Tigecycline Immunodetection Using Developed Group-Specific and Selective Antibodies for Drug Monitoring Purposes
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Synthesis of Conjugated Antigens
2.3. Antibody Preparation
2.4. Competitive Indirect ELISA
- Coating of antigens. GEL-based conjugates at the optimum concentration in CBB were coated on the plates (100 µL per well) overnight at 4 °C.
- Antibody binding (competitive step). Working antibody solution prepared in 1%BSA-PBST (100 µL) was added to the wells together with 100 µL of TGC standard (1000–0.01 ng/mL, and 0 ng/mL) in PBST or samples and incubated for 1 h at 25 °C.
- Detection of bound antibody using GAR-HRP. The latter reagent in 1%BSA-PBST was added in the amount of 100 µL per well and incubated for 1 h at 37 °C. All the steps described above were completed by washing three times with PBST to remove unreacted reagents.
- Enzymatic step. The substrate mixture (100 µL) was added to the wells, and after 0.5 h the reaction was terminated with 100 µL of the stop solution. Colored reaction product intensity was read at 450 nm using a LisaScan spectrophotometer (Erba Manheim, Czech Republic).
2.5. Sample Collection, Pretreatment and Recovery Examination
3. Results and Discussion
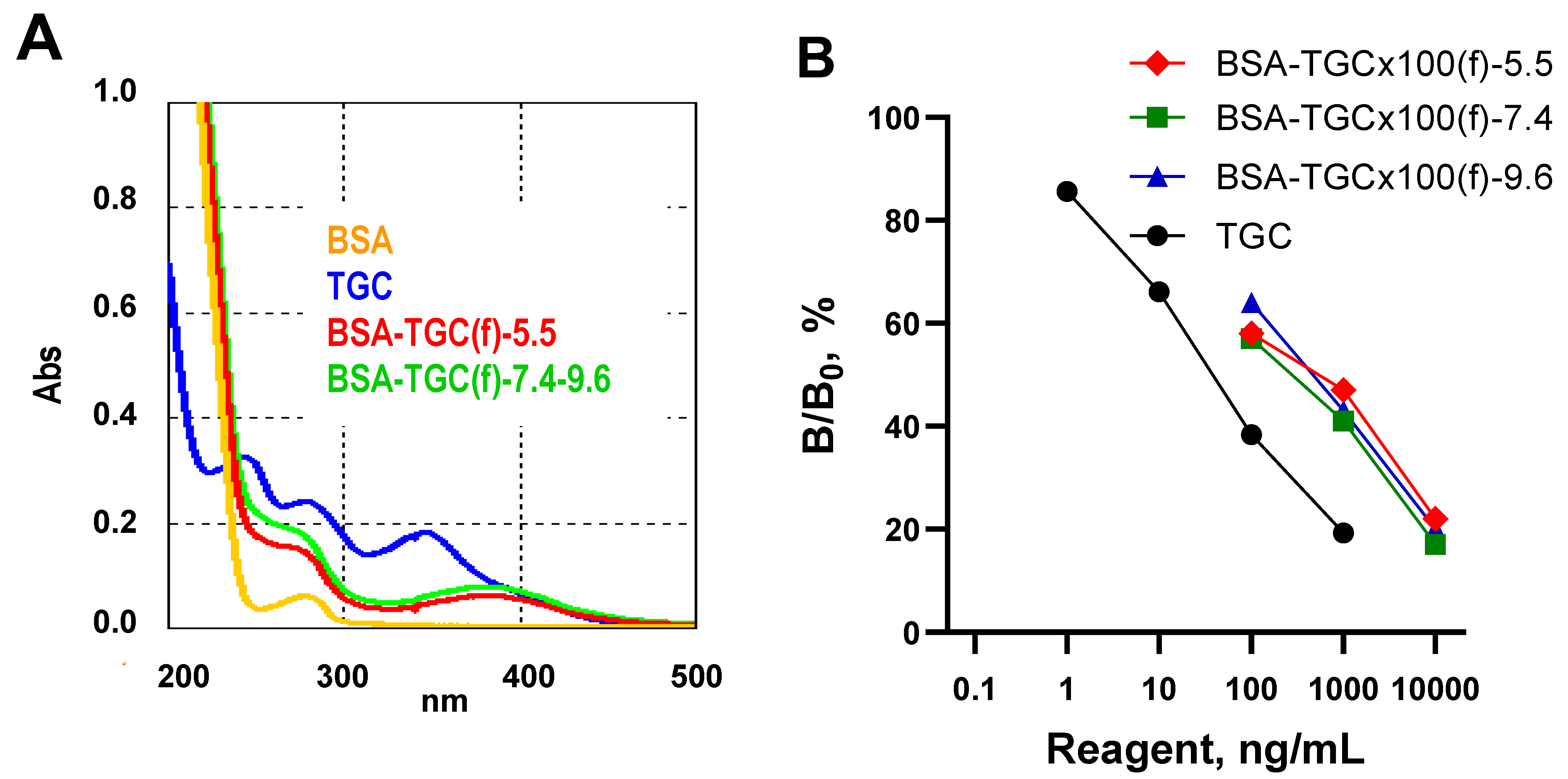
3.1. Preparation and Characteristics of Conjugated Antigens
3.2. Antibody Preparation, ELISA Development and Characteristics
3.3. Sample Pretreatment and Recovery Experiments
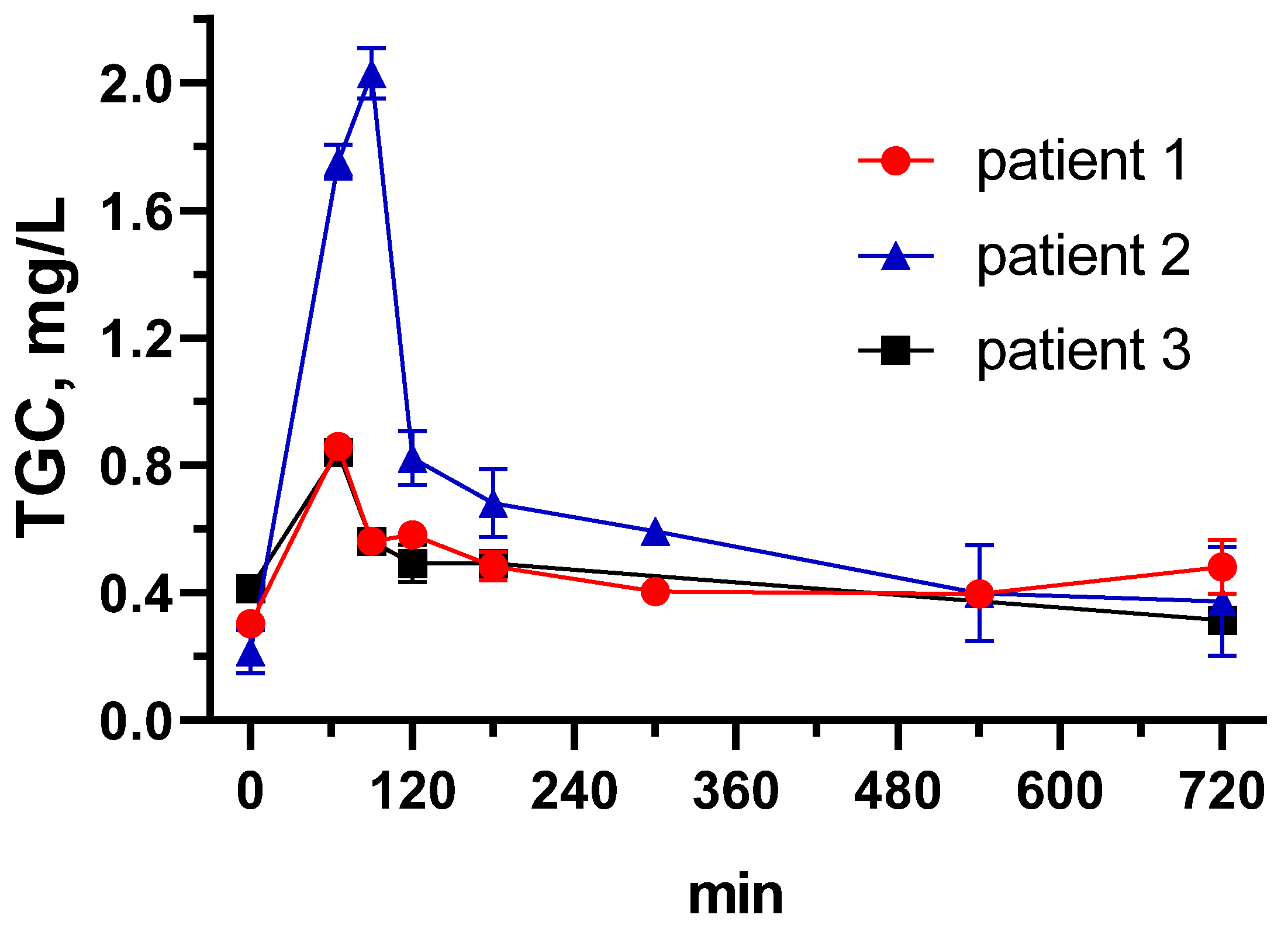
3.4. Tigecycline Pharmacokinetics in Critically Ill Patients Using the Developed ELISA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhanel, G.G.; Homenuik, K.; Nichol, K.; Noreddin, A.; Vercaigne, L.; Embil, J.; Gin, A.; Karlowsky, J.A. The glycylcyclines. Drugs 2004, 64, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Pan, L.; Guo, J.; French, N.; Villanueva, E.V.; Tefsen, B. Effectiveness and Safety of High Dose Tigecycline for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 1049–1064. [Google Scholar] [CrossRef]
