A Facile Glycerol-Assisted Synthesis of Low-Cu2+-Doped CoFe2O4 for Electrochemical Sensing of Acetaminophen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Structural Properties of Low-Cu2+-Doped CoFe2O4 Ferrite Nanoparticles, LCF NPs
2.2. Electrochemical Measurements
3. Results and Discussion
3.1. Synthesis of Low-Cu2+-Doped CoFe2O4 Nanoparticles LCF NPs
3.1.1. Structural Properties
3.1.2. Morphology and Surface Textural Properties
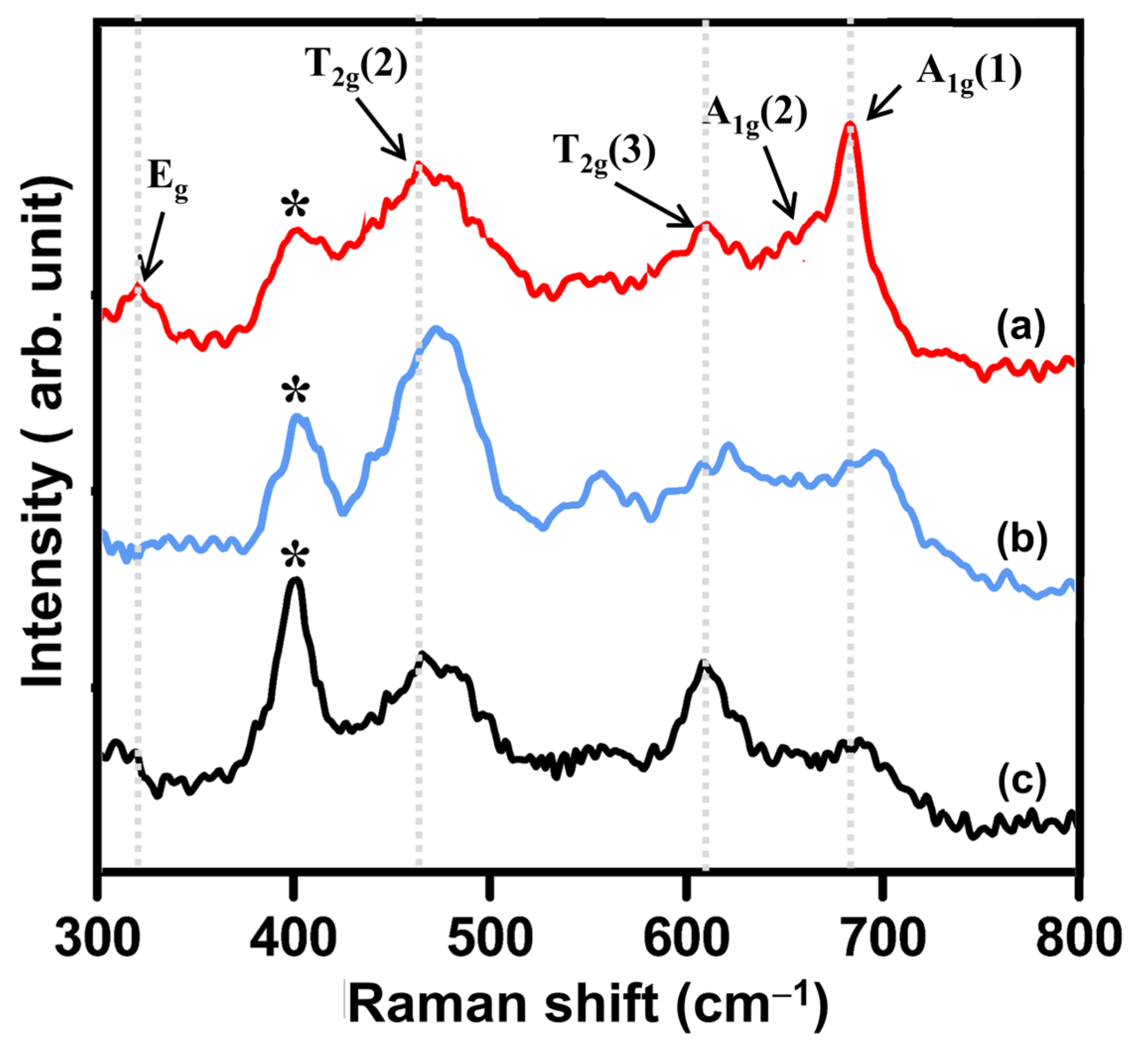
3.1.3. Vibrational Properties
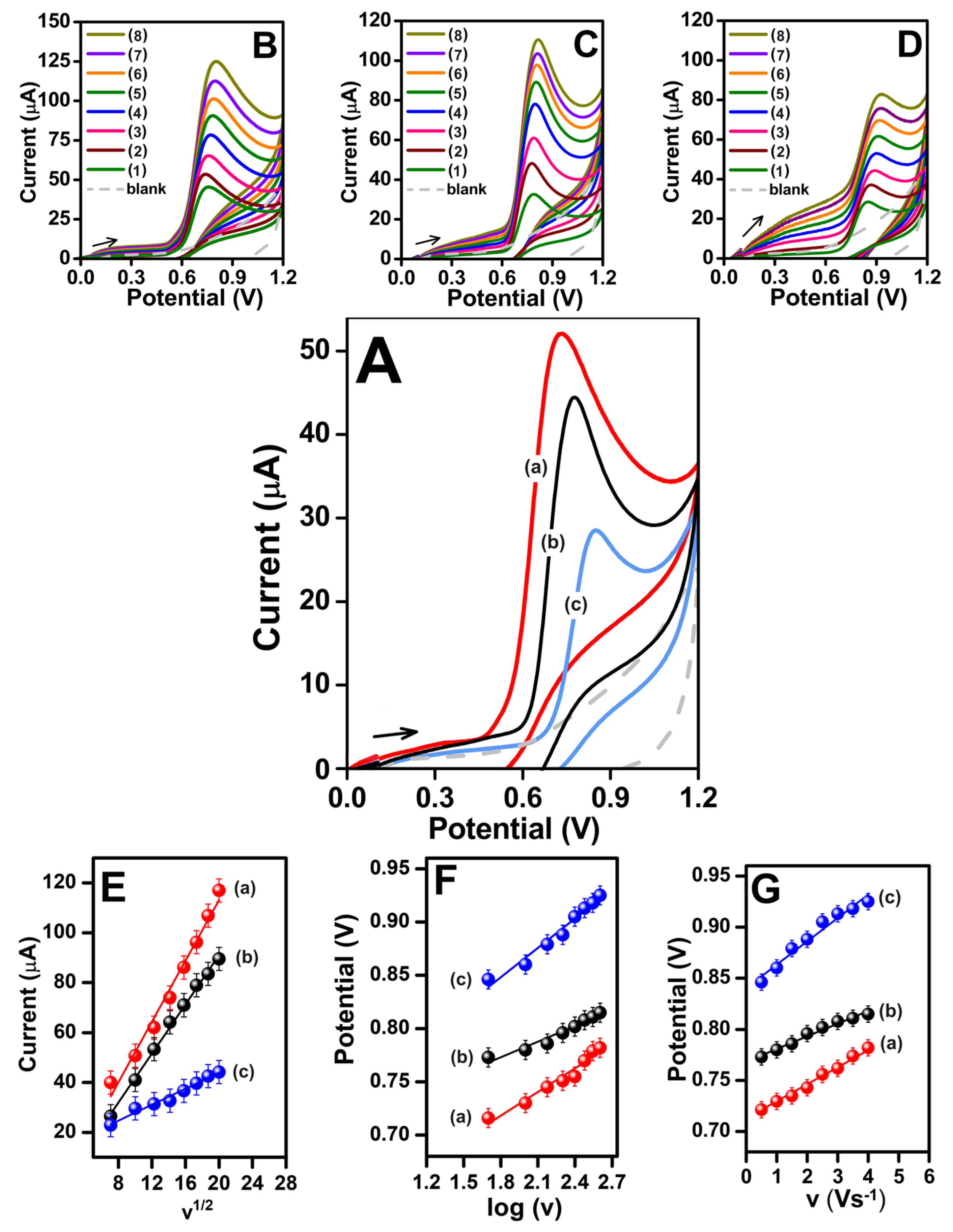
3.2. Electrochemical Properties of LCF NPs
Electrochemical Activity Measurements
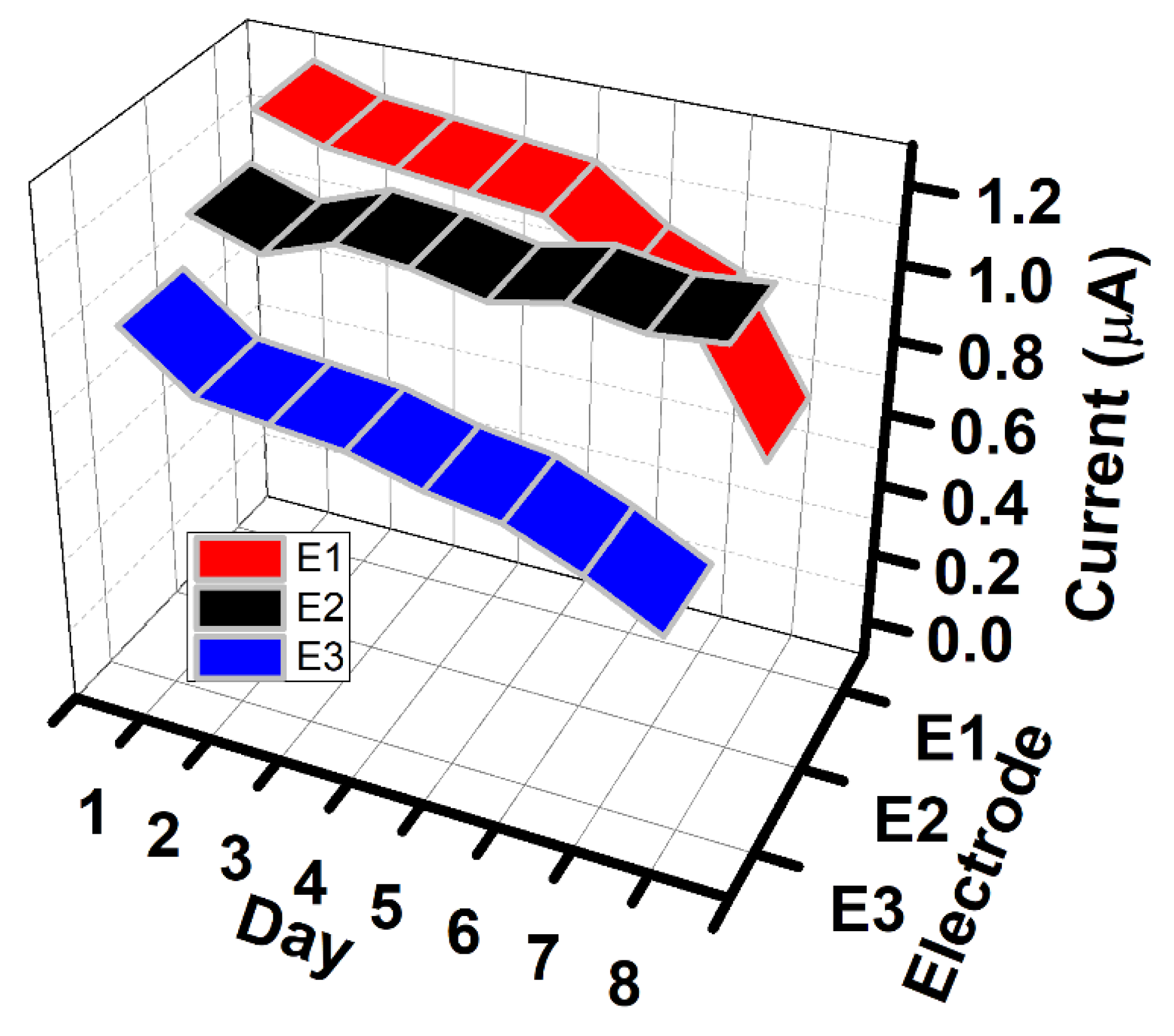
3.3. Improving Conditions for the Development of Sensitive and Selective LCF NPs/GCE Electrodes
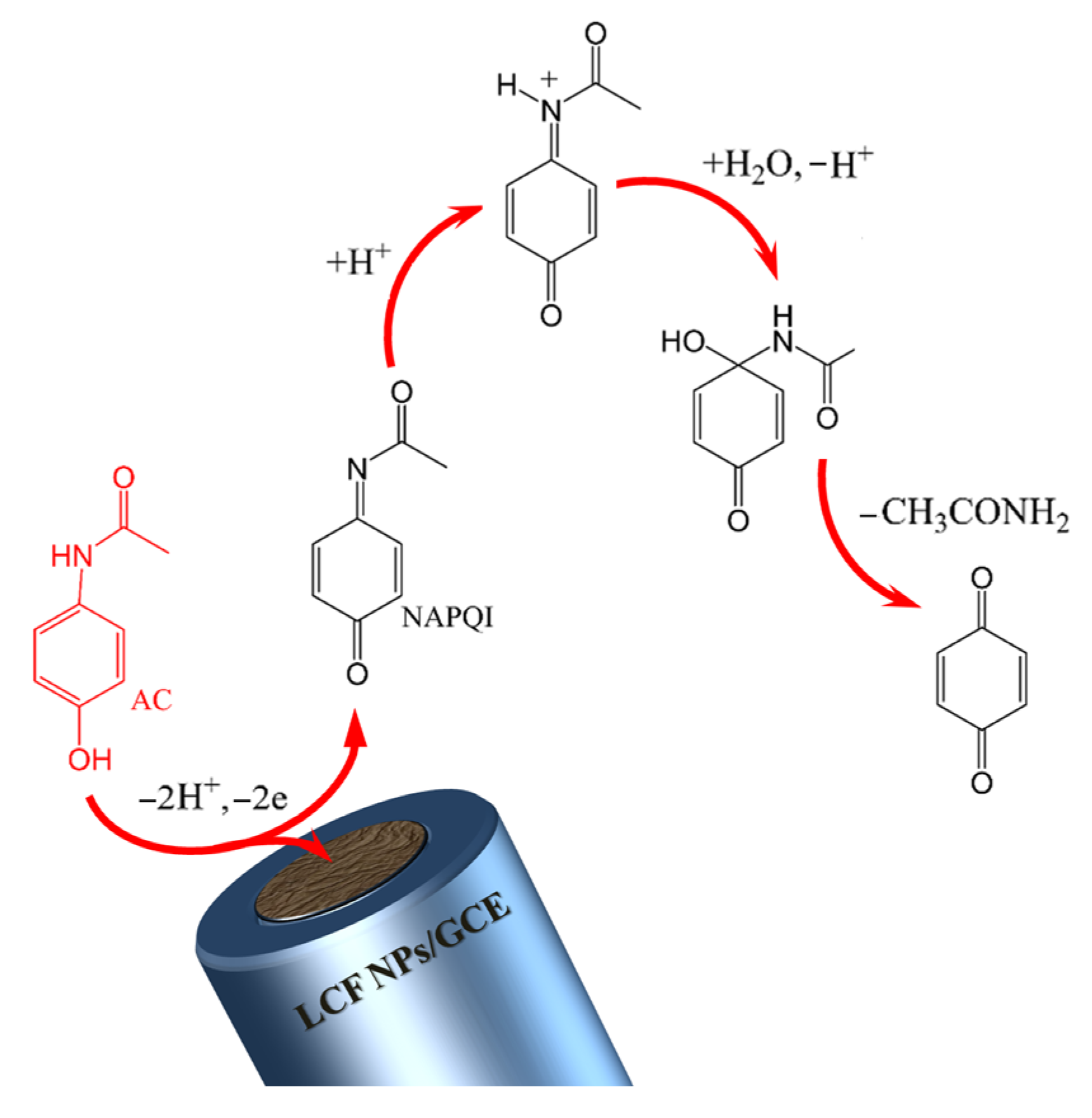
3.4. AC Determination on a Modified LCF NPs/GCE Electrode
3.4.1. Differential-Pulse Voltammetric DPV Determination of AC at the Modified LCF NPs/GCE Electrode
3.4.2. Analysis of Real Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rejithamol, R.; Beena, S. Carbon Paste Electrochemical Sensors for the Detection of Neurotransmitters. Front. Sens. 2022, 3, 901628. [Google Scholar] [CrossRef]
- Valencia-Valencia, M.G.; Alarcón-Ángeles, G.; Ramírez-Silva, M.T.; Gómez-Hernández, M.; Hurtado-Y-de-la-Peña, M. Interacción acetaminofén-bases nitrogenadas de DNA, un estudio electroquímico. Pädi Boletín Científico Cienc. Básicas Ing. ICBI 2023, 11, 10–15. [Google Scholar] [CrossRef]
- İslamoğlu, N.; Mülazımoğlu, İ.E.; Demir Mülazımoğlu, A. Sensitive and selective determination of paracetamol in antipyretic children’s syrup with a polyglycine modified glassy carbon electrode. Anal. Methods 2023, 15, 4149–4158. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Mabrouk, M.M.; Fekry, M.; Mansour, F.R. Glycerol-Based Green RP-HPLC Method for Simultaneous Determination of Methionine and Paracetamol in Pharmaceutical Tablets. Chromatographia 2023, 86, 707–716. [Google Scholar] [CrossRef]
- Vaas, A.P.J.P.; Yu, R.B.; Quirino, J.P. In-line sample concentration in capillary electrophoresis by cyclodextrin to admicelle microextraction. Anal. Bioanal. Chem. 2022, 414, 6671–6680. [Google Scholar] [CrossRef] [PubMed]
- Fahelelbom, K.M.; Saleh, A.; Al-Tabakha, M.M.A.; Ashames, A.A. Recent applications of quantitative analytical FTIR spectroscopy in pharmaceutical, biomedical, and clinical fields: A brief review. Rev. Anal. Chem. 2022, 41, 21–33. [Google Scholar] [CrossRef]
- Fateixa, S.; Mulandeza, O.; Nogueira, H.I.S.; Trindade, T. Raman imaging studies on the stability of Paracetamol tablets under different storage conditions. Vib. Spectrosc. 2023, 124, 103488. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Alabi, Z.O.; Akinyemi, A.O.; Oyelere, S.F.; Oluseye, A.B.; Owoyemi, B.C.D. Comparative evaluation of acetaminophen form (I) in commercialized paracetamol brands. Sci. Afr. 2023, 19, e01537. [Google Scholar] [CrossRef]
- Ben-Hander, G.M.; Gaeth, M.E.; Otmaan, N.A. Method Validation for Determination of Paracetamol in Tablet Dosage Form Using UV-Visible Spectrophotometer. Sirte J. Med. Sci. 2022, 1, 19–25. [Google Scholar]
- Abdallah, N.A.; Fathy, M.E.; Tolba, M.M.; El-Brashy, A.M.; Fawzia, A. Ibrahim Innovative localized surface plasmon resonance sensing technique for a green spectrofluorimetric assay of ketoprofen, paracetamol a chlorzoxazone in pharmaceutical preparations an biological fluids. RSC Adv. 2022, 12, 33540. [Google Scholar] [CrossRef]
