Immunosensors for Assay of Toxic Biological Warfare Agents
Abstract
:1. Introduction
2. Toxins as a Part of Biological Warfare Agents
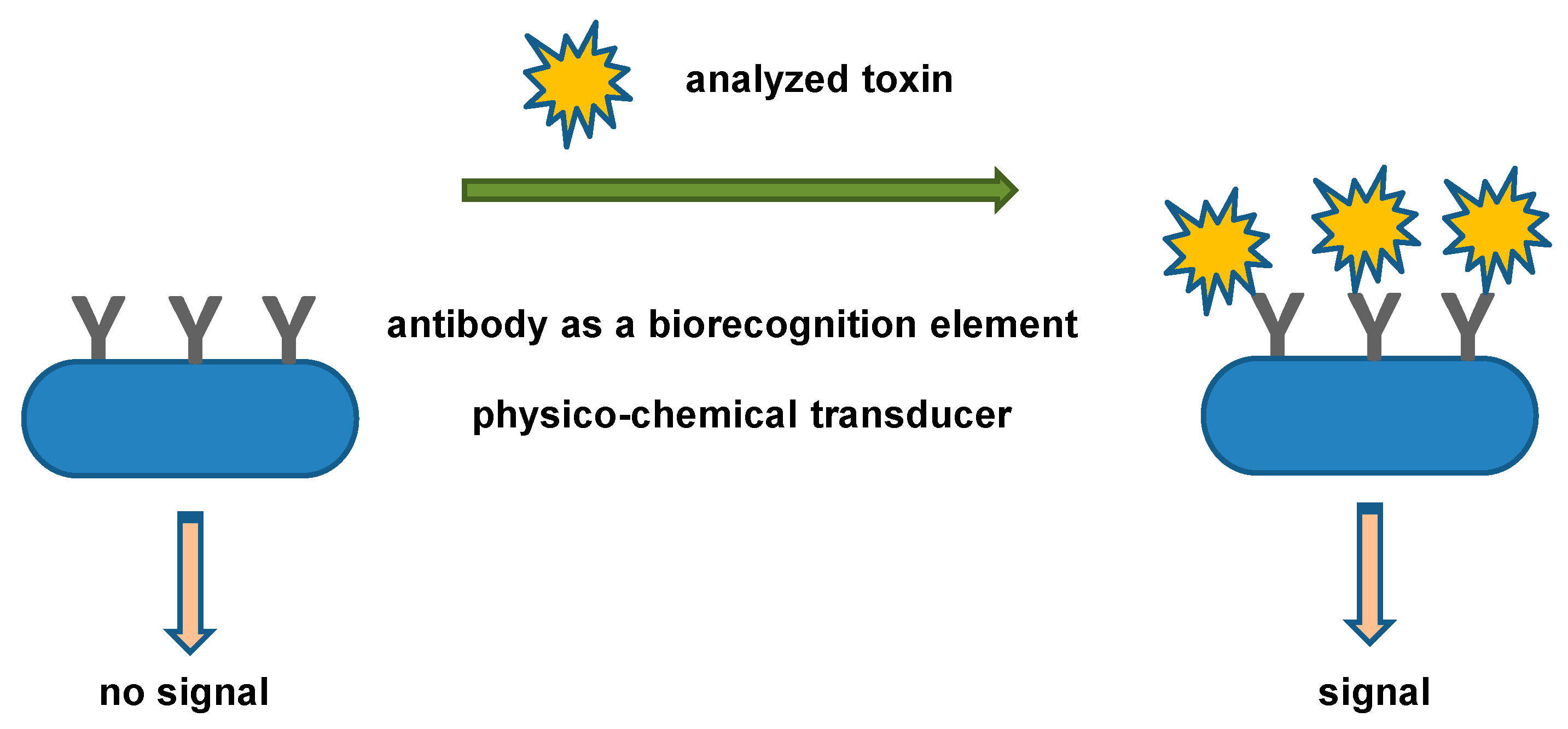
3. Biosensors for the Toxic Biological Warfare Agents Assay
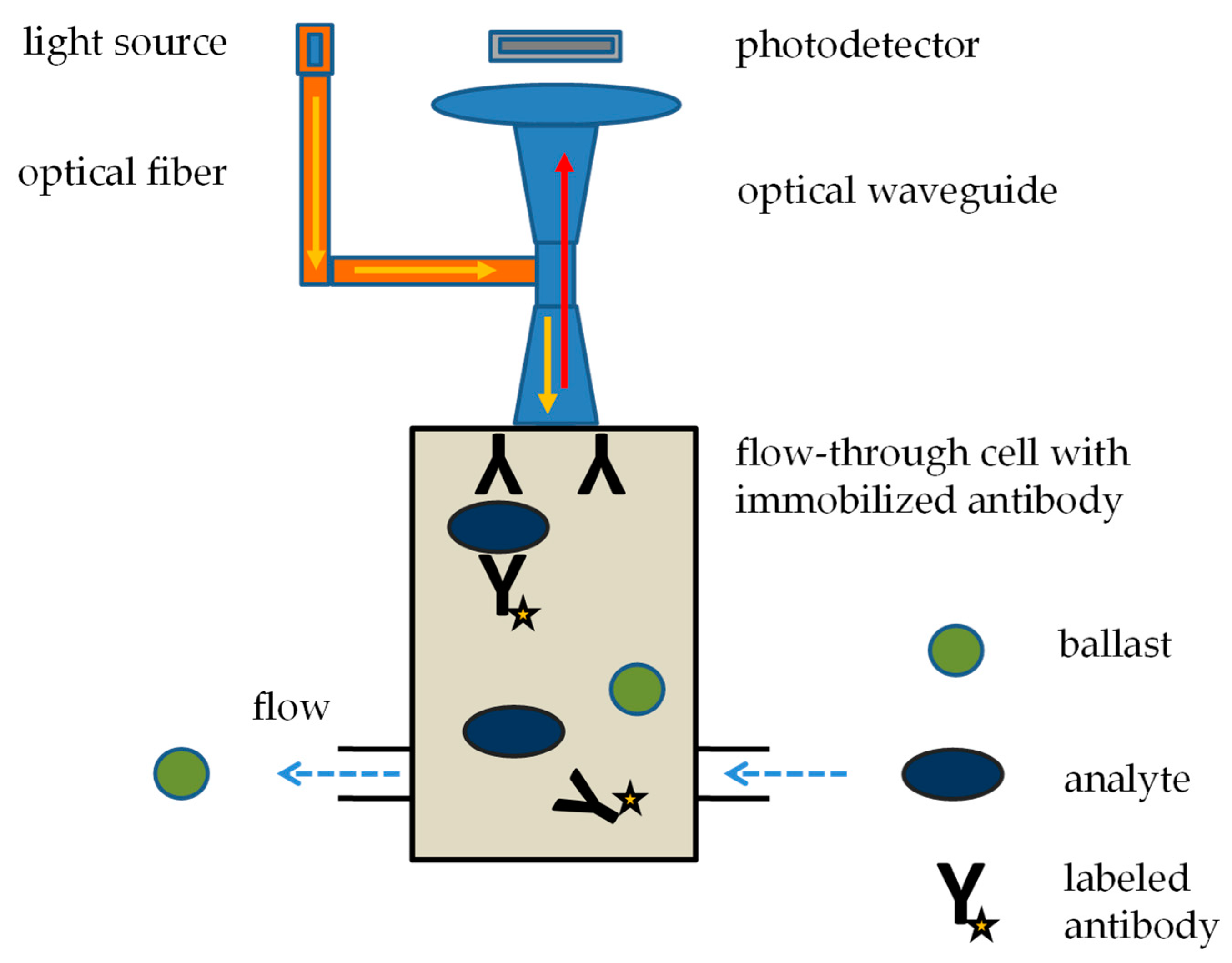
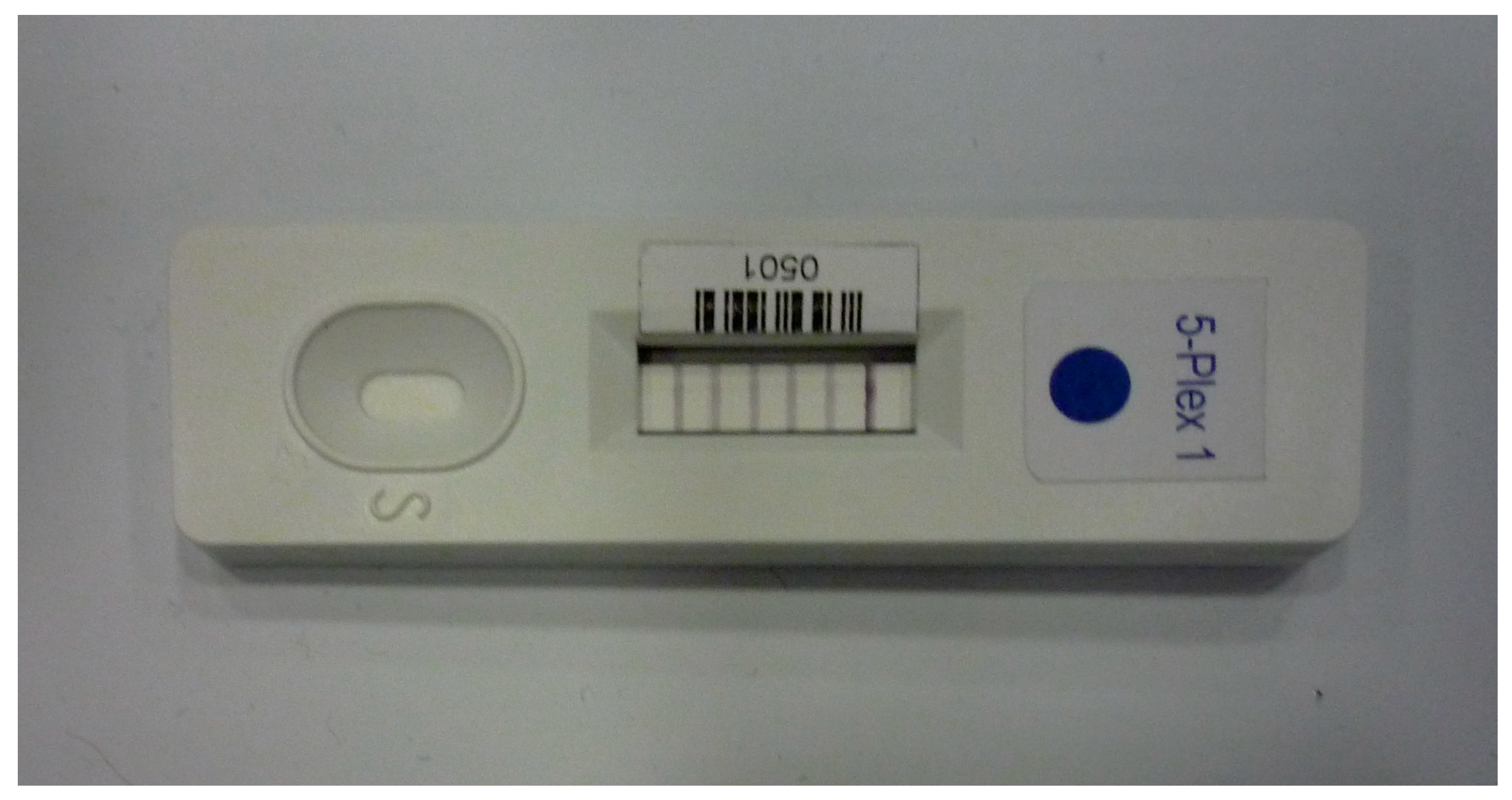
4. Commercial Immunosensors for Toxic Biological Warfare Agents
5. Progress on Immunosensors for Toxic Biological Warfare Agents Assay
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.W.; Su, H.; Shiea, J. Potential applications and challenges of novel ambient ionization mass spectrometric techniques in the emergency care for acute poisoning. Trac-Trends Anal. Chem. 2022, 157, 9. [Google Scholar] [CrossRef]
- De Girolamo, A.; Lippolis, V.; Pascale, M. Overview of recent liquid chromatography mass spectrometry-based methods for natural toxins detection in food products. Toxins 2022, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Huang, M.Z.; Shiea, J.T.; Lee, C.W. Thermal desorption ambient ionization mass spectrometry for emergency toxicology. Mass Spectrom. Rev. 2023, 20, e21784. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Cramer, B.; Dall’Asta, C.; Iha, M.H.; Lattanzio, V.M.T.; Maragos, C.; Solfrizzo, M.; Stranska, M.; Stroka, J.; Sumarah, M. Developments in mycotoxin analysis: An update for 2018-19. World Mycotoxin J. 2020, 13, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Liew, W.P.P.; Sabran, M.R. Recent advances in immunoassay-based mycotoxin analysis and toxicogenomic technologies. J. Food Drug Anal. 2022, 30, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Alam, F.; Dey, B.K.; Mandhadi, J.R.; Bin Emran, T.; Khandaker, M.U.; Safi, S.Z. Multidimensional chromatography and its applications in food products, biological samples and toxin products: A comprehensive review. Separations 2022, 9, 326. [Google Scholar] [CrossRef]
- Valdes, A.; Alvarez-Rivera, G.; Socas-Rodriguez, B.; Herrero, M.; Cifuentes, A. Capillary electromigration methods for food analysis and foodomics: Advances and applications in the period february 2019-february 2021. Electrophoresis 2022, 43, 37–56. [Google Scholar] [CrossRef]
- Bouteiller, P.; Lance, E.; Guerin, T.; Bire, R. Analysis of total-forms of cyanotoxins microcystins in biological matrices: A methodological review. Toxins 2022, 14, 550. [Google Scholar] [CrossRef]