- Prasad, P.; Sun, J.; Danner, R.L.; Natanson, C. Excess Deaths Associated With Tigecycline After Approval Based on Noninferiority Trials. Clin. Infect. Dis. 2012, 54, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Cai, H.; Li, Z.; Lu, Y.; Wang, G.; Lu, A. Tigecycline-induced coagulopathy: A literature review. Int. J. Clin. Pharm. Weekbl. 2019, 41, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Roberts, J.A.; Alobaid, A.S.; Roger, C.; Wang, Y.; Yang, Q.; Sun, J.; Dong, H.; Wang, X.; Xing, J.; et al. Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections. Antimicrob. Agents Chemother. 2017, 61, e00345-17. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Schmidt, S.; Ma, B.; Schiefelbein, L.; Rand, K.H.; Burkhardt, O.; Derendorf, H. Clinical Pharmacokinetics and Pharmacodynamics of Tigecycline. Clin. Pharmacokinet. 2009, 48, 575–584. [Google Scholar] [CrossRef]
- Hoffmann, M.; DeMaio, W.; Jordan, R.A.; Talaat, R.; Harper, D.; Speth, J.; Scatina, J. Metabolism, Excretion, and Pharmacokinetics of [14C]Tigecycline, a First-In-Class Glycylcycline Antibiotic, after Intravenous Infusion to Healthy Male Subjects. Drug Metab. Dispos. 2007, 35, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-C.; Huang, F.; Zhang, J.-M.; Zhuang, Y.-G. Population Pharmacokinetics of Tigecycline: A Systematic Review. Drug Des. Dev. Ther. 2022, 16, 1885–1896. [Google Scholar] [CrossRef]
- Rusu, A.; Buta, E.L. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef]
- Honore, P.M.; Jacobs, R.; De Waele, E.; Van Gorp, V.; Spapen, H.D. The blind spot in high-dose tigecycline pharmacokinetics in critically ill patients: Membrane adsorption during continuous extracorporeal treatment. Crit. Care 2015, 19, 24. [Google Scholar] [CrossRef]
- Leng, B.; Yan, G.; Wang, C.; Shen, C.; Zhang, W.; Wang, W. Dose optimisation based on pharmacokinetic/pharmacodynamic target of tigecycline. J. Glob. Antimicrob. Resist. 2021, 25, 315–322. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Lim, S.M.S.; Lipman, J.; Roberts, J.A. A personalised approach to antibiotic pharmacokinetics and pharmacodynamics in critically ill patients. Anaesth. Crit. Care Pain Med. 2021, 40, 100970. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, X.; Song, S.; Wu, A.; Liu, L.; Kuang, H.; Xu, C. Development of a monoclonal antibody-based immunochromatographic strip for the rapid detection of tigecycline in human serum. Anal. Methods 2021, 13, 817–824. [Google Scholar] [CrossRef]
- Nakane, P.K.; Kawaoi, A. Peroxidase-Labeled Antibody a New Method of Conjugation. J. Histochem. Cytochem. 1974, 22, 1084–1091. [Google Scholar] [CrossRef]
- Burkin, M.; Galvidis, I. Improved group determination of tetracycline antibiotics in competitive enzyme-linked immunosorbent assay. Food Agric. Immunol. 2009, 20, 245–252. [Google Scholar] [CrossRef]
- Burkin, M.A.; Moshcheva, A.G.; Galvidis, I.A. Immunoassay for Natamycin Trace Screening: Bread, Wine and Other Edibles Analysis. Biosensors 2022, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Burkin, M.A.; Surovoy, Y.A.; Arzumanian, V.G.; Galvidis, I.A. Development and application of amphotericin B immunoassay for pharmacokinetic studies and therapeutic drug monitoring in critically ill patients. J. Pharm. Biomed. Anal. 2022, 218, 114875. [Google Scholar] [CrossRef] [PubMed]
- Burkin, M.A.; Galvidis, I.A. Simultaneous immunodetection of ionophore antibiotics, salinomycin and narasin, in poultry products and milk. Anal. Methods 2021, 13, 1550–1558. [Google Scholar] [CrossRef]
- Galvidis, I.A.; Surovoy, Y.A.; Perevoznyuk, G.S.; Tsarenko, S.V.; Burkin, M.A. Unbound serum polymyxin B in patients with sepsis: Detection approaches and limited sampling strategy for clinical practice and research. J. Pharm. Biomed. Anal. 2022, 220, 114983. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Nuriev, R.; Galvidis, I.; Burkin, M. Immunochemical characteristics of Streptococcus pneumoniae type 3 capsular polysaccharide glycoconjugate constructs correlate with its immunogenicity in mice model. Vaccine 2020, 38, 8292–8301. [Google Scholar] [CrossRef]
