Fabrication of P and N Co-Doped Carbon Dots for Fe3+ Detection in Serum and Lysosomal Tracking in Living Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Measurements
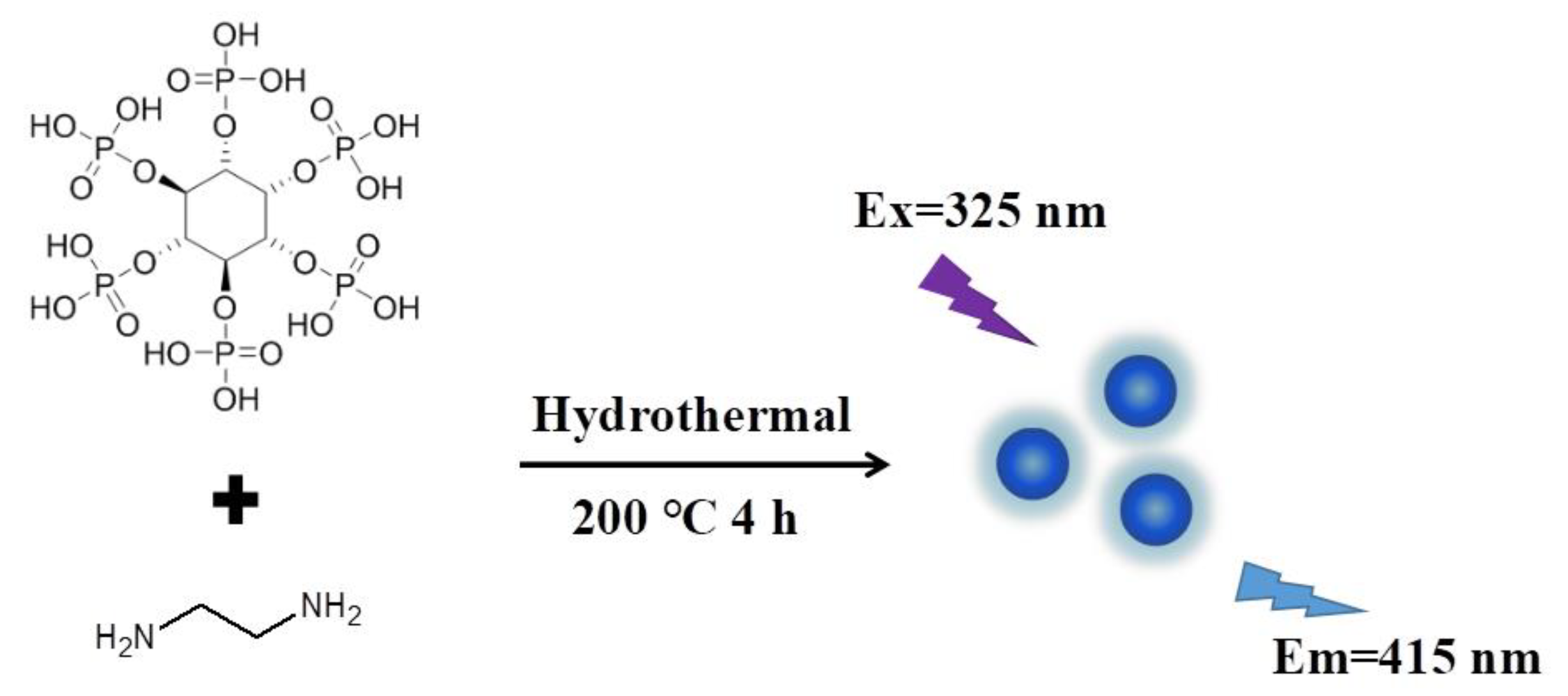
2.3. Preparation of PNCDs
2.4. PL Assay of Fe3+ with PNCDs
2.5. Detection of Fe3+ Content in Human Serum and SH-SY5Y Cells
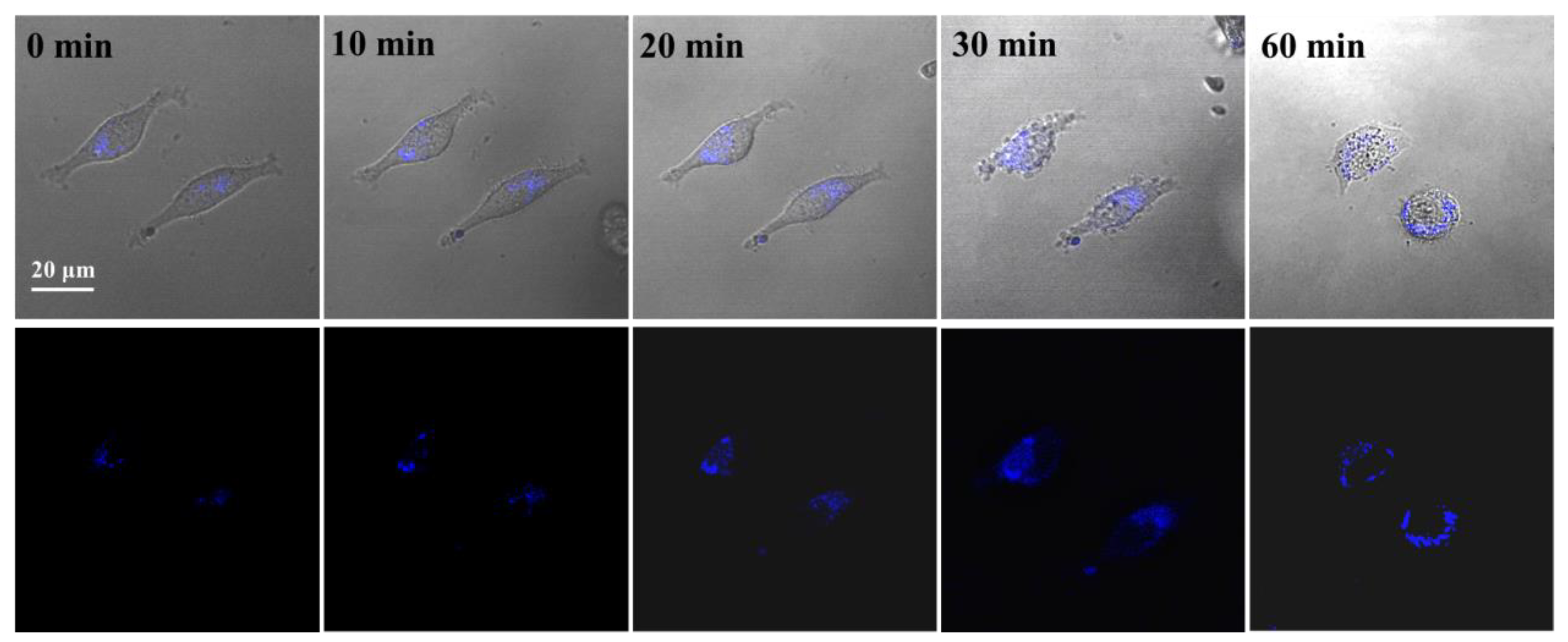
2.6. Cytotoxicity Assay
2.7. Subcellular Localization of PNCDs
2.8. Lysosomal Tracking
3. Results and Discussion
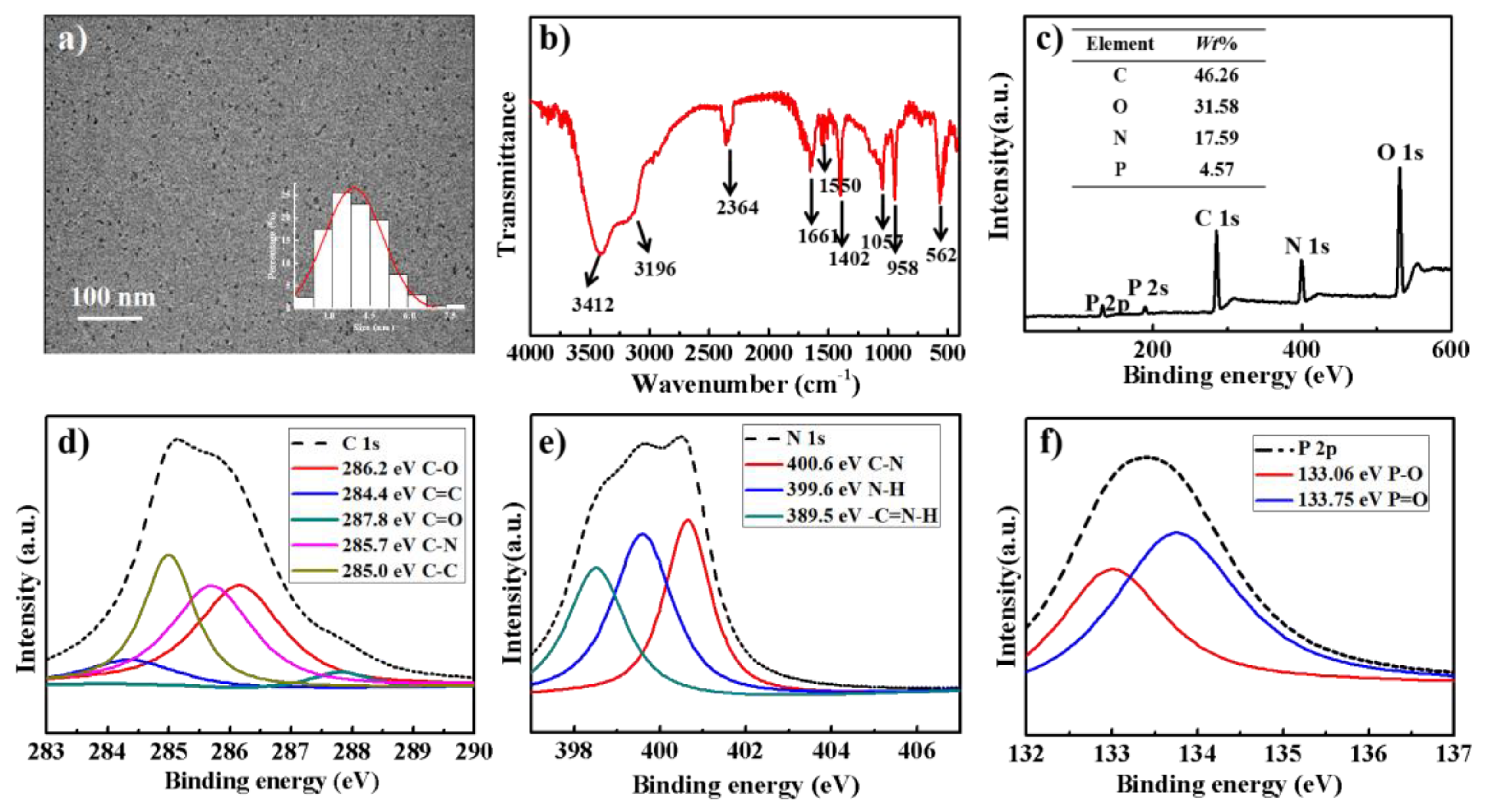
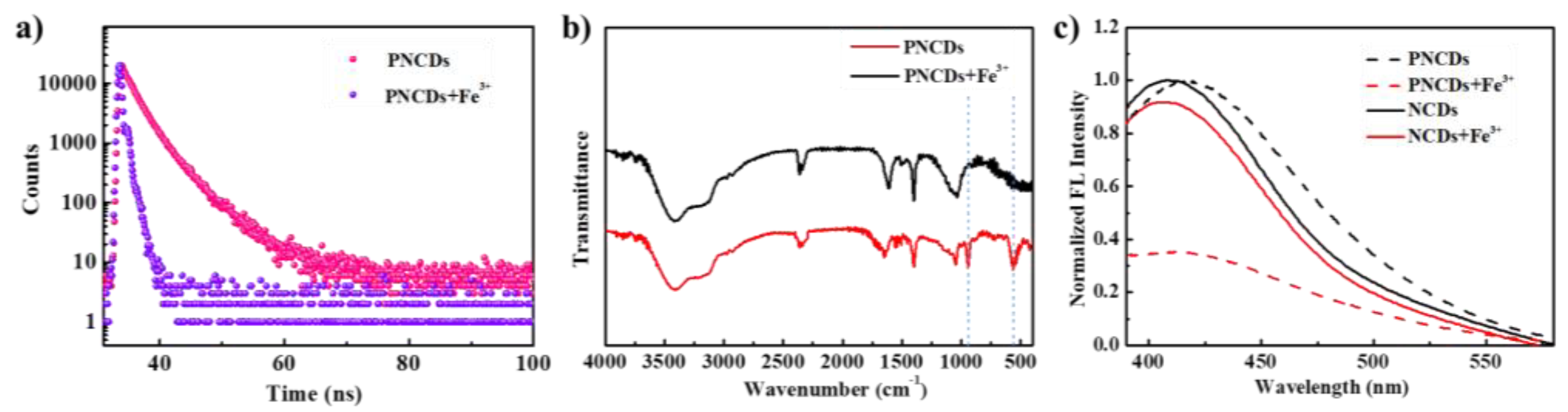
3.1. Preparation and Characterization of PNCDs
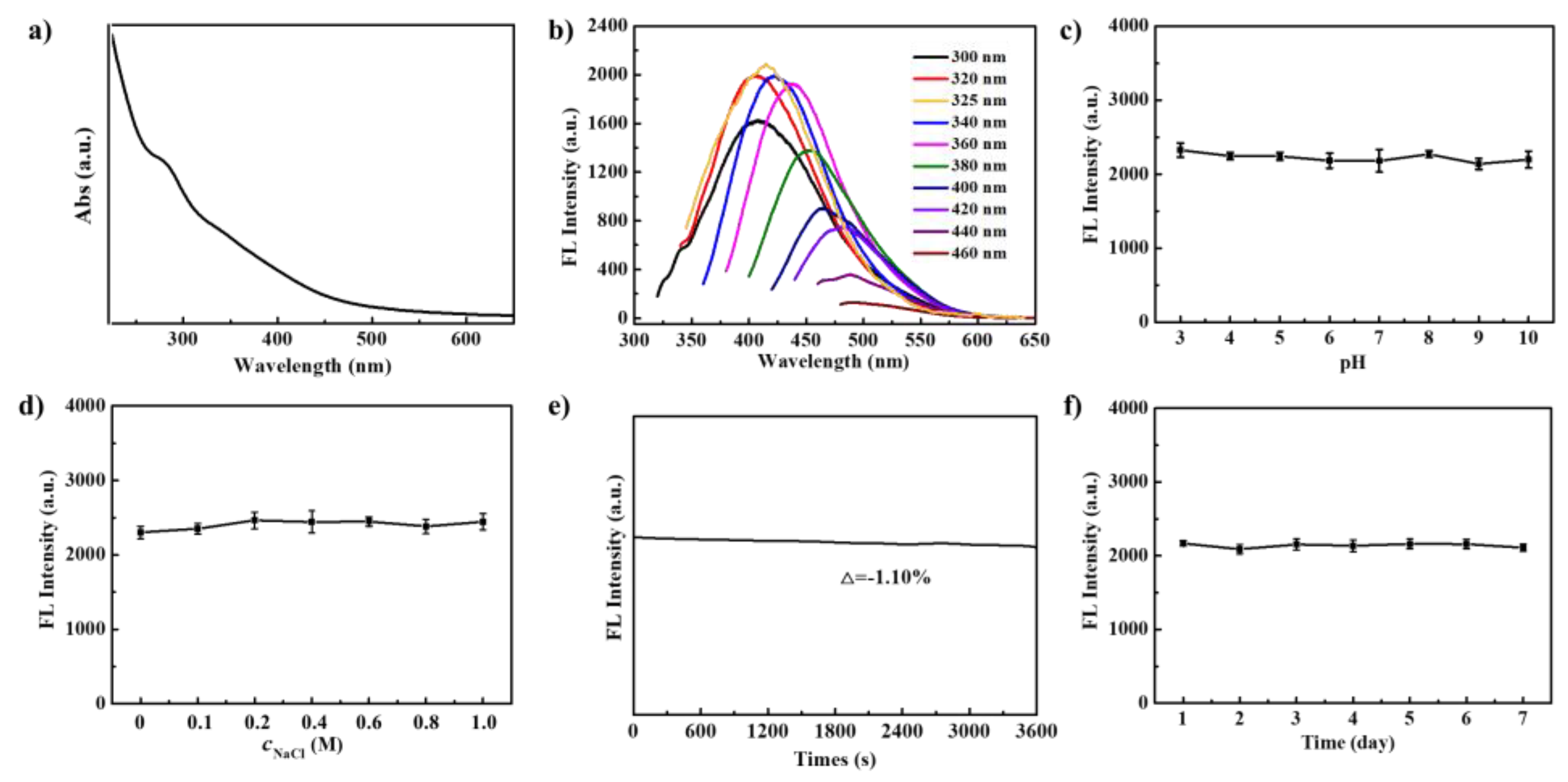
3.2. Optical Properties of PNCDs
3.3. Highly Sensitive Sensing of Fe3+ Using PNCDs
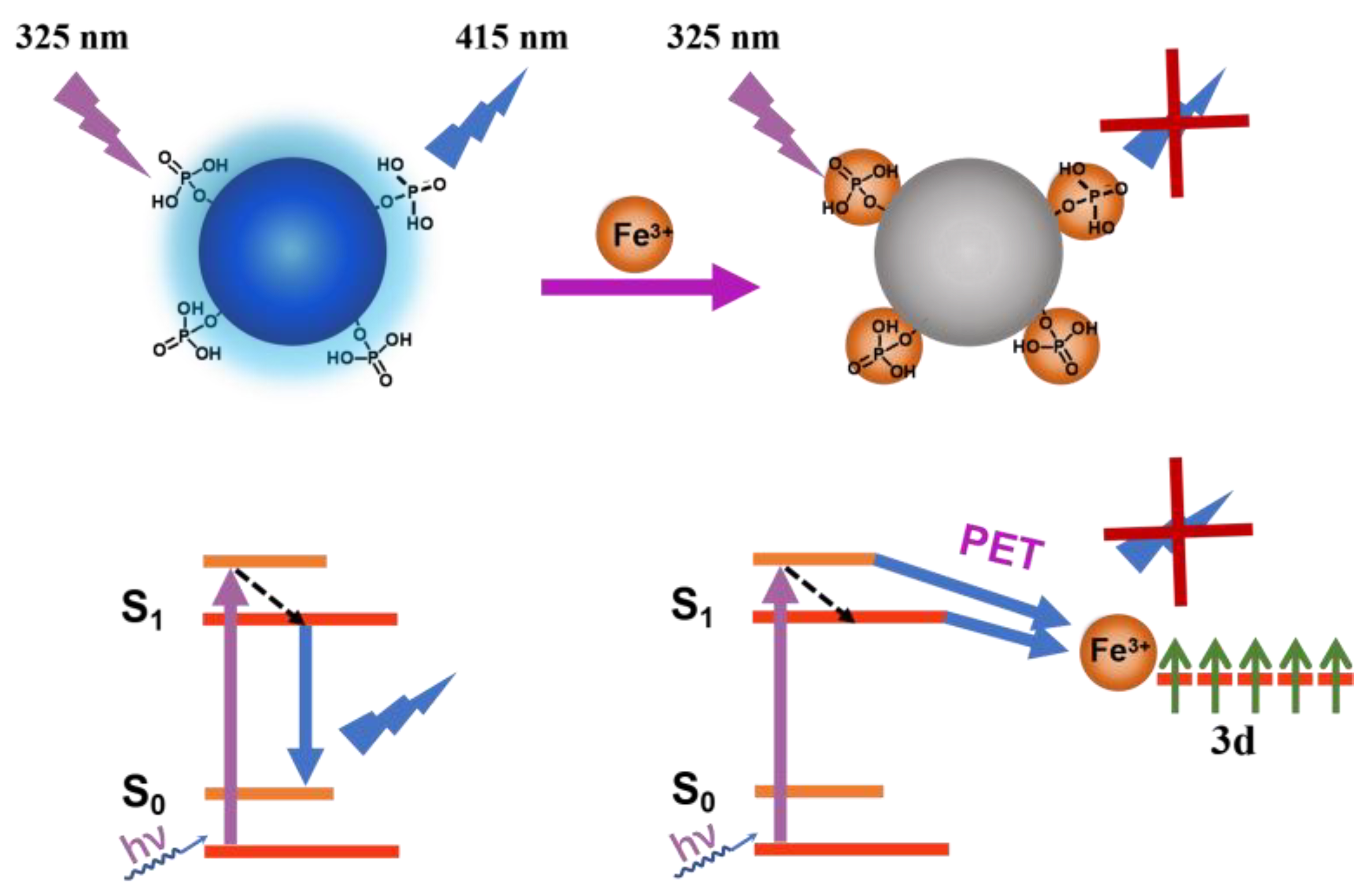
3.4. Quenching Mechanism of PNCDs by Fe3+
3.5. Determination of Fe3+ Contents in Human Serum and SH-SY5Y Cells
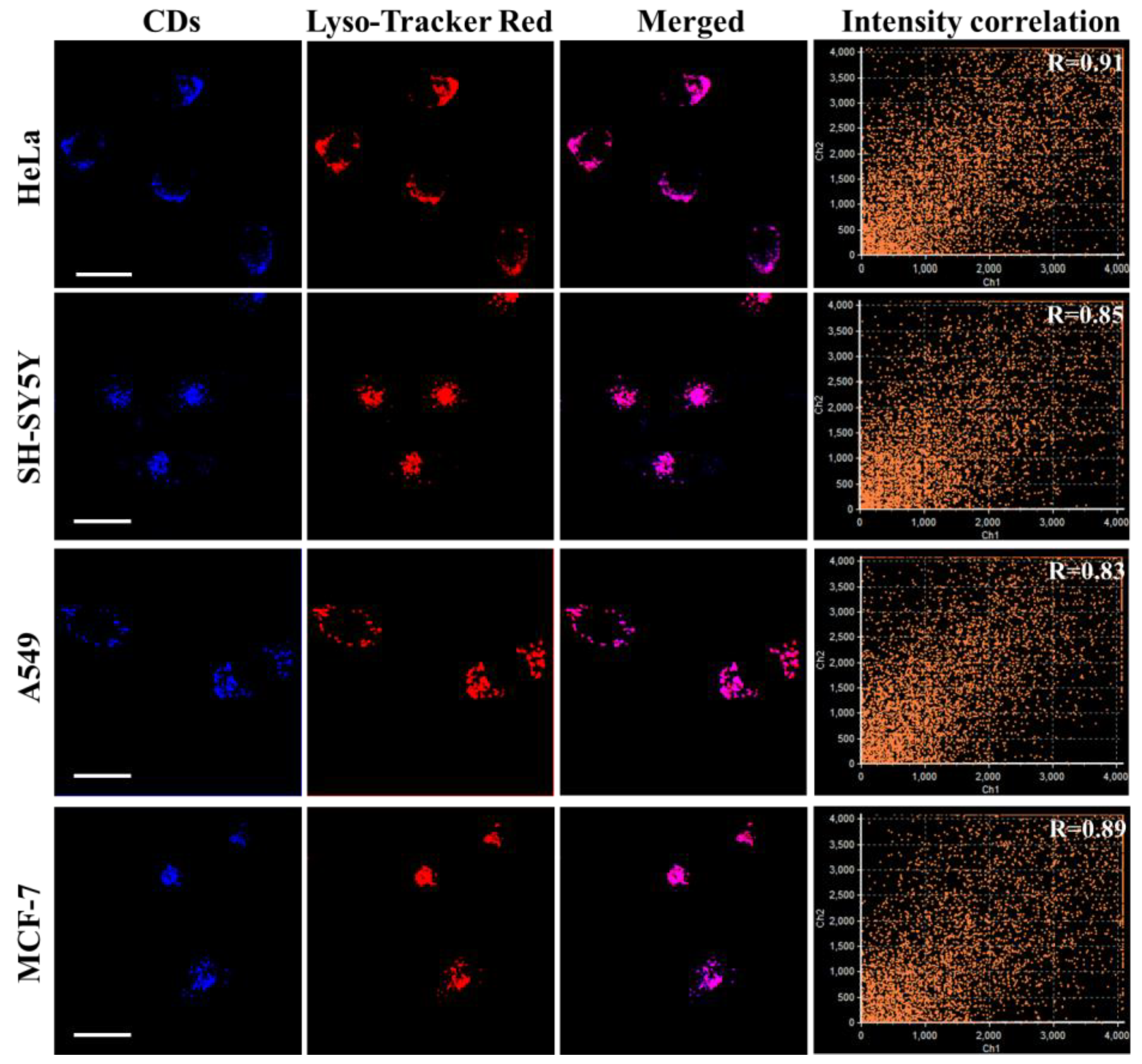
3.6. Intracellular Localization of PNCDs
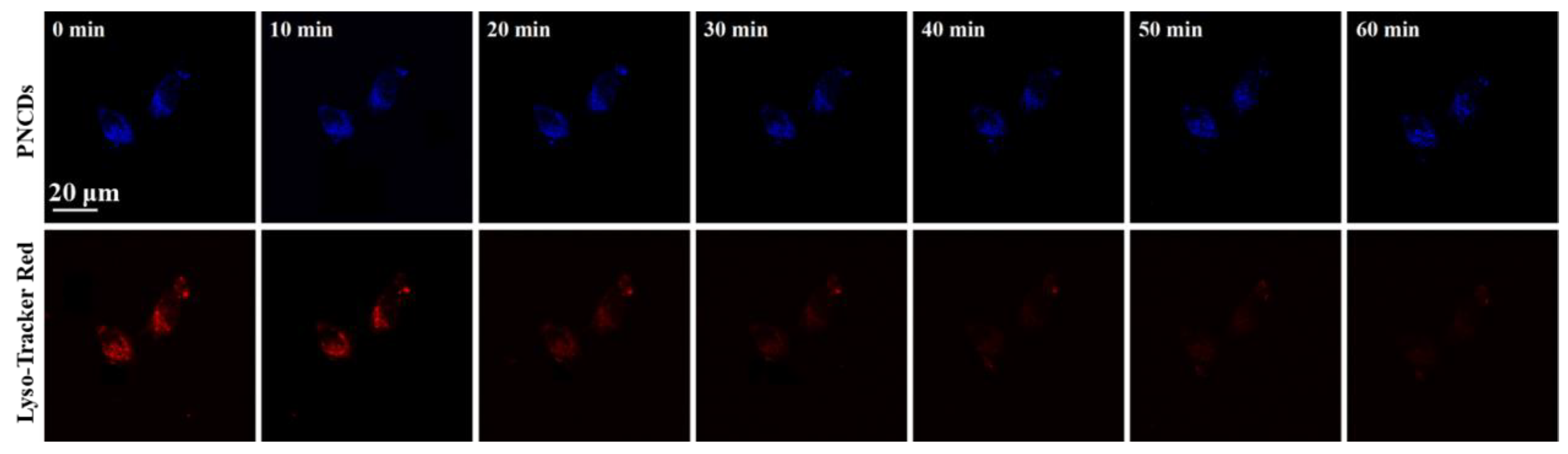
3.7. Lysosomal Tracking in Cells with PNCDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.Y.; Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zeng, Z.B.; Jiang, J.H.; Chang, Y.T.; Yuan, L. Discerning the chemistry in individual organelles with small-molecule fluorescent probes. Angew. Chem. Int. Ed. 2016, 55, 13658–13699. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Sloane, B.F. Near-infrared optical imaging of proteases in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef]
- Cai, Y.J.; Gui, C.; Samedov, K.; Su, H.F.; Gu, X.G.; Li, S.W.; Luo, W.W.; Sung, H.H.Y.; Lam, J.W.Y.; Kwok, R.T.K.; et al. An acidic pH independent piperazine-TPE AIEgen as a unique bioprobe for lysosome tracing. Chem. Sci. 2017, 8, 7593–7603. [Google Scholar] [CrossRef]
- Soreghan, B.; Thomas, S.N.; Yang, A.J. Aberrant sphingomyelin/ceramide metabolic-induced neuronal endosomal/lysosomal dysfunction: Potential pathological consequences in age-related neurodegeneration. Adv. Drug Deliv. Rev. 2003, 55, 1515–1524. [Google Scholar] [CrossRef]
- Ge, W.; Li, D.X.; Gao, Y.P.; Cao, X.T. The roles of lysosomes in inflammation and autoimmune diseases. Int. Rev. Immunol. 2015, 34, 415–431. [Google Scholar] [CrossRef]