- Qi, H.; Li, Q.; Jing, J.; Jing, T.; Liu, C.; Qiu, L.; Sami, R.; Helal, M.; Ismail, K.A.; Aljahani, A.H. Construction of N-CDs and Calcein-Based Ratiometric Fluorescent Sensor for Rapid Detection of Arginine and Acetaminophen. Nanomaterials 2022, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Gharous, M.; Bounab, L.; Pereira, F.J.; Choukairi, M.; López, R.; Aller, A.J. Electrochemical Kinetics and Detection of Paracetamol by Stevensite-Modified Carbon Paste Electrode in Biological Fluids and Pharmaceutical Formulations. Int. J. Mol. Sci. 2023, 24, 11269. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.P.; Ribas, L.S.; Napoli, J.S.; Abreu, E.; Diaz de Tuesta, J.L.; Gomes, H.T.; Tusset, A.M.; Lenzi, G.G. Green Magnetic Nanoparticles CoFe2O4@Nb5O2 Applied in Paracetamol Removal. Magnetochemistry 2023, 9, 200. [Google Scholar] [CrossRef]
- Yohannes, Y.B.; Mekonnen, A.N.; Assefa, M. Voltammetric determination of paracetamol at glycine modified carbon paste electrode. Ethiopi. J. Sci. Technol. 2022, 15, 293–311. [Google Scholar]
- Garkani Nejad, F.; Beitollahi, H.; Sheikhshoaie, I. A UiO-66-NH2 MOF/PAMAM Dendrimer Nanocomposite for Electrochemical Detection of Tramadol in the Presence of Acetaminophen in Pharmaceutical Formulations. Biosensors 2023, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Chu, Z.; Wang, Q.; Cao, Y.; Cao, J.; Li, J.; Lei, W.; Zhang, B.; Si, W. Fe–Decorated Nitrogen–Doped Carbon Nanospheres as an Electrochemical Sensing Platform for the Detection of Acetaminophen. Molecules 2023, 28, 3006. [Google Scholar] [CrossRef]
- Afshari, M.; Carabineiro, S.A.C.; Gorjizadeh, M. Sulfonated Silica Coated CoFe2O4 Magnetic Nanoparticles for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-One and Octahydroquinazoline Derivatives. Catalysts 2023, 13, 989. [Google Scholar] [CrossRef]
- Shen, L.; Dong, J.; Wen, B.; Wen, X.; Li, J. Facile Synthesis of Hollow Fe3O4-rGO Nanocomposites for the Electrochemical Detection of Acetaminophen. Nanomaterials 2023, 13, 707. [Google Scholar] [CrossRef]
- Gomes, E.C.; Ribeiro, C.L.; Santos, V.O., Jr.; Paterno, L.G. Differential Pulse Voltammetric Detection of Acetaminophen Using Nickel Phthalocyanine/CeO2-Modified ITO Electrodes. Chemosensors 2023, 11, 154. [Google Scholar] [CrossRef]
- Mishra, S.; Acharya, R.; Parida, K. Spinel-Ferrite-Decorated Graphene-Based Nanocomposites for Enhanced Photocatalytic Detoxification of Organic Dyes in Aqueous Medium: A Review. Water 2023, 15, 81. [Google Scholar] [CrossRef]
- Zeng, X.-L.; Sivanesarajah, I.; Hartmann, U. Conversion of Hard to Soft Magnetic Ferrite Nanowires by Paramagnetic Shielding. Solids 2023, 4, 304–315. [Google Scholar] [CrossRef]
- Aysal, N.; Kurt, Y.; Öztürk, H.; Ildiz, G.O.; Yesiltas, M.; Laçin, D.; Öngen, S.; Nikitin, T.; Fausto, R. Crystallization Kinetics: Relationship between Crystal Morphology and the Cooling Rate—Applications for Different Geological Materials. Crystals 2023, 13, 1130. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Afzia, M.; Khan, R.A.; Ismail, B.; Zaki, M.E.A.; Althagafi, T.M.; Alanazi, A.A.; Khan, A.U. Correlation between Magnetic and Dielectric Response of CoFe2O4:Li1+/Zn2+ Nanopowders Having Improved Structural and Morphological Properties. Molecules 2023, 28, 2824. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic Nanoparticle Integrated Ternary Polymer Blend of PVA/Starch/Glycerol: A Promising Antimicrobial Food Packaging Material. Polymers 2022, 14, 1379. [Google Scholar] [CrossRef]