- Galvidis, I.A.; Eremin, S.A.; Burkin, M.A. Three for the price of one! Immunodetection of three amphenicols in foodstuffs using a universal standard curve. Anal. Methods 2020, 12, 1728–1735. [Google Scholar] [CrossRef]
- Surovoy, Y.A.; Burkin, M.A.; Galvidis, I.A.; Sobolev, M.A.; Rende, O.C.; Tsarenko, S.V. Comparative polymyxin B pharmacokinetics in critically ill patients with renal insufficiency and in continuous veno-venous hemodialysis. Eur. J. Clin. Pharmacol. 2022, 79, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, G.; Micalizzi, M.; Speth, J.; Raible, D.; Troy, S. Pharmacokinetics of Tigecycline after Single and Multiple Doses in Healthy Subjects. Antimicrob. Agents Chemother. 2005, 49, 220–229. [Google Scholar] [CrossRef]
- Yao, F.; Wang, Y.; Hou, Y.; Wang, X.; Lan, J.; Wu, Z.; Wang, Y.; Chen, C. Establishment and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Tigecycline in Critically Ill Patients. Int. J. Anal. Chem. 2020, 2020, 6671392. [Google Scholar] [CrossRef]
- Bayliss, M.A.; Rigdova, K.; Kyriakides, M.; Grier, S.; Lovering, A.M.; Ellery, K.; Griffith, D.C.; MacGowan, A. Challenges in the bioanalysis of tetracyclines: Epimerisation and chelation with metals. J. Chromatogr. B 2019, 1134, 121807. [Google Scholar] [CrossRef] [PubMed]



| Analyte |  | |||||||
|---|---|---|---|---|---|---|---|---|
| R | R1 | R2 | R3 | R4 | R5 | |||
| TC EVC TGC CTC LC MNC DC OTC MTC | H NHCOCH2N(CH2)4 NHCOCH2NHC(CH3)3 H H H H H H | H F N(CH3)2 Cl H N(CH3)2 H H H | OH H OH OH OH H OH H | CH2 | CH3 H CH3 CH3 CH3 H CH3 CH3 | H H H H H H H OH H | H H H H Me-N-Lys H H H H | |
| Anti-BSA-TC(f) Ab #32 | anti-BSA-TGC(f) Ab #100 | |||||||
| Analyte | IC50, ng/mL | CR, % | IC50, ng/mL | CR, % | ||||
| TC | 1.25 | 126.5 | >1000 | <0.02 | ||||
| EVC | 1.33 | 119.3 | >1000 | <0.02 | ||||
| TGC | 1.59 | 100 | 0.23 | 100 | ||||
| CTC | 1.90 | 83.5 | >1000 | <0.02 | ||||
| LC | 5.58 | 28.4 | >1000 | <0.02 | ||||
| MNC | 9.08 | 17.5 | >1000 | <0.02 | ||||
| DC | 13.08 | 12.1 | >1000 | <0.02 | ||||
| OTC | 27.05 | 5.9 | >1000 | <0.02 | ||||
| MTC | 62.11 | 2.6 | >1000 | <0.02 | ||||