- Platt, F.M. Sphingolipid lysosomal storage disorders. Nature 2014, 510, 68–75. [Google Scholar] [CrossRef]
- Wan, Q.Q.; Chen, S.M.; Shi, W.; Li, L.H.; Ma, H.M. Lysosomal pH rise during heat shock monitored by a lysosom-targeting near-infrared ratiometric fluorescent probe. Angew. Chem. 2014, 126, 11096–11100. [Google Scholar] [CrossRef]
- Moore, M.N. Cytochemical demonstration of latency of lysosomal hydrolases in digestive cells of the common mussel, Mytilus edulis, and changes induced by thermal stress. Cell Tissue Res. 1976, 175, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lekube, X.; Cajaraville, M.P.; Marigómez, I. Use of polyclonal antibodies for the detection of changes induced by cadmium in lysosomes of aquatic organisms. Sci. Total Environ. 2000, 247, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Koukouzika, N.; Dimitriadis, V.K. Multiple biomarker comparison in Mytilus galloprovincialis from the Greece coast: “lysosomal membrane stability, neutral red retention, micronucleus frequency and stress on stress”. Ecotoxicology 2005, 14, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.L.; Dong, H.J.; Hu, M.M.; Wang, J.Y.; Zhang, H.; Zhu, H.; Sun, W.; Peng, X.J. Fluorescence imaging lysosomal changes during cell division and apoptosis observed using Nile Blue based near-infrared emission. Chem. Commun. 2013, 50, 882–884. [Google Scholar] [CrossRef]
- Wu, J.S.; Liu, W.M.; Ge, J.C.; Zhang, H.Y.; Wang, P.F. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- Proos, V.N.; Lundberg, M. Protein effects in non-heme iron enzyme catalysis: Insights from multiscale models. J. Biol. Inorg. Chem. 2016, 21, 645–657. [Google Scholar] [CrossRef]
- Korolnek, T.; Hamza, I. Macrophages and iron trafficking at the birth and death of red cells. Blood J. Am. Soc. Hematol. 2015, 125, 2893–2897. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.Y.; Fan, S.Y.; Li, Z.J.; Wang, G.L.; Gu, Z.G.; Liu, J.K. D-penicillamine-functionalized graphene quantum dots for fluorescent detection of Fe3+ in iron supplement oral liquids. Sens. Actuators B 2017, 243, 211–220. [Google Scholar] [CrossRef]
