Fullerton, J.M.; Ahmed, M.; Hartmann, S.; Papiol, S.; Heilbronner, U.; Degenhardt, F.; Tekola-Ayele, F. Association of polygenic score and the involvement of cholinergic and glutamatergic pathways with lithium treatment response in patients with bipolar disorder. Mol. Psychiatry 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sultzer, D.L.; Lim, A.C.; Gordon, H.L.; Yarns, B.C.; Melrose, R.J. Cholinergic receptor binding in unimpaired older adults, mild cognitive impairment, and Alzheimer’s disease dementia. Alzheimer’s Res. Ther. 2022, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Kusaka, M.; Mori, J.; Horikawa, A.; Hasegawa, Y. Simple method for the determination of choline and acetylcholine by pyrolysis gas chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1979, 164, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.; Meek, J.; Neff, N.H. Acetylcholine and choline in neuronal tissue measured by HPLC with electrochemical detection. J. Neurochem. 1983, 41, 188–194. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Venkataraman, S.; Rajendran, D.S.; Kumar, V.V.; Kumar, V.V.; Rangasamy, G. Acetylcholinesterase biosensors for electrochemical detection of neurotoxic pesticides and acetylcholine neurotransmitter: A literature review. Environ. Res. 2023, 227, 115724. [Google Scholar] [CrossRef]
- Walker, M.C.; van der Donk, W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef]
- Alessandri, B.; Bullock, R. Glutamate, and its receptors in the pathophysiology of brain and spinal cord injuries. Prog. Brain Res. 1998, 116, 303–330. [Google Scholar] [PubMed]
- May, P.C.; Gray, P.N. The mechanism of glutamate-induced degeneration of cultured Huntington’s disease and control fibroblasts. J. Neurol. Sci. 1985, 70, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.; Fu, P.; Zhang, Z.; Lin, K.; Ko, J.K.-S.; Yung, K.K.-L. Roles of glutamate receptors in Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 4391. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-S.; Huang, T.-H.; Lai, M.-C.; Huang, C.-W. The Role of Glutamate Receptors in Epilepsy. Biomedicines 2023, 11, 783. [Google Scholar] [CrossRef]
- Budczies, J.; Pfitzner, B.M.; Györffy, B.; Winzer, K.J.; Radke, C.; Dietel, M.; Fiehn, O.; Denkert, C. Glutamate enrichment as new diagnostic opportunity in breast cancer. Int. J. Cancer 2015, 136, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Jung, E.S.; Park, H.M.; Jeong, S.J.; Kim, K.; Chon, S.; Yu, S.-Y.; Woo, J.-T.; Lee, C.H. Plasma glutamine and glutamic acid are potential biomarkers for predicting diabetic retinopathy. Metabolomics 2018, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Monge-Acuña, A.A.; Fornaguera-Trías, J. A high performance liquid chromatography method with electrochemical detection of gamma-aminobutyric acid, glutamate and glutamine in rat brain homogenates. J. Neurosci. Methods 2009, 183, 176–181. [Google Scholar] [CrossRef]
- Church, W.H.; Lee, C.S.; Dranchak, K.M. Capillary electrophoresis of glutamate and aspartate in rat brain dialysate improvements in detection and analysis time using cyclodextrins. J. Chromatogr. B Biomed. Sci. Appl. 1997, 700, 67–75. [Google Scholar] [CrossRef]
- Buck, K.; Voehringer, P.; Ferger, B. Rapid analysis of GABA and glutamate in microdialysis samples using high performance liquid chromatography and tandem mass spectrometry. J. Neurosci. Methods 2009, 182, 78–84. [Google Scholar] [CrossRef]