- Hempel, B.F.; Damm, M.; Petras, D.; Kazandjian, T.D.; Szentiks, C.A.; Fritsch, G.; Nebrich, G.; Casewell, N.R.; Klein, O.; Sussmuth, R.D. Spatial venomics-cobra venom system reveals spatial differentiation of snake toxins by mass spectrometry imaging. J. Proteome Res. 2023, 22, 26–35. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Hengchao, E.; Tian, E.J.; Fan, T.T.; Yang, X.L.; Li, X.B.; Zhang, Y.M.; Li, X.J.; Chen, A.L.; Zhou, C.Y.; et al. Structural annotation and discovery of toxic cyclopeptides and their analogues in lethal mushroom amanita and lepiota species using uplc-hrms and molecular networking strategy. Food Control 2023, 146, 9. [Google Scholar] [CrossRef]
- Zhou, J.J.; Lv, X.Q.; Jia, J.L.; Din, Z.U.; Cai, S.Q.; He, J.L.; Xie, F.; Cai, J. Nanomaterials-based electrochemiluminescence biosensors for food analysis: Recent developments and future directions. Biosensors 2022, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Progress in biosensors for the point-of-care diagnosis of COVID-19. Sensors 2022, 22, 7423. [Google Scholar] [CrossRef] [PubMed]
- Hignett, S.; Hancox, G.; Otter, M.E. Chemical, biological, radiological, nuclear and explosive (cbrne) events systematic literature review of evacuation, triage and decontamination for vulnerable people. Int. J. Emerg. Serv. 2019, 8, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Razak, S.; Hignett, S.; Barnes, J. Emergency department response to chemical, biological, radiological, nuclear, and explosive events: A systematic review. Prehospital Disaster Med. 2018, 33, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Mueller, K. The methodology of mass destruction: Assessing threats in the new world order. J. Strateg. Stud. 2000, 23, 163–187. [Google Scholar] [CrossRef]
- Peintner, L.; Wagner, E.; Shin, A.; Tukhanova, N.; Turebekov, N.; Abdiyeva, K.; Spaiser, O.; Serebrennikova, Y.; Tintrup, E.; Dmitrovskiy, A.; et al. Eight years of collaboration on biosafety and biosecurity issues between kazakhstan and germany as part of the german biosecurity programme and the g7 global partnership against the spread of weapons and materials of mass destruction. Front. Public Health 2021, 9, 14. [Google Scholar] [CrossRef]
- Graham, A.T. The nuclear non-proliferation treaty: Delayed review—Issues old and new. J. Peace Nucl. Disarm. 2021, 4, 186–195. [Google Scholar] [CrossRef]
- Vogel, H. Weapons of mass destruction, wmd. Eur. J. Radiol. 2007, 63, 205–213. [Google Scholar] [CrossRef]
- Priego, A. Mass-destruction weapons proliferation in the national security strategy 2013. Rev. UNISCI 2014, 35, 189–204. [Google Scholar]
- Janik, E.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Biological toxins as the potential tools for bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef] [Green Version]