- Li, G.C.; Tang, J.; Ding, P.G.; Ye, Y. A rhodamine-benzimidazole based chemosensor for Fe3+ and its application in living cells. J. Fluoresc. 2016, 26, 155–161. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.W.; Li, J.X.; Zeng, M.Y.; Xu, J.Z.; Wang, X.K.; Hu, W.P. Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 2014, 6, 4157–4162. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.C.; Cao, J.; Zhu, J.; Fan, L.Z.; Li, X.H. Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Siahcheshm, P.; Heiden, P. High quantum yield carbon quantum dots as selective fluorescent turn-off probes for dual detection of Fe2+/Fe3+ ions. J. Photochem. Photobiol. A 2023, 435, 114284. [Google Scholar] [CrossRef]
- Iqbal, A.; Tian, Y.J.; Wang, X.D.; Gong, D.Y.; Guo, Y.L.; Iqbal, K.; Wang, Z.P.; Liu, W.S.; Qin, W.W. Carbon dots prepared by solid state method via citric acid and 1, 10-phenanthroline for selective and sensing detection of Fe2+ and Fe3+. Sens. Actuators B 2016, 237, 408–415. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Kumar, Y.A.; Mani, G.; Nallapureddy, R.R.; Parvathala, A.; Albaqami, M.D.; Karamic, A.M.; Joo, S.W. A novel hybridized needle-like Co3O4/N-CNO composite for superior energy storage asymmetric supercapacitors. J. Alloys Compd. 2022, 908, 164447. [Google Scholar] [CrossRef]
- Hu, Q.; Wujcik, E.K.; Kelarakis, A.; Cyriac, J.; Gong, X.J. Carbon-based nanomaterials as novel nanosensors. J. Nanomater. 2017, 1, 323–347. [Google Scholar] [CrossRef]
- Rajaji, U.; Govindasamy, M.; Sha, R.; Alshgari, R.A.; Juang, R.S.; Liu, T.Y. Surface engineering of 3D spinel Zn3V2O8 wrapped on sulfur doped graphitic nitride composites: Investigation on the dual role of electrocatalyst for simultaneous detection of antibiotic drugs in biological fluids. Compos. Part B 2022, 242, 110017. [Google Scholar] [CrossRef]
- Liu, H.Y.; Sun, Y.Q.; Yang, J.; Hu, Y.L.; Yang, R.; Li, Z.H.; Qu, L.B.; Lin, Y.H. High performance fluorescence biosensing of cysteine in human serum with superior specificity based on carbon dots and cobalt-derived recognition. Sens. Actuators B 2019, 28, 62–68. [Google Scholar] [CrossRef]
- Shi, X.X.; Meng, H.M.; Sun, Y.Q.; Qu, L.B.; Lin, Y.H.; Li, Z.H. Du, D. Far-red to near-infrared carbon dots: Preparation and applications in biotechnology. Small 2019, 15, 1901507. [Google Scholar] [CrossRef]