- Ramos, C.P.; Lopes, E.O.; Oliveira Júnior, C.A.; Diniz, A.N.; Lobato, F.C.F.; Silva, R.O.S. Immunochromatographic test and ELISA for the detection of glutamate dehydrogenase (GDH) and A/B toxins as an alternative for the diagnosis of Clostridioides (Clostridium) difficile–associated diarrhea in foals and neonatal piglets. Braz. J. Microbiol. 2020, 51, 1459–1462. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, J.; Shi, R.; Waterhouse, G.I.; Pan, J.; Du, Z.; Yao, Q.; Wu, L.-Z.; Tung, C.-H.; Xie, J. Evolution of thiolate-stabilized Ag nanoclusters from Ag-thiolate cluster intermediates. Nat. Commun. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Nakamula, I.; Yamanoi, Y.; Yonezawa, T.; Imaoka, T.; Yamamoto, K.; Nishihara, H. Nanocage catalysts-rhodium nanoclusters encapsulated with dendrimers as accessible and stable catalysts for olefin and nitroarene hydrogenations. Chem. Commun. 2008, 5716–5718. [Google Scholar] [CrossRef] [PubMed]
- Javani, S.; Lorca, R.; Latorre, A.; Flors, C.; Cortajarena, A.L.; Somoza, A. Antibacterial Activity of DNA-Stabilized Silver Nanoclusters Tuned by Oligonucleotide Sequence. ACS Appl. Mater. Interfaces 2016, 8, 10147–10154. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Garg, S.; Karmakar, T. Insights into the Interactions of Peptides with Monolayer-Protected Metal Nanoclusters. ACS Appl. Bio Mater. 2023. [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Luminescent metal nanoclusters for potential chemosensor applications. Chemosensors 2017, 5, 36. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X.; Li, Z.; Li, J.; Ran, X.; Yang, L. Water-soluble amino pillar [5] arene functionalized gold nanoclusters as fluorescence probes for the sensitive determination of dopamine. Microchem. J. 2019, 150, 104084. [Google Scholar] [CrossRef]
- Guo, X.; Wu, F.; Ni, Y.; Kokot, S. Synthesizing a nano-composite of BSA-capped Au nanoclusters/graphitic carbon nitride nanosheets as a new fluorescent probe for dopamine detection. Anal. Chim. Acta 2016, 942, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bhamore, J.R.; Malek, N.I.; Murthy, Z.V.P.; Kailasa, S.K. Trypsin mediated one-pot reaction for the synthesis of red fluorescent gold nanoclusters: Sensing of multiple analytes (carbidopa, dopamine, Cu2+, Co2+ and Hg2+ ions). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 215, 209–217. [Google Scholar] [CrossRef]
- Vikraman, A.E.; Jose, A.R.; Jacob, M.; Kumar, K.G. Thioglycolic acid capped CdS quantum dots as a fluorescent probe for the nanomolar determination of dopamine. Anal. Methods 2015, 7, 6791–6798. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.; Xie, X.; Wang, X.; Wang, D.; Wang, P.; Li, H.; Yang, J.; Li, Y. Doping effect and fluorescence quenching mechanism of N-doped graphene quantum dots in the detection of dopamine. Talanta 2019, 196, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Ankireddy, S.R.; Kim, J. Selective detection of dopamine in the presence of ascorbic acid via fluorescence quenching of InP/ZnS quantum dots. Int. J. Nanomed. 2015, 10, 113–119. [Google Scholar]
- Baluta, S.; Cabaj, J.; Malecha, K. Neurotransmitters detection using a fluorescence-based sensor with graphene quantum dots. Opt. Appl. 2017, 47, 225–231. [Google Scholar]
- Zhao, J.; Zhao, L.; Lan, C.; Zhao, S. Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sens. Actuators B Chem. 2016, 223, 246–251. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Xie, Z.; Chen, P.; Sun, X.; Yan, J.; Zhou, S. In situ bifunctionalized carbon dots with boronic acid and amino groups for ultrasensitive dopamine detection. Anal. Methods 2016, 8, 3236–3241. [Google Scholar] [CrossRef]
- Gupta, A.; Nandi, C.K. PC12 live cell ultrasensitive neurotransmitter signaling using high quantum yield sulphur doped carbon dots and its extracellular Ca2+ ion dependence. Sens. Actuators B Chem. 2017, 245, 137–145. [Google Scholar] [CrossRef]
- Tiwari, A.; Walia, S.; Sharma, S.; Chauhan, S.; Kumar, M.; Gadly, T.; Randhawa, J.K. High quantum yield carbon dots and nitrogen-doped carbon dots as fluorescent probes for spectroscopic dopamine detection in human serum. J. Mater. Chem. B 2023, 11, 1029–1043. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Ma, Q. Fluorescence detection of dopamine based on nitrogen-doped graphene quantum dots and visible paper-based test strips. Anal. Methods 2017, 9, 2246–2251. [Google Scholar] [CrossRef]