- Olsnes, S. The history of ricin, abrin and related toxins. Toxicon 2004, 44, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M.; Qamar, F.; Saifi, M.; Abdin, M.Z. Natural inhibitors: A sustainable way to combat aflatoxins. Front. Microbiol. 2022, 13, 9. [Google Scholar] [CrossRef]
- Pohanka, M. Botulinum toxin as a biological warfare agent: Poisoning, diagnosis and countermeasures. Mini-Rev. Med. Chem. 2020, 20, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Zadeh, F.A.; Ebrahimzadeh, F.; Jafari-Sales, A.; Gholami, S. Globally vibrio cholera antibiotics resistance to rna and DNA effective antibiotics: A systematic review and meta-analysis. Microb. Pathog. 2022, 172, 15. [Google Scholar] [CrossRef]
- Morris, W.E.; Fernandez-Miyakawa, M.E. Toxins of clostridium perfringens. Rev. Argent. Microbiol. 2009, 41, 251–260. [Google Scholar]
- Dao, F.Y.; Yang, H.; Su, Z.D.; Yang, W.R.T.; Wu, Y.; Ding, H.; Chen, W.; Tang, H.; Lin, H. Recent advances in conotoxin classification by using machine learning methods. Molecules 2017, 22, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schollenberger, M.; Drochner, W.; Muller, H.M. Fusarium toxins of the scirpentriol subgroup: A review. Mycopathologia 2007, 164, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M.A. Fusarium langsethiae—A ht-2 and t-2 toxins producer that needs more attention. J. Phytopathol. 2013, 161, 1–10. [Google Scholar] [CrossRef]
- Welten, R.D.; Meneely, J.P.; Elliott, C.T. A comparative review of the effect of microcystin-lr on the proteome. Expo. Health 2020, 12, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, Y.V.; Sudarkina, O.Y.; Kurmanova, A.G. Ribosome-inactivating lectins of plants. Mol. Biol. 2006, 40, 711–723. [Google Scholar] [CrossRef]
- Akbar, M.A.; Yusof, N.Y.M.; Tahir, N.I.; Ahmad, A.; Usup, G.; Sahrani, F.K.; Bunawan, H. Biosynthesis of saxitoxin in marine dinoflagellates: An omics perspective. Mar. Drugs 2020, 18, 103. [Google Scholar] [CrossRef] [Green Version]
- Bergan, J.; Lingelem, A.B.D.; Simm, R.; Skotland, T.; Sandvig, K. Shiga toxins. Toxicon 2012, 60, 1085–1107. [Google Scholar] [CrossRef]
- Leal, M.; de Mejia, E.G. Review: Toxicological and nutritional implications of t-2 toxin. Food Sci. Technol. Int. 1997, 3, 241–250. [Google Scholar] [CrossRef]
- Makarova, M.; Rycek, L.; Hajicek, J.; Baidilov, D.; Hudlicky, T. Tetrodotoxin: History, biology, and synthesis. Angew. Chem.-Int. Edit. 2019, 58, 18338–18387. [Google Scholar] [CrossRef]
- Maltseva, D.V.; Gerasimov, V.M.; Sakharov, D.A.; Shkurnikov, M.Y. Target cell glycosylation determines the biodistribution of plant lectin viscumin. Bull. Exp. Biol. Med. 2017, 163, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Musiani, S.; Buonamici, L.; Santi, S.; Riccio, M.; Maraldi, N.M.; Girbes, T.; Stirpe, F. Interaction of volkensin with hela cells: Binding, uptake, intracellular localization, degradation and exocytosis. Cell. Mol. Life Sci. 2004, 61, 1975–1984. [Google Scholar] [CrossRef]