- Li, T.Z.; Wang, J.H.; Chen, X.W. Regulating the properties of carbon dots via a solvent-involved molecule fusion strategy for improved sensing selectivity. Anal. Chim. Acta 2019, 1088, 107–115. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Q.L.; Chang, H.T. Recent advances and sensing applications of carbon dots. Small Methods 2020, 4, 1900387. [Google Scholar] [CrossRef]
- Li, R.Y.; Li, Z.J.; Sun, X.L.; Ji, J.; Liu, L.; Gu, Z.G.; Wang, G.L. Graphene quantum dot-rare earth upconversion nanocages with extremely high efficiency of upconversion luminescence, stability and drug loading towards controlled delivery and cancer theranostics. Chem. Eng. J. 2020, 382, 122992. [Google Scholar] [CrossRef]
- Zhai, Y.C.; Wang, Y.; Li, D.; Zhou, D.; Jing, P.T.; Shen, D.Z.; Qu, S.N. Red carbon dots-based phosphors for white light-emitting diodes with color rendering index of 92. J. Colloid Interface Sci. 2018, 528, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, S.J.; Li, C.X.; Huang, H.; Liu, Y.; Kang, Z.H. Tuning laccase catalytic activity with phosphate functionalized carbon dots by visible light. ACS Appl. Mater. Interfaces 2015, 7, 10004–10012. [Google Scholar] [CrossRef]
- Zheng, A.Q.; Guo, T.T.; Guan, F.L.; Chen, X.W.; Shu, Y.; Wang, J.H. Ionic liquid mediated carbon dots: Preparations, properties and applications. Trends Anal. Chem. 2019, 119, 115638. [Google Scholar] [CrossRef]
- Wang, F.T.; Wang, L.N.; Xu, J.; Huang, K.J.; Wu, X. Synthesis and modification of carbon dots for advanced biosensing application. Analyst 2021, 146, 4418–4435. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.L.; Jiang, S.C.; Park, S.J.; Meng, L.Y. A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 2020, 49, 6915–6938. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Li, X.L.; Ling, Y.F.; Huang, C.; Jia, N.Q. Morpholine derivative-functionalized carbon dots-based fluorescent probe for highly selective lysosomal imaging in living cells. ACS Appl. Mater. Interfaces 2017, 9, 28222–28232. [Google Scholar] [CrossRef]