- Wang, L.; Jana, J.; Chung, J.S.; Hur, S.H. Glutathione modified N-doped carbon dots for sensitive and selective dopamine detection. Dye. Pigment. 2021, 186, 109028. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Liu, Y.; Gao, R.; Wu, X.; Gao, X. Highly sensitive detection of dopamine based on gold nanoflowers enhanced-Tb (III) fluorescence. Talanta 2022, 249, 123700. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Xiong, H.; Wen, W.; Cheng, N. A ratiometric fluorometric epinephrine and norepinephrine assay based on carbon dot and CdTe quantum dots nanocomposites. Microchem. J. 2019, 146, 66–72. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S. A novel fluorescent biosensor for adrenaline detection and tyrosinase inhibitor screening. Anal. Bioanal. Chem. 2018, 410, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Sivasankaran, U.; Kumar, K.G. A cost effective strategy for dual channel optical sensing of adrenaline based on ‘in situ’formation of copper nanoparticles. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 223, 117292. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Dutta, R.K. Ethylene glycol and alanine anhydride based nitrogen doped fluorescent carbon nanoparticles as probe for detection of epinephrine, nor-epinephrine and dopamine. Dye. Pigment. 2022, 203, 110314. [Google Scholar] [CrossRef]
- Mann, F.A.; Herrmann, N.; Meyer, D.; Kruss, S. Tuning selectivity of fluorescent carbon nanotube-based neurotransmitter sensors. Sensors 2017, 17, 1521. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Reddy, A.S.; Kim, J.; Yun, K. Sensitive detection of epinephrine in human serum via fluorescence enhancement of gold nanoclusters. Appl. Surf. Sci. 2019, 498, 143837. [Google Scholar] [CrossRef]
- Wei, F.; Xu, G.; Wu, Y.; Wang, X.; Yang, J.; Liu, L.; Zhou, P.; Hu, Q. Molecularly imprinted polymers on dual-color quantum dots for simultaneous detection of norepinephrine and epinephrine. Sens. Actuators B Chem. 2016, 229, 38–46. [Google Scholar] [CrossRef]
- Ghasemi, F.; Hormozi-Nezhad, M.R.; Mahmoudi, M. Identification of catecholamine neurotransmitters using fluorescence sensor array. Anal. Chim. Acta 2016, 917, 85–92. [Google Scholar] [CrossRef]
- Wei, F.; Wu, Y.; Xu, G.; Gao, Y.; Yang, J.; Liu, L.; Zhou, P.; Hu, Q. Molecularly imprinted polymer based on CdTe@SiO2 quantum dots as a fluorescent sensor for the recognition of norepinephrine. Analyst 2014, 139, 5785–5792. [Google Scholar] [CrossRef]
- Nejad, M.A.F.; Ghasemi, F.; Hormozi-Nezhad, M.R. A wide-color-varying ratiometric nanoprobe for detection of norepinephrine in urine samples. Anal. Chim. Acta 2018, 1039, 124–131. [Google Scholar] [CrossRef]
- RosináJose, A.; GirisháKumar, K. A colorimetric and fluorometric sensor for the determination of norepinephrine. Anal. Methods 2016, 8, 5801–5805. [Google Scholar]
- Wang, Z.; Zhang, Y.; Zhang, B.; Lu, X. Mn2+ doped ZnS QDs modified fluorescence sensor based on molecularly imprinted polymer/sol-gel chemistry for detection of Serotonin. Talanta 2018, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Sun, B.; Yi, C.; Guan, R.; Fei, J.; Hu, Z.; Liu, B.; Liu, X. A fluorescence turn-on biosensor based on transferrin encapsulated gold nanoclusters for 5-hydroxytryptamine detection. Sens. Actuators B Chem. 2019, 294, 177–184. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Li, H.; Ge, Q.-M.; Cong, H.; Liu, M.; Tao, Z.; Zhao, J.-L. A chemo-sensor constructed by nanohybrid of multifarene [3, 3] and rGO for serotonin hydrochloride with dual response in both fluorescence and voltammetry. Microchem. J. 2020, 158, 105145. [Google Scholar] [CrossRef]