- Darling, R.G.; Catlett, C.L.; Huebner, K.D.; Jarrett, D.G. Threats in bioterrorism i: Cdc category a agents. Emerg. Med. Clin. N. Am. 2002, 20, 273. [Google Scholar] [CrossRef]
- Bhalla, D.K.; Warheit, D.B. Biological agents with potential for misuse: A historical perspective and defensive measures. Toxicol. Appl. Pharmacol. 2004, 199, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Senveli, S.U.; Tigli, O. Biosensors in the small scale: Methods and technology trends. IET Nanobiotechnol. 2013, 7, 7–21. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A critical review of electrochemical glucose sensing: Evolution of biosensor platforms based on advanced nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, E.B.; Aydin, M.; Sezginturk, M.K. Advances in electrochemical immunosensors. Adv. Clin. Chem. 2019, 92, 1–57. [Google Scholar] [PubMed]
- Tokranova, N.; Cady, N.; Lamphere, A.; Levitsky, I.A. Highly sensitive fentanyl detection based on nanoporous electrochemical immunosensors. IEEE Sens. J. 2022, 22, 20165–20170. [Google Scholar] [CrossRef] [PubMed]
- Nunez, F.A.; Castro, A.C.H.; de Oliveira, V.L.; Lima, A.C.; Oliveira, J.R.; de Medeiros, G.X.; Sasahara, G.L.; Santos, K.S.; Lanfredi, A.J.C.; Alves, W.A. Electrochemical immunosensors based on zinc oxide nanorods for detection of antibodies against SARS-CoV-2 spike protein in convalescent and vaccinated individuals. ACS Biomater. Sci. Eng. 2023, 16, 458–473. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.X.; Pei, J.; Tian, Y.Z.; Liu, J.Y. A reagentless electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on the interface with redox probe-modified electron transfer wires and effectively immobilized antibody. Front. Chem. 2022, 10, 12. [Google Scholar] [CrossRef]
- You, Y.; Luo, B.; Wang, C.; Dong, H.T.; Wang, X.D.; Hou, P.C.; Sun, L.J.; Li, A.X. An ultrasensitive probe-free electrochemical immunosensor for gibberellins employing polydopamine-antibody nanoparticles modified electrode. Bioelectrochemistry 2023, 150, 8. [Google Scholar] [CrossRef]
- Sadique, M.A.; Yadav, S.; Khare, V.; Khan, R.; Tripathi, G.K.; Khare, P.S. Functionalized titanium dioxide nanoparticle-based electrochemical immunosensor for detection of SARS-CoV-2 antibody. Diagnostics 2022, 12, 2612. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, J.; Lee, D.; Jang, J. Paper-based electrochemical peptide sensor for label-free and rapid detection of airborne bacillus anthracis simulant spores. Sens. Actuator B-Chem. 2022, 355, 8. [Google Scholar] [CrossRef]