- Mao, Q.X.; Yuan, X.L.; Kong, X.L.; Chen, X.W.; Wang, J.H. Targeted imaging of the lysosome and endoplasmic reticulum and their pH monitoring with surface regulated carbon dots. Nanoscale 2018, 10, 12788–12796. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Rais, A.; Rawat, K.; Prasad, T.; Bohidar, H.B. Boron-doped carbon quantum dots: A ‘turn-off’fluorescent probe for dopamine detection. Nanotechnology 2020, 32, 025501. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Yun, S.; Kang, E.S.; Kim, J.W.; Park, J.H.; Choi, J.S. Effect of heteroatoms on the optical properties and enzymatic activity of N-doped carbon dots. RSC Adv. 2021, 11, 18776–18782. [Google Scholar] [CrossRef] [PubMed]
- Zhi, B.; Gallagher, M.J.; Frank, B.P.; Lyons, T.Y.; Qiu, T.A.; Da, J.; Mensch, A.C.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H.; et al. Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact. Carbon 2018, 129, 438–449. [Google Scholar] [CrossRef]
- Luo, K.; Wen, Y.M.; Kang, X.H. Halogen-Doped Carbon Dots: Synthesis, Application, and Prospects. Molecules 2022, 27, 4620. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, Y.B.; Shi, H.X.; Yan, Y.J.; Liu, E.Z.; Hu, X.Y.; Fan, J. A strong blue fluorescent nanoprobe for highly sensitive and selective detection of mercury (II) based on sulfur doped carbon quantum dots. Mater. Chem. Phys. 2019, 232, 145–151. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, Y.M.; Wang, J.; Liu, H.; Liu, X.Q.; Wang, L.F.; Liu, X.G.; Xue, W.D.; Ma, N. Electrochemical synthesis of phosphorus-doped graphene quantum dots for free radical scavenging. Phys. Chem. Chem. Phys. 2017, 19, 11631–11638. [Google Scholar] [CrossRef]
- Shangguan, J.F.; Huang, J.; He, D.G.; He, X.X.; Wang, K.M.; Ye, R.Z.; Yang, X.; Qing, T.P.; Tang, J.H. Highly Fe3+-selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef]
- Sun, X.C.; Bruückner, C.; Lei, Y. One-pot and ultrafast synthesis of nitrogen and phosphorus co-doped carbon dots possessing bright dual wavelength fluorescence emission. Nanoscale 2015, 7, 17278–17282. [Google Scholar] [CrossRef]
- He, S.L.; Qi, S.W.; Sun, Z.C.; Zhu, G.S.; Zhang, K.; Chen, W.W. Si, N-codoped carbon dots: Preparation and application in iron overload diagnosis. J. Mater. Sci. 2019, 54, 4297–4305. [Google Scholar] [CrossRef]
- Xu, L.N.; Mao, W.; Huang, J.R.; Li, S.H.; Huang, K.; Li, M.; Xia, J.L.; Chen, Q. Economical, green route to highly fluorescence intensity carbon materials based on ligninsulfonate/graphene quantum dots composites: Application as excellent fluorescent sensing platform for detection of Fe3+ ions. Sens. Actuators B Chem. 2016, 230, 54–60. [Google Scholar] [CrossRef]