- Thakur, S.; Neogi, S.; Ray, A.K. Morphology-Controlled Synthesis of ZnO Nanostructures for Caffeine Degradation and Escherichia coli Inactivation in Water. Catalysts 2021, 11, 63. [Google Scholar] [CrossRef]
- Lazarova, T.; Kovacheva, D.; Georgieva, M.; Tzankov, D.; Tyuliev, G.; Spassova, I.; Naydenova, A. Tunable nanosized spinel manganese ferrites synthesized by solution combustion method. Appl. Surf. Sci. 2019, 496, 143571. [Google Scholar] [CrossRef]
- Bartunek, V.; Sedmidubský, D.; Huber, Š.; Švecová, M.; Ulbrich, P.; Jankovský, O. Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel. Materials 2018, 11, 1241. [Google Scholar] [CrossRef]
- Flood-Garibay, J.A.; Méndez-Rojas, M.A. Effects of Co-Solvent Nature and Acid Concentration in the Size and Morphology of Wrinkled Mesoporous Silica Nanoparticles for Drug Delivery Applications. Molecules 2021, 26, 4186. [Google Scholar] [CrossRef]
- Al-Malwi, S.D.; Al-Ammari, R.H.; Alshehri, A.; Narasimharao, K. Influence of Synthesis Conditions on Physicochemical and Photocatalytic Properties of Ag Containing Nanomaterials. Catalysts 2022, 12, 1226. [Google Scholar] [CrossRef]
- Saikova, S.; Pavlikov, A.; Karpov, D.; Samoilo, A.; Kirik, S.; Volochaev, M.; Trofimova, T.; Velikanov, D.; Kuklin, A. Copper Ferrite Nanoparticles Synthesized Using Anion-Exchange Resin: Influence of Synthesis Parameters on the Cubic Phase Stability. Materials 2023, 16, 2318. [Google Scholar] [CrossRef] [PubMed]
- Al-Kadhi, N.S.; Al-Senani, G.M.; Almufarij, R.S.; Abd-Elkader, O.H.; Deraz, N.M. Green Synthesis of Nanomagnetic Copper and Cobalt Ferrites Using Corchorus Olitorius. Crystals 2023, 13, 758. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F.; Almufarij, R.S.; Abd-Elkader, O.H.; Deraz, N.M. Magnetic Behavior of Virgin and Lithiated NiFe2O4 Nanoparticles. Crystals 2023, 13, 69. [Google Scholar] [CrossRef]
- Fahim, A.M.; Abouzeid, R.E.; Al Kiey, S.A.; Dacrory, S. Development of semiconductive foams based on cellulose-benzenesulfonate/CuFe2O4-nanoparticles and theoretical studies with DFT/B3PW91/LANDZ2 basis set. J. Mol. Struct. 2022, 1247, 131390. [Google Scholar] [CrossRef]
- Khairy, M.; Bayoumy, W.A.; Selima, S.S.; Mousa, M.A. Studies on characterization, magnetic and electrochemical properties of nano-size pure and mixed ternary transition metal ferrites prepared by the auto-combustion method. J. Mater. Res. 2020, 35, 2652–2663. [Google Scholar] [CrossRef]
- Dadaei, M.; Naeimi, H. An Environment-Friendly Method for Green Synthesis of Pyranopyrazole Derivatives Catalyzed by CoCuFe2O4 Magnetic Nanocrystals under Solvent-Free Conditions. Polycycl. Aromat. Compd. 2022, 42, 204–217. [Google Scholar] [CrossRef]
- Ding, G.; Chen, C.; Tai, H.; Tang, Z.; Wang, Z.; Cheng, G.; Wan, X. The effect of Co2+, Cu2+ element ratio on the microwave absorption properties of CoxCu1-xFe2O4/RGO composites. Mater. Lett. 2021, 289, 129423. [Google Scholar] [CrossRef]