- Song, S.P.; Wang, L.H.; Li, J.; Zhao, J.L.; Fan, C.H. Aptamer-based biosensors. Trac-Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, G.F.; Feng, W.J.; He, P.G.; Fang, Y.Z. A thermodynamic investigation into the binding affinity between aptamer-DNA and aptamer-protein. Acta Chim. Sin. 2010, 68, 1427–1430. [Google Scholar]
- Mazzaracchio, V.; Neagu, D.; Porchetta, A.; Marcoccio, E.; Pomponi, A.; Faggioni, G.; D’Amore, N.; Notargiacomo, A.; Pea, M.; Moscone, D. A label-free impedimetric aptasensor for the detection of bacillus anthracis spore simulant. Biosens. Bioelectron. 2019, 126, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, S.G.; Lahousse, M.; Park, H.Y.; Park, H.C.; Jeong, B.; Kim, J.; Kim, S.K.; Yoon, M.Y. Screening and characterization of high-affinity ssdna aptamers against anthrax protective antigen. J. Biomol. Screen 2011, 16, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.L.; Duan, J.Q.; Wang, M.; Cao, J.; She, Y.X.; Cao, Z.; Li, G.Y.; Jin, F.; Wang, J.; Abd El-Aty, A.M. Application of molecularly imprinted electrochemical biomimetic sensors for detecting small molecule food contaminants. Polymers 2023, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Maria, C.G.A.; Varghese, A.; Nidhin, M. Recent advances in nanomaterials based molecularly imprinted electrochemical sensors. Crit. Rev. Anal. Chem. 2023, 53, 88–97. [Google Scholar]
- Cui, B.C.; Liu, P.; Liu, X.J.; Liu, S.Z.; Zhang, Z.H. Molecularly imprinted polymers for electrochemical detection and analysis: Progress and perspectives. J. Mater. Res. Technol-JMRT 2020, 9, 12568–12584. [Google Scholar] [CrossRef]
- Saxena, K.; Murti, B.T.; Yang, P.K.; Malhotra, B.D.; Chauhan, N.; Jain, U. Fabrication of a molecularly imprinted nano-interface-based electrochemical biosensor for the detection of caga virulence factors of h. Pylori. Biosensors 2022, 12, 1066. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Q.; Wang, F.; Yang, H.F. Dummy molecularly imprinted solid-phase extraction-sers determination of afb1 in peanut. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2023, 288, 9. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Soylak, M.; Erk, N. Innovative molecularly imprinted electrochemical sensor for the nanomolar detection of tenofovir as an anti-hiv drug. Talanta 2023, 253, 11. [Google Scholar] [CrossRef]
- Carinelli, S.; Kuhnemund, M.; Nilsson, M.; Pividori, M.I. Yoctomole electrochemical genosensing of ebola virus cdna by rolling circle and circle to circle amplification. Biosens. Bioelectron. 2017, 93, 65–71. [Google Scholar] [CrossRef]