- Zhao, F.; Yoo, J.; Kim, J. Fabrication of the optical Fiber GABA sensor based on the NADP+-functionalized quantum dots. J. Nanosci. Nanotechnol. 2016, 16, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Su, Z.; Tu, Y.; Yan, J. Determination of dopamine based on its enhancement of gold-silver nanocluster fluorescence. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 252, 119519. [Google Scholar] [CrossRef]
- Aparna, R.; Devi, J.A.; Nebu, J.; Syamchand, S.; George, S. Rapid response of dopamine towards insitu synthesised copper nanocluster in presence of H2O2. J. Photochem. Photobiol. A Chem. 2019, 379, 63–71. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Yu, Y.; Li, C.; Chen, C.; Huang, H.; Liang, C.; Lou, Y.; Chen, X.-B.; Shi, Z.; Feng, S. Saccharomyces-derived carbon dots for biosensing pH and vitamin B 12. Talanta 2019, 195, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, H.; Li, Z.; Tedesco, A.C.; Bi, H. Carbon dots derived from tea polyphenols as photosensitizers for photodynamic therapy. Molecules 2022, 27, 8627. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable photoluminescence across the entire visible spectrum from carbon dots excited by white light. Angew. Chem. Int. Ed. 2015, 54, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, F.; Zhao, X.; Chen, L.; Su, X. 3-Aminophenyl boronic acid-functionalized CuInS2 quantum dots as a near-infrared fluorescence probe for the determination of dopamine. Biosens. Bioelectron. 2013, 47, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal. Chem. 2014, 54, 83–102. [Google Scholar] [CrossRef]
- Liu, H.; Li, N.; Zhang, H.; Zhang, F.; Su, X. A simple and convenient fluorescent strategy for the highly sensitive detection of dopamine and ascorbic acid based on graphene quantum dots. Talanta 2018, 189, 190–195. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, T.; Bi, L. A dual-channel ratiometric fluorescent probe for determination of the activity of tyrosinase using nitrogen-doped graphene quantum dots and dopamine-modified CdTe quantum dots. Microchim. Acta 2019, 186, 635. [Google Scholar] [CrossRef]
- Chatterjee, M.; Nath, P.; Kadian, S.; Kumar, A.; Kumar, V.; Roy, P.; Manik, G.; Satapathi, S. Highly sensitive and selective detection of dopamine with boron and sulfur co-doped graphene quantum dots. Sci. Rep. 2022, 12, 9061. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mahnashi, M.H.; Alkahtani, S.A.; El-Wekil, M.M. Nitrogen and sulfur co-doped graphene quantum dots/nanocellulose nanohybrid for electrochemical sensing of anti-schizophrenic drug olanzapine in pharmaceuticals and human biological fluids. Int. J. Biol. Macromol. 2020, 165, 2030–2037. [Google Scholar] [CrossRef]
- Tammina, S.K.; Yang, D.; Koppala, S.; Cheng, C.; Yang, Y. Highly photoluminescent N, P doped carbon quantum dots as a fluorescent sensor for the detection of dopamine and temperature. J. Photochem. Photobiol. B Biol. 2019, 194, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Yang, M.; Kong, W.; Huang, H.; Liu, Y. Highly sensitive, stable, and precise detection of dopamine with carbon dots/tyrosinase hybrid as fluorescent probe. RSC Adv. 2014, 4, 46437–46443. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Liu, J.; Zhang, Y.; Yang, Y.; Huang, H.; Liu, Y.; Kang, Z. A practical and highly sensitive C3N4-TYR fluorescent probe for convenient detection of dopamine. Nanoscale 2015, 7, 12068–12075. [Google Scholar] [CrossRef] [PubMed]
- Sangubotla, R.; Kim, J. A facile enzymatic approach for selective detection of γ-aminobutyric acid using corn-derived fluorescent carbon dots. Appl. Surf. Sci. 2019, 490, 61–69. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Ding, S.; Lyu, Z.; Ebrahimi, G.; Du, D.; Dolatabadi, J.E.N.; Torbati, M.; Lin, Y. Aptamer functionalized nanomaterials for biomedical applications: Recent advances and new horizons. Nano Today 2021, 39, 101177. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Loth, C.; Lutz, J.-F. Chemical conjugation of nucleic acid aptamers and synthetic polymers. Polym. Chem. 2021, 12, 3498–3509. [Google Scholar] [CrossRef]