- Aswad, M.A.; Mutlak, F.A.-H.; Jabir, M.S.; Abdulridha, S.K.; Ahmed, A.F.; Nayef, U.M. Laser assisted hydrothermal synthesis of magnetic ferrite nanoparticles for biomedical applications. J. Phys. Conf. Ser. 2021, 1795, 012030. [Google Scholar] [CrossRef]
- Patil, K.; Phadke, S.; Mishra, A. Effect of D-block element Co2+ substitution on structural and vibrational properties of spinel ferrites. AIP Conf. Proc. 2021, 2369, 020015. [Google Scholar]
- Mahmood, N.B.; Saeed, F.R.; Gbashi, K.R.; Hamodi, A.; Jaffar, Z.M. Structural properties of CoxCu1−xFe2O4 solid solution. J. Mech. Behav. Mater. 2021, 30, 220–227. [Google Scholar] [CrossRef]
- Anju; Yadav, R.S.; Pötschke, P.; Pionteck, J.; Krause, B.; Kuřitka, I.; Vilčáková, J.; Škoda, D.; Urbánek, P.; Machovský, M.; et al. CuxCo1−xFe2O4 (x = 0.33, 0.67, 1) Spinel Ferrite Nanoparticles Based Thermoplastic Polyurethane Nanocomposites with Reduced Graphene Oxide for Highly Efficient Electromagnetic Interference Shielding. Int. J. Mol. Sci. 2022, 23, 2610. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwarlu, C.; Ramesh, M.; Vinod, G.; Reddy, Y.S.; Rajashekhar, K.; Naresh, B.; Ramesh, P.; Dasharatha, U.; Venkatesh, B.; Naik, J.L. The Structural and Electrical Studies on Cu-Co Ferrites. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1221, 012014. [Google Scholar] [CrossRef]
- Patil, K.; Phadke, S.; Mishra, A. Structure and ac conductivity of (Cu/Co)Fe2O4 spinel materials. Mater. Today Proc. 2022, 66, 2070–2074. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Ramadan, R.; El-Masry, M.M. Synthesis and characterization of mixed ternary transition metal ferrite nanoparticles comprising cobalt, copper and binary cobalt–copper for high-performance supercapacitor applications. Appl. Phys. A 2022, 128, 473. [Google Scholar] [CrossRef]
- Alzoubi, G.M. The Effect of Co-Doping on the Structural and Magnetic Properties of Single-Domain Crystalline Copper Ferrite Nanoparticles. Magnetochemistry 2022, 8, 164. [Google Scholar] [CrossRef]
- Hunyek, A.; Sirisathitkul, C.; Koyvanich, K. Tapioca starch in the sol-gel synthesis of cobalt ferrites with divalent cation substitutions. Karbala Int. J. Mod. Sci. 2022, 8, 397–405. [Google Scholar] [CrossRef]
- Mohamed, W.; Hadia, N.; Al Bakheet, B.; Alzaid, M.; Abu-Dief, A.M. Impact of Cu2+ cations substitution on structural, morphological, optical and magnetic properties of Co1−xCuxFe2O4 nanoparticles synthesized by a facile hydrothermal approach. Solid. State Sci. 2022, 125, 106841. [Google Scholar] [CrossRef]
- Saleem, S.; Irfan, M.; Naz, M.Y.; Shukrullah, S.; Munir, M.A.; Ayyaz, M.; Alwadie, A.S.; Legutko, S.; Petru, J.; Rahman, S. Investigating the Impact of Cu2+ Doping on the Morphological, Structural, Optical, and Electrical Properties of CoFe2O4 Nanoparticles for Use in Electrical Devices. Materials 2022, 15, 3502. [Google Scholar] [CrossRef]
- Morales-Morales, J.A.; Silgado-Cortázar, D.G.; Rosero-Martínez, J.M.; Basante-Delgado, S.F.; Mendez-Albores, E.; Aperador-Chaparro, W.A.; Gómez-Cuaspud, J.A. Copper and cobalt co-doped ferrites as effective agents for the electrochemical detection of N-acetyl-p-aminophenol. ECS Trans. 2021, 100, 117. [Google Scholar] [CrossRef]
- Ramadan, R.; El-Masry, M.M. Effect of (Co and Zn) doping on structural, characterization and the heavy metal removal efficiency of CuFe2O4 nanoparticles. J. Aust. Ceram. Soc. 2023, 1–16. [Google Scholar] [CrossRef]