- Ilkhani, H.; Farhad, S. A novel electrochemical DNA biosensor for ebola virus detection. Anal. Biochem. 2018, 557, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; Bandara, A.B.; Wang, Y.M.; Wang, A.B.; Inzana, T.J. Photonic biosensor assays to detect and distinguish subspecies of francisella tularensis. Sensors 2011, 11, 3004–3019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Euler, M.; Wang, Y.J.; Heidenreich, D.; Patel, P.; Strohmeier, O.; Hakenberg, S.; Niedrig, M.; Hufert, F.T.; Weidmann, M. Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents. J. Clin. Microbiol. 2013, 51, 1110–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarova, E.; Aldissi, M.; Bogomolova, A. Direct electrochemical sensor for fast reagent-free DNA detection. Biosens. Bioelectron. 2005, 21, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Hao, R.Z.; Song, H.B.; Zuo, G.M.; Yang, R.F.; Wei, H.P.; Wang, D.B.; Cui, Z.Q.; Zhang, Z.P.; Cheng, Z.X.; Zhang, X.E. DNA probe functionalized qcm biosensor based on gold nanoparticle amplification for bacillus anthracis detection. Biosens. Bioelectron. 2011, 26, 3398–3404. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Zhen, S.J.; Huang, C.Z.; Li, Y.F. Ultrasensitive ratiometric electrochemiluminescence for detecting atxa mrna using luminol-encapsulated liposome as effectively amplified signal labels. Biosens. Bioelectron. 2021, 186, 6. [Google Scholar] [CrossRef]
- Wasiewska, L.A.; Diaz, F.G.; Shao, H.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Highly sensitive electrochemical sensor for the detection of shiga toxin-producing e. Coli (stec) using interdigitated micro-electrodes selectively modified with a chitosan-gold nanocomposite. Electrochim. Acta 2022, 426, 11. [Google Scholar] [CrossRef]
- Pohanka, M. COVID-19 molecular level laboratory diagnoses. Bratisl. Med. J. 2021, 122, 11–17. [Google Scholar] [CrossRef]
- Pohanka, M. Point-of-care diagnosis of COVID-19 disease based on antigen tests. Bratisl. Med. J. 2021, 122, 763–770. [Google Scholar] [CrossRef]
- Pickering, S.; Betancor, G.; Galão, R.P.; Merrick, B.; Signell, A.W.; Wilson, H.D.; Kia Ik, M.T.; Seow, J.; Graham, C.; Acors, S. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. PLoS Pathog. 2020, 16, e1008817. [Google Scholar] [CrossRef]