- Teniou, A.; Rhouati, A.; Catanante, G. A simple fluorescent aptasensing platform based on graphene oxide for dopamine determination. Appl. Biochem. Biotechnol. 2022, 194, 1925–1937. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, K.; Wang, S.; Kang, W.; Cheng, C.; Niu, L.M.; Guo, Z. A novel label-free fluorescence aptasensor for dopamine detection based on an Exonuclease III-and SYBR Green I-aided amplification strategy. Sens. Actuators B Chem. 2020, 305, 127348. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, S.; Zhang, J.; Liang, N.; Zhao, L. A facile aptamer-based sensing strategy for dopamine detection through the fluorescence energy transfer between dye and single-wall carbon nanohorns. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2022, 279, 121415. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, Y.; Zhang, H.; Chen, X.; Qiu, H. Fluorometric dopamine assay based on an energy transfer system composed of aptamer-functionalized MoS2 quantum dots and MoS2 nanosheets. Microchim. Acta 2019, 186, 58. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, S.; Gao, X.; Gao, R.; Zhu, Y.; Wu, X.; Zang, R.; Yao, T. A universal label-free fluorescent aptasensor based on Ru complex and quantum dots for adenosine, dopamine and 17β-estradiol detection. Biosens. Bioelectron. 2016, 79, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hu, Y.; Zhang, Z.; Tang, Y. Universal fluorometric aptasensor platform based on water-soluble conjugated polymers/graphene oxide. Anal. Bioanal. Chem. 2018, 410, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, J.; Li, J.; Lei, L.; Zhang, W.; Yan, S.; Li, Y. Fluorescence detection of dopamine based on the polyphenol oxidase–mimicking enzyme. Anal. Bioanal. Chem. 2020, 412, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Ovechkina, V.S.; Zakian, S.M.; Medvedev, S.P.; Valetdinova, K.R. Genetically encoded fluorescent biosensors for biomedical applications. Biomedicines 2021, 9, 1528. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Nasu, Y.; Shkolnikov, I.; Kim, A.; Campbell, R.E. Engineering genetically encoded fluorescent indicators for imaging of neuronal activity: Progress and prospects. Neurosci. Res. 2020, 152, 3–14. [Google Scholar] [CrossRef]
- Kim, H.; Ju, J.; Lee, H.N.; Chun, H.; Seong, J. Genetically encoded biosensors based on fluorescent proteins. Sensors 2021, 21, 795. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Liebscher, I. Mutations in G protein–coupled receptors: Mechanisms, pathophysiology and potential therapeutic approaches. Pharmacol. Rev. 2021, 73, 89–119. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The role of amino acids in neurotransmission and fluorescent tools for their detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef]
- Patriarchi, T.; Cho, J.R.; Merten, K.; Howe, M.W.; Marley, A.; Xiong, W.-H.; Folk, R.W.; Broussard, G.J.; Liang, R.; Jang, M.J. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360, eaat4422. [Google Scholar] [CrossRef]
- Nakamoto, C.; Goto, Y.; Tomizawa, Y.; Fukata, Y.; Fukata, M.; Harpsøe, K.; Gloriam, D.E.; Aoki, K.; Takeuchi, T. A genetically encoded red fluorescence dopamine biosensor enables dual imaging of dopamine and norepinephrine. bioRxiv 2020. [CrossRef]
- Nakamoto, C.; Goto, Y.; Tomizawa, Y.; Fukata, Y.; Fukata, M.; Harpsøe, K.; Gloriam, D.E.; Aoki, K.; Takeuchi, T. A novel red fluorescence dopamine biosensor selectively detects dopamine in the presence of norepinephrine in vitro. Mol. Brain 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, C.; Lischinsky, J.E.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 2019, 102, 745–761.e748. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.K.; Keller, J.P.; Altermatt, M.; Liang, R.; Matsui, A.; Dong, C.; Hon, O.J.; Yao, Z.; Sun, J.; Banala, S. Directed evolution of a selective and sensitive serotonin sensor via machine learning. Cell 2020, 183, 1986–2002.e26. [Google Scholar] [CrossRef] [PubMed]