- Morales-Morales, J.A. Synthesis of Hematite α-Fe2O3 Nano Powders by the controlled precipitation method. Cienc. Desarro. 2017, 8, 99–107. [Google Scholar] [CrossRef]
- Hammad, T.M.; Kuhn, S.; Amsha, A.A.; Hempelmann, R. Investigation of structural, optical, and magnetic properties of Co2+ ions substituted CuFe2O4 spinel ferrite nanoparticles prepared via precipitation approach. J. Aust. Ceram. Soc. 2021, 57, 543–553. [Google Scholar] [CrossRef]
- Basante-Delgado, S.F.; González-Vidal, D.; Morales-Morales, J.A.; Aperador-Chaparro, W.A.; Gómez-Cuaspud, J.A. A preliminary study of oxides of Fe doped with Ba, Co, Cu and synthetized by the citrate sol–gel combustion route. J. Phys. Conf. Ser. 2020, 1541, 012013. [Google Scholar] [CrossRef]
- Ghosh, M.P.; Mukherjee, S. Microstructural, magnetic, and hyperfine characterizations of Cu-doped cobalt ferrite nanoparticles. J. Am. Ceram. Soc. 2019, 102, 7509–7520. [Google Scholar] [CrossRef]
- Dhyani, R.; Srivastava, R.C.; Dixit, G. Study of Magnetic and Temperature-Dependent Dielectric Properties of Co-CuFe2O4 Nanoferrites. J. Electron. Mater. 2022, 51, 5492–5507. [Google Scholar] [CrossRef]
- Kadam, A.B.; Mande, V.K.; Kadam, S.B.; Kadam, R.H.; Shirsath, S.E.; Borade, R.B. Influence of gadolinium (Gd3+) ion substitution on structural, magnetic, and electrical properties of cobalt ferrites. J. Alloys Compd. 2020, 840, 155669. [Google Scholar] [CrossRef]
- Tukaram, V.; Shinde, S.S.; Borade, R.B.; Kadam, A.B. Study of cation distribution, structural and electrical properties of Al–Zn substituted Ni–Co ferrite. Phys. B 2020, 577, 411783. [Google Scholar] [CrossRef]
- Vermaa, K.C.; Goyal, N.; Singh, M.; Singhb, M.; Kotnala, R.K. Hematite α-Fe2O3 induced magnetic and electrical behavior of NiFe2O4 and CoFe2O4 ferrite nanoparticles. Results Phys. 2019, 13, 102212. [Google Scholar] [CrossRef]
- Van Ngo, H.; Nguyen, H.T.; Le, H.T.; Le, T.H.; Vu, T.T.; Pham, K.T.; Thinh, X.P. Enhancement of electrochemical properties of porous rGO by controlled growth CoFe2O4 nanoparticles. J. Mater. Sci. Mater. Electron. 2023, 34, 1707. [Google Scholar] [CrossRef]
- Hamdi, N.; Belam, W. Structural, Electrical and Magnetic Properties of Copper-Substituted Co0.8−XNi0.2 Ferrites Synthesized by Sol–Gel Autocombustion Process. J. Electron. Mater. 2023, 52, 5996–6008. [Google Scholar] [CrossRef]
- Basak, M.; Rahman, M.L.; Ahmed, M.F.; Biswas, B.; Sharmin, N. Calcination effect on structural, morphological and magnetic properties of nanosized CoFe2O4 developed by a simple co-precipitation technique. Mater. Chem. Phys. 2021, 264, 124442. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Deac, I.G.; Neag, E.; Cadar, O. Influence of Cu2+, Ni2+, and Zn2+ ions doping on the structure, mor-phology, and magnetic properties of Co-ferrite embedded in SiO2 matrix obtained by an innovative sol-gel route. Nanomaterials 2020, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.S.; Abu-Dief, A.M. Synthesis, characterization and photocatalysis enhancement of Eu2O3-ZnO mixed oxide nanoparticles. J. Phys. Chem. Solid. 2018, 116, 375–385. [Google Scholar] [CrossRef]
- More, P.; Kadam, S.; Lokhande, P.; Jangam, G.; Patange, S.; Satpute, D. Effect of sintering temperature on the structural, morphological, and the magnetic properties of Ni0.25Cu0.55Zn0.20Fe2O4 nano ferrite. J. Magn. Magn. Mater. 2023, 586, 17119. [Google Scholar] [CrossRef]