- Ristic, M.; Nikolic, N.; Cabarkapa, V.; Turkulov, V.; Petrovic, V. Validation of the standard q COVID-19 antigen test in vojvodina, serbia. PLoS ONE 2021, 16, e0247606. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Ahmed, R.; Iqbal, S.M.A.; Chowdhury, R.; Paulmurugan, R.; Demirci, U.; Asghar, W. Diagnosis for COVID-19: Current status and future prospects. Expert Rev. Mol. Diagn. 2021, 21, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sun, H.; Tian, J.P.; Song, Q.M.; Zhang, W.W. Paper-based point-of-care testing of SARS-CoV-2. Front. Bioeng. Biotechnol. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.A.; Kramer, M.F.; Lim, D.V. A rapid detection method for vaccinia virus, the surrogate for smallpox virus. Biosen. Bioelectron. 2004, 20, 322–327. [Google Scholar] [CrossRef]
- Nath, N.; Eldefrawi, M.; Wright, J.; Darwin, D.; Huestis, M. A rapid reusable fiber optic biosensor for detecting cocaine metabolites in urine. J. Anal. Toxicol. 1999, 23, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Narang, U.; Anderson, G.P.; Ligler, F.S.; Burans, J. Fiber optic-based biosensor for ricin. Biosens. Bioelectron. 1997, 12, 937–945. [Google Scholar] [CrossRef]
- Cao, L.K.; Anderson, G.P.; Ligler, F.S.; Ezzell, J. Detection of yersinia pestis fraction 1 antigen with a fiber optic biosensor. J. Clin. Microbiol. 1995, 33, 336–341. [Google Scholar] [CrossRef] [Green Version]
- DeMarco, D.R.; Saaski, E.W.; McCrae, D.A.; Lim, D.V. Rapid detection of escherichia coli o157:H7 in ground beef using a fiber-optic biosensor. J. Food. Prot. 1999, 62, 711–716. [Google Scholar] [CrossRef]
- Tempelman, L.A.; King, K.D.; Anderson, G.P.; Ligler, F.S. Quantitating staphylococcal enterotoxin b in diverse media using a portable fiber-optic biosensor. Anal. Biochem. 1996, 233, 50–57. [Google Scholar] [CrossRef]
- Anderson, G.P.; King, K.D.; Gaffney, K.L.; Johnson, L.H. Multi-analyte interrogation using the fiber optic biosensor. Biosens. Bioelectron. 2000, 14, 771–777. [Google Scholar] [CrossRef]
- Pohanka, M. Current trends in the biosensors for biological warfare agents assay. Materials 2019, 12, 2303. [Google Scholar] [CrossRef] [Green Version]
- Gwyn, S.; Mitchell, A.; Dean, D.; Mkocha, H.; Handali, S.; Martin, D.L. Lateral flow-based antibody testing for chlamydia trachomatis. J. Immunol. Methods 2016, 435, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shome, R.; Kalleshamurthy, T.; Shome, B.R.; Sahay, S.; Natesan, K.; Bambal, R.G.; Sairiwal, L.; Mohandoss, N.; Barbuddhe, S.B. Lateral flow assay for brucellosis testing in multiple livestock species. J. Microbiol. Methods 2018, 148, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Machiesky, L.; Cote, O.; Kirkegaard, L.H.; Mefferd, S.C.; Larkin, C. A rapid lateral flow immunoassay for identity testing of biotherapeutics. J. Immunol. Methods 2019, 474, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Wang, Y.B.; Yang, J.F.; Sun, Z.K.; Yue, Z.H.; Li, L.L.; He, L.; Hu, X.F. An immunochromatographic lateral flow strip test for the rapid detection of danofloxacin in milk. Food Anal. Meth. 2019, 12, 2430–2437. [Google Scholar] [CrossRef]