- Jadhav, R.W.; Khobrekar, P.P.; Bugde, S.T.; Bhosale, S.V. Nanoarchitectonics of neomycin-derived fluorescent carbon dots for selective detection of Fe3+ ions. Anal. Methods 2022, 14, 3289–3298. [Google Scholar] [CrossRef]
- Michalke, B.; Willkommen, D.; Venkataramani, V. Setup of capillary electrophoresis-inductively coupled plasma mass spectrometry (CE-ICP-MS) for quantification of iron redox species (Fe (II), Fe (III)). JoVE 2020, 159, e61055. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, C.; Han, Z.; Xiao, Y. A photostable near-infrared fluorescent tracker with pH-independent specificity to lysosomes for long time and multicolor imaging. ACS Appl. Mater. Interfaces 2014, 6, 21669–21676. [Google Scholar] [CrossRef]
- Wu, L.L.; Li, X.L.; Huang, C.; Jia, N.Q. Dual-modal colorimetric/fluorescence molecular probe for ratiometric sensing of pH and its application. Anal. Chem. 2016, 88, 8332–8338. [Google Scholar] [CrossRef]
- Wu, L.L.; Wang, Y.; James, T.D.; Jia, N.Q.; Huang, C.S. A hemicyanine based ratiometric fluorescence probe for mapping lysosomal pH during heat stroke in living cells. Chem. Commun. 2018, 54, 5518–5521. [Google Scholar] [CrossRef]
- Zhou, X.F.; Su, F.Y.; Lu, H.G.; Senechal-Willis, P.; Tian, Y.Q.; Johnson, R.H.; Meldrum, D.R. An FRET-based ratiometric chemosensor for in vitro cellular fluorescence analyses of pH. Biomaterials 2012, 33, 171–180. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, Y.; Tian, W.M.; Deng, L.Z. Activatable rotor for quantifying lysosomal viscosity in living cells. J. Am. Chem. Soc. 2013, 135, 2903–2906. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.Y.; Sun, Y.Q.; Geng, X.; Zhao, K.R.; Meng, H.M.; Yang, R.; Qu, L.B.; Li, Z.H. A wash-free lysosome targeting carbon dots for ultrafast imaging and monitoring cell apoptosis status. J. Am. Chem. Soc. 2020, 1106, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Wang, X.; Han, J. Resistance to tumor necrosis factor-induced cell death mediated by PMCA4 deficiency. Mol. Cell. Biol. 2001, 21, 8276–8288. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.J.; Lin, M.; Yang, T.; Li, R.S.; Huang, C.Z.; Wang, J.; Li, Y.F. Nitrogen and phosphorus doped polymer carbon dots as a sensitive cellular mapping probe of nitrite. J. Mater. Chem. B 2019, 7, 2074–2080. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.P.; Hu, Q.; Guo, X.Q.; Li, L.; Shuang, S.M.; Gong, X.J.; Dong, C. Carbon quantum dots doped with phosphorus and nitrogen are a viable fluorescent nanoprobe for determination and cellular imaging of vitamin B12 and cobalt (II). Microchim. Acta 2019, 186, 506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ruan, S.B.; Liu, S.; Sun, T.T.; Qu, D.; Zhao, H.F.; Xie, Z.G.; Gao, H.L.; Jing, X.B.; Sun, Z.C. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Omer, K.M. Highly passivated phosphorous and nitrogen co-doped carbon quantum dots and fluorometric assay for detection of copper ions. Anal. Bioanal. Chem. 2018, 410, 6331–6336. [Google Scholar] [CrossRef]
- Tammina, S.K.; Yang, D.; Koppala, S.; Cheng, C.; Yang, Y. Highly photoluminescent N, P doped carbon quantum dots as a fluorescent sensor for the detection of dopamine and temperature. J. Photochem. Photobiol. B 2019, 194, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.P.; Wang, Y.H.; Xiao, Y.L.; Liu, W. Hydrothermal synthesis of carbon dots codoped with nitrogen and phosphorus as a turn-on fluorescent probe for cadmium (II). Microchim. Acta 2019, 186, 147. [Google Scholar] [CrossRef]
- Qie, X.W.; Zan, M.H.; Li, L.; Gui, P.; Chang, Z.M.; Ge, M.F.; Wang, R.S.; Guo, Z.Z.; Dong, W.F. High photoluminescence nitrogen, phosphorus co-doped carbon nanodots for assessment of microbial viability. Colloids Surf. B 2020, 191, 110987. [Google Scholar] [CrossRef]
- Pang, S.; Liu, S.Y. Dual-emission carbon dots for ratiometric detection of Fe3+ ions and acid phosphatase. Anal. Chim. Acta 2020, 1105, 155–161. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.X.; Liu, L.; Duan, Z.Q.; Liu, Y.P.; Zhang, W.Y.; Cui, J.J.; Rong, Y.F.; Dong, C. One-step synthesis of nitrogen/fluorine co-doped carbon dots for use in ferric ions and ascorbic acid detection. Nanomaterials 2022, 12, 2377. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wang, Y.M.; He, B.; Chen, X.Y.; Sun, J. Chitin-based carbon dots with tunable photoluminescence for Fe3+ detection. ACS Appl. Nano Mater. 2022, 5, 7502–7511. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Xiong, H.M. Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 2014, 6, 13817–13823. [Google Scholar] [CrossRef] [PubMed]