- Patil, K.; Kadam, S.; Lokhande, P.; Balgude, S.; More, P. The effects of cobalt and magnesium co-doping on the structural and magnetic properties of ZnFe2O4 synthesized using a sonochemical process. Solid State Commun. 2021, 337, 114435. [Google Scholar] [CrossRef]
- Prabhakaran, T.; Mangalaraja, R.V.; Denardin, J.C.; Varaprasa, K. The effect of capping agents on the structural and magnetic properties of cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 11774–11782. [Google Scholar] [CrossRef]
- Thu, P.T.K.; Trinh, N.D.; Hoan, N.T.V.; Du, D.X.; Mau, T.X.; Trung, V.H.; Phong, N.H.; Toan, T.T.T.; Khieu, D.Q. Synthesis of cobalt ferrite and simultaneous determination of ascorbic acid, acetaminophen and caffeine by voltammetric method using cobalt ferrite modified electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 17245–17261. [Google Scholar] [CrossRef]
- Soleymani, J.; Hasanzadeh, M.; Shadjou, N.; Jafari, M.K.; Gharamaleki, J.V.; Yadollahi, M.; Jouyban, A. A new kinetic–mechanistic approach to elucidate electrooxidation of doxorubicin hydrochloride in unprocessed human fluids using magnetic graphene-based nanocomposite modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 61, 638–650. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Richtera, L. Preparation and Characterization of Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes and Its Application in a Sensitive Assay of Antihyperlipidemic Simvastatin in Biological Samples. Molecules 2019, 24, 2215. [Google Scholar] [CrossRef]
- Ali, M.F.B.; Abdel-aal, F.A.M. In situ polymerization and FT-IR characterization of poly-glycine on pencil graphite electrode for sensitive determination of anti-emetic drug, granisetron in injections and human plasma. RSC Adv. 2019, 9, 4325–4335. [Google Scholar] [CrossRef]
- Bagherinasab, Z.; Beitollahi, H.; Yousefi, M.; Bagherzadeh, M.; Hekmati, M. A sensitive voltammetric morphine nanosensor based on BaFe12O19 nanoparticle-modified screen-printed electrodes. J. Iran. Chem. Soc. 2019, 17, 717–724. [Google Scholar] [CrossRef]
- Lang, J.; Wang, W.; Zhou, Y.; Guo, D.; Shi, R.; Zhou, N. Electrochemical Behavior and Direct Quantitative Determination of Paclitaxel. Front. Chem. 2022, 10, 834154. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley Interscience: New York, NY, USA, 2004. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19. [Google Scholar] [CrossRef]
- Zaouak, A.; Jelassi, H. Electrochemical behavior of methocarbamol on platinum electrode by cyclic voltammetry. J. Mater. Environ. Sci. 2020, 11, 2025–2033. [Google Scholar]
- Mousty, C.; Farhat, H. Recent advances in layered double hydroxides-based electrochemical sensors: Insight in transition metal contribution. Electroanalysis 2023, 35, e202200527. [Google Scholar] [CrossRef]
- Patil, D.G.; Gokavi, N.M.; Bagoji, A.M.; Nandibewoor, T.S. Electrochemical Characterization and determination of paracetamol and tramadol drug using graphite pencil electrode. Anal. Bioanal. Electrochem. 2016, 8, 78–91. [Google Scholar]
- Gete, G.T.; Kassaw, M.M. Determination of paracetamol and kinetics Parameters using Nickel hexacynoferrate modified carbon paste electrode. Int. J. Adv. Res. 2016, 4, 1087–1093. [Google Scholar] [CrossRef]