| Assay | IC50 | IC20–IC80 | IC90 (LOD) | Reference |
|---|---|---|---|---|
| ELISA LFIA ELISA-Ab#32 | 2.30 nd 1.59 | nd nd 0.22–18.9 | 0.22 15.03 0.05 | [13] [13] Present work |
| ELISA-Ab#100 | 0.23 | 0.04–2.83 | 0.02 | Present work |
| Sample Pretreatment | TGC Spike Level, ng/mL | Ab #32 | Ab #100 | ||
|---|---|---|---|---|---|
| RC, % | RSD, % | RC, % | RSD, % | ||
| PBST | 1500 | 81.4 | 8.6 | 102.0 | 9.4 |
| 300 | 98.1 | 3.4 | 90.8 | 11.5 | |
| TCA | 1500 | 82.0 | 6.0 | 88.7 | 2.2 |
| 300 | 73.6 | 9.7 | 80.1 | 4.1 | |
| MeOH | 1500 | 24.1 | 16.1 | 25.9 | 15.5 |
| 300 | 28.5 | 7.1 | 24.7 | 22.7 | |
| ACN | 1500 | 35.8 | 24.3 | 35.1 | 14.7 |
| 300 | 34.8 | 38.8 | 69.3 | 1.2 | |
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Dose, mg/12 h | 100 | 100 | 50 |
| AUC0-24 h, mg*h/L | 11.08 | 15.38 | 10.60 |
| Cmax, mg/L | 0.86 | 2.03 | 0.84 |
| CL, L/h | 18.04 | 13.01 | 9.43 |
| Vss, L | 975.49 | 149.96 | 189.56 |
| Causative microorganism | K. pneumoniae | E. faecium | A. baumannii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvidis, I.A.; Surovoy, Y.A.; Tsarenko, S.V.; Burkin, M.A. Tigecycline Immunodetection Using Developed Group-Specific and Selective Antibodies for Drug Monitoring Purposes. Biosensors 2023, 13, 343. https://doi.org/10.3390/bios13030343
Galvidis IA, Surovoy YA, Tsarenko SV, Burkin MA. Tigecycline Immunodetection Using Developed Group-Specific and Selective Antibodies for Drug Monitoring Purposes. Biosensors. 2023; 13(3):343. https://doi.org/10.3390/bios13030343
Chicago/Turabian StyleGalvidis, Inna A., Yury A. Surovoy, Sergei V. Tsarenko, and Maksim A. Burkin. 2023. "Tigecycline Immunodetection Using Developed Group-Specific and Selective Antibodies for Drug Monitoring Purposes" Biosensors 13, no. 3: 343. https://doi.org/10.3390/bios13030343
APA StyleGalvidis, I. A., Surovoy, Y. A., Tsarenko, S. V., & Burkin, M. A. (2023). Tigecycline Immunodetection Using Developed Group-Specific and Selective Antibodies for Drug Monitoring Purposes. Biosensors, 13(3), 343. https://doi.org/10.3390/bios13030343