- Ramanarayanan, R.; Swaminathan, S. Synthesis and characterisation of green luminescent carbon dots from guava leaf extract. Mater. Today Proc. 2020, 33, 2223–2227. [Google Scholar] [CrossRef]
- Zhu, J.T.; Chu, H.Y.; Wang, T.S.; Wang, C.Z.; Wei, Y.M. Fluorescent probe based nitrogen doped carbon quantum dots with solid-state fluorescence for the detection of Hg2+ and Fe3+ in aqueous solution. Microchem. J. 2020, 158, 105142. [Google Scholar] [CrossRef]
- Pu, Z.F.; Wen, Q.L.; Yang, Y.J.; Cui, X.M.; Ling, J.; Liu, P.; Cao, Q.E. Fluorescent carbon quantum dots synthesized using phenylalanine and citric acid for selective detection of Fe3+ ions. Spectrochim. Acta Part A 2020, 229, 117944. [Google Scholar] [CrossRef]
- Senol, A.M.; Bozkurt, E. Facile green and one-pot synthesis of seville orange derived carbon dots as a fluorescent sensor for Fe3+ ions. Microchem. J. 2020, 159, 105357. [Google Scholar] [CrossRef]

| Sample | Determined (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%, n = 11) |
|---|---|---|---|---|---|
| Serum 1 | 2.93 ± 0.23 | 5 | 7.44 ± 0.17 | 90.27 ± 1.70 | 1.31 |
| Serum 2 | 3.22 ± 0.86 | 5 | 8.88 ± 0.78 | 113.23 ± 1.77 | 1.26 |
| Serum 3 | 3.86 ± 0.05 | 5 | 8.65 ± 0.03 | 95.76 ± 0.47 | 0.94 |
| Serum 4 | 4.04 ± 0.39 | 5 | 9.58 ± 0.22 | 110.33 ± 3.58 | 1.74 |
| SH-SY5Y cells | 2.58 ± 0.25 | 5 | 7.70 ± 0.31 | 102.33 ± 6.07 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Yang, M.; Chen, X. Fabrication of P and N Co-Doped Carbon Dots for Fe3+ Detection in Serum and Lysosomal Tracking in Living Cells. Biosensors 2023, 13, 230. https://doi.org/10.3390/bios13020230
Xing Y, Yang M, Chen X. Fabrication of P and N Co-Doped Carbon Dots for Fe3+ Detection in Serum and Lysosomal Tracking in Living Cells. Biosensors. 2023; 13(2):230. https://doi.org/10.3390/bios13020230
Chicago/Turabian StyleXing, Yanzhi, Mei Yang, and Xuwei Chen. 2023. "Fabrication of P and N Co-Doped Carbon Dots for Fe3+ Detection in Serum and Lysosomal Tracking in Living Cells" Biosensors 13, no. 2: 230. https://doi.org/10.3390/bios13020230