- Caiwei, L.; Weimeng, S.; Yuting, W.; Cheng, Z.; Wu, L.; Qingli, H. Electrochemical Determination of Paracetamol at Poly(3-Methylthiophene)/Reduced Graphene Oxide Modified Glassy Carbon Electrode. NANO Brief. Rep. Rev. 2018, 13, 1850104. [Google Scholar]
- Qin, L.; Xiaozhi, K.; Lanzhi, X.; Zhixiang, Y.; Yingchun, Y. A facile synthesis of nanostructured CoFe2O4 for the electrochemica.l sensing of bisphenol A. RSC Adv. 2020, 10, 6156. [Google Scholar]
- Monteiro, M.K.S.; Santos, E.C.M.M.; Silva, D.R.; Martínez-Huitle, C.A.; dos Santo, E.V. Simultaneous determination of paracetamol and caffeine in pharmaceutical formulations and synthetic urine using cork-modified graphite electrodes. J. Solid. State Electrochem. 2020, 24, 1789–1800. [Google Scholar] [CrossRef]
- Islam, M.T.; Quispe, C.; Islam, M.A.; Ali, E.S.; Saha, S.; Asha, U.H.; Mondal, M.; Razis, A.F.A.; Sunusi, U.; Kamal, R.M.; et al. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomed. Pharmacother. 2021, 140, 111732. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur, M.; Rabenirina, G.; Schifano, N.; Odou, P.; Ethgen, S.; Lebuffe, G.; Foulon, C. Determination of acetaminophen and its main metabolites in urine by capillary electrophoresis hyphenated to mass spectrometry. Talanta 2019, 205, 120108. [Google Scholar] [CrossRef]
- Katzman, B.; Levine, M.A. Massive Acetaminophen Overdose with Unusual Pharmacokinetics. Can. J. Gen. Intern. Med. 2023, 18, 9–13. [Google Scholar] [CrossRef]
- Foroozan, H.; Masoumeh, T.; Somayeh, T. Ultra-sensitive electrochemical sensing of acetaminophen and codeine in biological fluids using CuO/CuFe2O4 nanoparticles as a novel electrocatalyst. J. Food Drug Anal. 2018, 26, 879–886. [Google Scholar]
- Naguib, I.A.; Majed, M.; Albogami, M.; Alshehri, M.; Bukhari, A.; Alshabani, H.; Alsalahat, I.; Abd-ElSalam, H.-A.H. Greenness Assessment of HPLC Analytical Methods with Common Detectors for Assay of Paracetamol and Related Materials in Drug Products and Biological Fluids. Separations 2023, 10, 283. [Google Scholar] [CrossRef]
- Zheng, M.X.; Gao, F.; Wang, Q.X.; Cai, X.L.; Jiang, S.L.; Huang, L.Z.; Gao, F. Electrocatalytic oxidation and sensitive determination of acetaminophen on glassy carbon electrode modified with graphene–chitosan composite. Mater. Sci. Eng. C 2013, 33, 1514–1520. [Google Scholar] [CrossRef]
- Wan, Q.J.; Wang, X.W.; Yu, F.; Wang, X.X.; Yang, N.J. Effects of capacitance and resistance of MWNT-film coated electrodes on voltammetric detection of acetaminophen. J. Appl. Electrochem. 2009, 39, 1145–1151. [Google Scholar] [CrossRef]
- Xu, C.; Huang, K.; Fan, Y.; Wu, Z.; Li, J. Electrochemical determination of acetaminophen based on TiO2-graphene/poly(methyl red) composite film modified electrode. J. Mol. Liq. 2012, 165, 32–37. [Google Scholar] [CrossRef]
- Kumar, S.A.; Tang, C.F.; Chen, S.M. Electroanalytical determination of acetaminophen using nano-TiO2/polymer coated electrode in the presence of dopamine. Talanta 2008, 76, 997–1005. [Google Scholar] [CrossRef]
- Goyal, R.N.; Singh, S.P. Voltammetric determination of paracetamol at C60-modified glassy carbon electrode. Electrochim. Acta 2006, 51, 3008–3012. [Google Scholar] [CrossRef]