- Marvin, J.S.; Shimoda, Y.; Magloire, V.; Leite, M.; Kawashima, T.; Jensen, T.P.; Kolb, I.; Knott, E.L.; Novak, O.; Podgorski, K. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat. Methods 2019, 16, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zeng, J.; Jing, M.; Zhou, J.; Feng, J.; Owen, S.F.; Luo, Y.; Li, F.; Wang, H.; Yamaguchi, T. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 2018, 174, 481–496.e19. [Google Scholar] [CrossRef]
- Marvin, J.S.; Borghuis, B.G.; Tian, L.; Cichon, J.; Harnett, M.T.; Akerboom, J.; Gordus, A.; Renninger, S.L.; Chen, T.-W.; Bargmann, C.I. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 2013, 10, 162–170. [Google Scholar] [CrossRef]
- Magloire, V.; Savtchenko, L.P.; Jensen, T.P.; Sylantyev, S.; Kopach, O.; Cole, N.; Tyurikova, O.; Kullmann, D.M.; Walker, M.C.; Marvin, J.S. Volume-transmitted GABA waves pace epileptiform rhythms in the hippocampal network. Curr. Biol. 2023, 33, 1249–1264.e7. [Google Scholar] [CrossRef]
- Wan, J.; Peng, W.; Li, X.; Qian, T.; Song, K.; Zeng, J.; Deng, F.; Hao, S.; Feng, J.; Zhang, P. A genetically encoded sensor for measuring serotonin dynamics. Nat. Neurosci. 2021, 24, 746–752. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, P.; Wang, G.; Feng, J.; Mesik, L.; Zeng, J.; Jiang, H.; Wang, S.; Looby, J.C.; Guagliardo, N.A. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat. Biotechnol. 2018, 36, 726–737. [Google Scholar] [CrossRef]
- Share, Growth Analysis, By Technology (Piezoelectric electrochemical) By end User (Bioreactor, Medical) Industry Forecast 2023–2030. Global Biosensor Market Size; 2023; 157p. Available online: https://www.skyquestt.com/report/biosensors-market (accessed on 26 November 2023).
- Global Carbon Quantum Dots (CQD) Market Share (2023–2030). Research Report World; 2023; Volume 92. Available online: https://www.linkedin.com/pulse/global-carbon-quantum-dots-cqd-market-share (accessed on 26 November 2023).







| Sensor Code | Analyte | EC50 | ΔF/F0 (%) | Ref. |
|---|---|---|---|---|
| dLight1.1 | DA | 330 ± 30 nM | 230 ± 9 | [163] |
| dLight1.2 | DA | 770 ± 10 nM | 340 ± 20 | |
| dLight1.3a | DA | 2300 ± 20 nM | 660 ± 30 | |
| dLight1.3b | DA | 1680 ± 10 nM | 930 ± 30 | |
| dLight1.4 | DA | 4.1 ± 0.2 nM | 170 ± 10 | |
| dLight1.5 | DA | 110 ± 10 nM | 180 ± 10 | |
| R-GenGAR-DA | DA | 0.7 µM | −50 | [164] |
| R-GenGAR-DA | DA | 0.92 µM | −43 | [165] |
| GRABNE1h | NEP | 83 nM | 130 | [166] |
| GRABNE1m | NEP | 930 nM | 230 | |
| iSeroSnFR | Serotonin | 10 mM | 87 ± 20 | [167] |
| iGABASnFR | GABA | 240 µM | [168] | |
| GRABDA1m | DA | 130 nM | 90 | [169] |
| IGluSnFR | Glutamate | 4 µM | 4.5 | [170] |
| iGABASnFR2 | GABA | 1.1 µM | 0.074 ± 0.006 | [171] |
| GRAB 5HT | Serotonin | 22 nM | 280 | [172] |
| GAch 2.0 | ACh | 2 µM | 100.65 ± 7.61 | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindaraju, R.; Govindaraju, S.; Yun, K.; Kim, J. Fluorescent-Based Neurotransmitter Sensors: Present and Future Perspectives. Biosensors 2023, 13, 1008. https://doi.org/10.3390/bios13121008
Govindaraju R, Govindaraju S, Yun K, Kim J. Fluorescent-Based Neurotransmitter Sensors: Present and Future Perspectives. Biosensors. 2023; 13(12):1008. https://doi.org/10.3390/bios13121008
Chicago/Turabian StyleGovindaraju, Rajapriya, Saravanan Govindaraju, Kyusik Yun, and Jongsung Kim. 2023. "Fluorescent-Based Neurotransmitter Sensors: Present and Future Perspectives" Biosensors 13, no. 12: 1008. https://doi.org/10.3390/bios13121008
APA StyleGovindaraju, R., Govindaraju, S., Yun, K., & Kim, J. (2023). Fluorescent-Based Neurotransmitter Sensors: Present and Future Perspectives. Biosensors, 13(12), 1008. https://doi.org/10.3390/bios13121008