- Wan, Q.; Wang, X.; Yu, F.; Wang, X.; Yang, N. Poly(taurine)/MWNT-modified glassy carbon electrodes for the detection of acetaminophen. J. Appl. Electrochem. 2009, 39, 785–790. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Granja-Banguera, C.P.; Silgado-Cortázar, D.G.; Morales-Morales, J.A. Transition Metal Substituted Barium Hexaferrite Modified Electrode: Application as Electrochemical Sensor of Acetaminophen. Molecules 2022, 27, 1550. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Kung, C.W.; Wei, H.Y.; Boopathi, K.M.; Chu, C.W.; Ho, K.C. A high performance electrochemical sensor for acetaminophen based on a rGO-PEDOT nanotube composite modified electrode. J. Mater. Chem. A 2014, 2, 7229–7237. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lai, C.L.; Zhang, Y.Y.; Deng, J.H.; Li, H.T.; Yao, S.Z. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, H.; Gao, X.; Ma, H. Electrochemical behavior and voltammetric determination of acetaminophen based on glassy carbon electrodes modified with poly(4-aminobenzoic acid)/electrochemically reduced graphene oxide composite films. Mater. Sci. Eng. C 2014, 45, 21–28. [Google Scholar] [CrossRef]
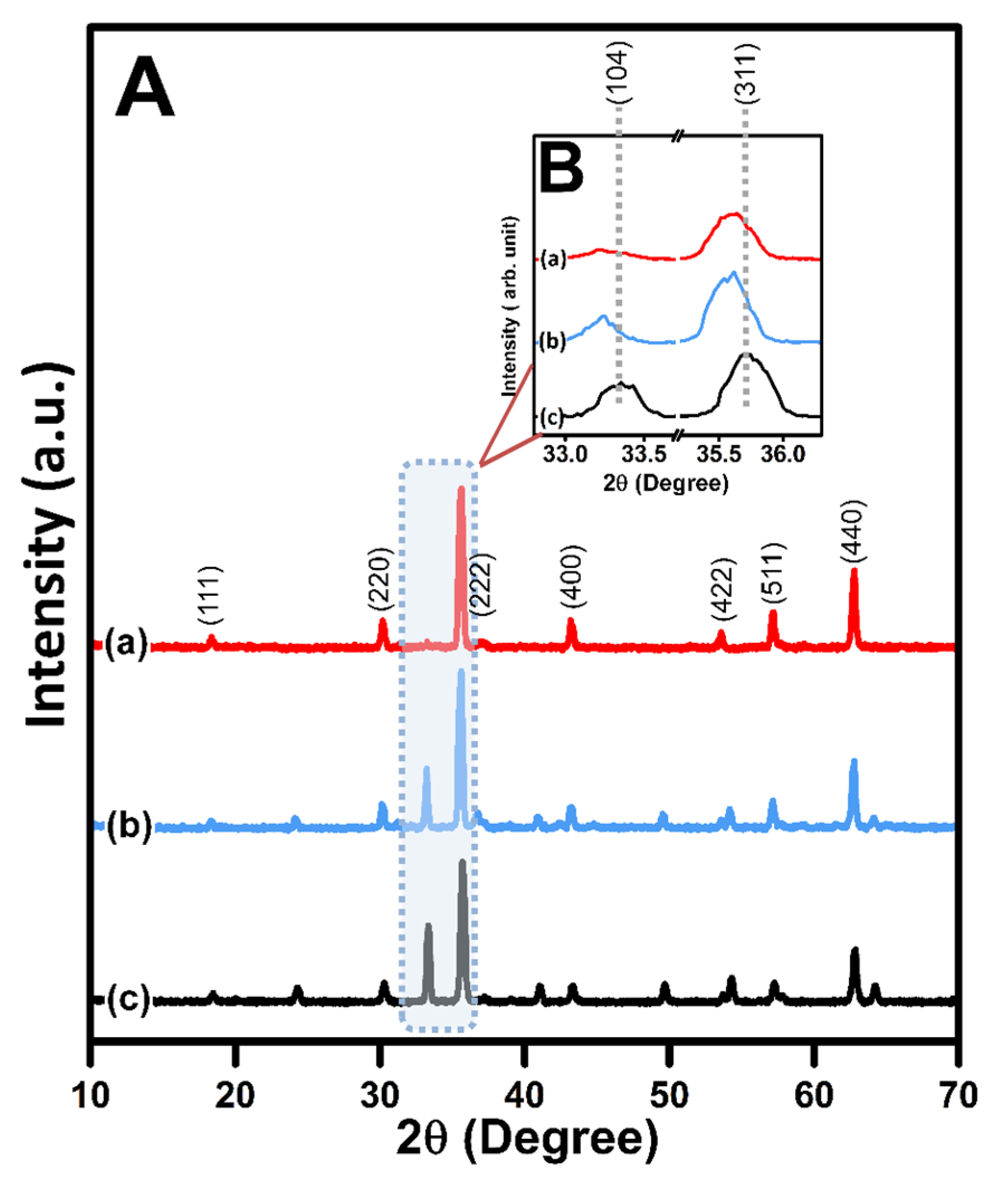
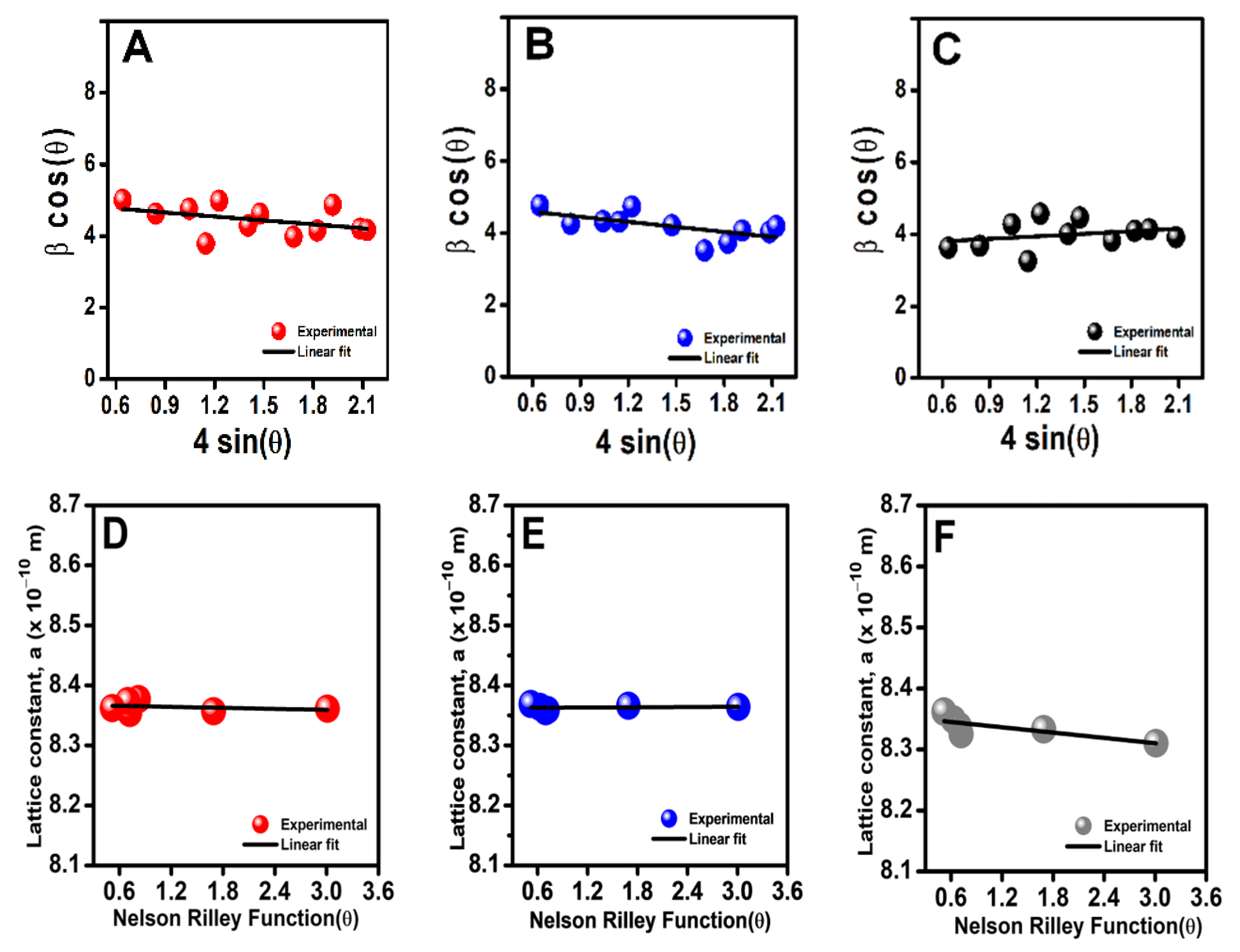



| Sample | L (nm) | † ε (×10−4) | ψ a (Å) | dXRD (g cm−3) | δ dbulk (g cm−3) | * P (%) | ϕ SSA (m2 g−1) |
|---|---|---|---|---|---|---|---|
| S-009 | 29.7 | −4.7 | ‡ (8.36) 8.35 | (5.34) 5.36 | 5.19 | 2.81 | (37.83) 37.69 |
| S-013 | 29.0 | −3.7 | (8.37) 8.36 | (5.33) 5.35 | 5.20 | 2.44 | (38.81) 38.67 |
| S-028 | 39.5 | +2.3 | (8.35) 8.32 | (5.37) 5.43 | 5.21 | 2.98 | (28.29) 27.97 |
| Urine | Added (μM) | (a) Detected (µM) | Recovery | SD ± RSD (%) | |||
|---|---|---|---|---|---|---|---|
| S-013/GCE | GCE | S-013/GCE | GCE | S-013/GCE | GCE | ||
| Sample 1 | 1.0 | 1.01 | 0.88 | 101.0 | 88.01 | 0.04 ± 3.96 | 0.04 ± 4.54 |
| Sample 2 | 2.0 | 1.93 | 1.78 | 96.50 | 89.03 | 0.09 ± 4.66 | 0.06 ± 3.37 |
| Sample 3 | 4.0 | 3.94 | 3.26 | 98.50 | 81.50 | 0.13 ± 3.28 | 0.09 ± 2.76 |
| Sample 4 | 8.0 | 7.98 | 7.14 | 99.75 | 89.25 | 0.15 ± 1.88 | 0.12 ± 1.68 |
| Sample 5 | 16.0 | 15.96 | 14.48 | 99.75 | 90.50 | 0.11 ± 0.69 | 0.21 ± 1.45 |
| (b) Sample 6 | - | 10.75 | 8.63 | - | - | 0.39 ± 3.68 | 0.38 ± 4.40 |
| 15 | 24.81 | 22.38 | 99.24 | 89.52 | 0.27 ± 1.09 | 0.37 ± 1.65 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso-González, J.G.; Granja-Banguera, C.P.; Morales-Morales, J.A.; Dector, A. A Facile Glycerol-Assisted Synthesis of Low-Cu2+-Doped CoFe2O4 for Electrochemical Sensing of Acetaminophen. Biosensors 2023, 13, 997. https://doi.org/10.3390/bios13120997
Alfonso-González JG, Granja-Banguera CP, Morales-Morales JA, Dector A. A Facile Glycerol-Assisted Synthesis of Low-Cu2+-Doped CoFe2O4 for Electrochemical Sensing of Acetaminophen. Biosensors. 2023; 13(12):997. https://doi.org/10.3390/bios13120997
Chicago/Turabian StyleAlfonso-González, José Guillermo, Claudia Patricia Granja-Banguera, Jimmy Alexander Morales-Morales, and Andrés Dector. 2023. "A Facile Glycerol-Assisted Synthesis of Low-Cu2+-Doped CoFe2O4 for Electrochemical Sensing of Acetaminophen" Biosensors 13, no. 12: 997. https://doi.org/10.3390/bios13120997
APA StyleAlfonso-González, J. G., Granja-Banguera, C. P., Morales-Morales, J. A., & Dector, A. (2023). A Facile Glycerol-Assisted Synthesis of Low-Cu2+-Doped CoFe2O4 for Electrochemical Sensing of Acetaminophen. Biosensors, 13(12), 997. https://doi.org/10.3390/bios13120997