- Tel, O.Y.; Gurbilek, S.E.; Keskin, O.; Yucetepe, A.G.; Karadenizli, A. Development of lateral flow test for serological diagnosis of tularemia. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 579–584. [Google Scholar]
- Peto, T.; Uk, C.-L.F.O. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine 2021, 36, 7. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Xu, X.X.; Xu, L.G.; Kuang, H.; Xu, C.L.; Guo, L.L. Gold nanoparticle-based lateral flow immunoassay for the rapid and on-site detection of wheat allergen in milk. Food Biosci. 2023, 51, 8. [Google Scholar] [CrossRef]
- Slotved, H.C.; Sparding, N.; Tanassi, J.T.; Steenhard, N.R.; Heegaard, N.H.H. Evaluating 6 ricin field detection assays. Biosecur. Bioterror. 2014, 12, 186–189. [Google Scholar] [CrossRef]
- Gessler, F.; Pagel-Wieder, S.; Avondet, M.A.; Bohnel, H. Evaluation of lateral flow assays for the detection of botulinum neurotoxin type a and their application in laboratory diagnosis of botulism. Diagn. Microbiol. Infect. Dis. 2007, 57, 243–249. [Google Scholar] [CrossRef]
- Jia, X.F.; Wang, K.L.; Li, X.Y.; Liu, Z.Z.; Liu, Y.; Xiao, R.; Wang, S.Q. Highly sensitive detection of three protein toxins via sers-lateral flow immunoassay based on sio2@au nanoparticles. Nanomed.-Nanotechnol. Biol. Med. 2022, 41, 11. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Kumar, A.; Chauhan, N.; Khanuja, M.; Malhotra, B.D.; Jain, U. Electrochemical immunosensor for detection of h. Pylori secretory protein vaca on g-c3n4/zno nanocomposite-modified au electrode. ACS Omega 2023, 7, 32292–32301. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.S.; Hu, W.; Pei, F.B.; Liu, Z.W.; Du, B.; Mu, X.H.; Liu, B.; Hao, Q.L.; Lei, W.; Tong, Z.Y. A highly sensitive fluorescence and screen-printed electrodes-electrochemiluminescence immunosensor for ricin detection based on cdse/zns qds with dual signal. Toxins 2022, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, M.; Vasileva, N.; Godjevargova, T. Determination of aflatoxin m1 in milk by a magnetic nanoparticle-based fluorescent immunoassay. Anal. Lett. 2017, 50, 452–469. [Google Scholar] [CrossRef]
- Peltomaa, R.; Abbas, A.; Yli-Mattila, T.; Lamminmaki, U. Single-step noncompetitive immunocomplex immunoassay for rapid aflatoxin detection. Food Chem. 2022, 392, 7. [Google Scholar] [CrossRef]
- Cheng, H.P.; Chuang, H.S. Rapid and sensitive nano-immunosensors for botulinum. ACS Sens. 2019, 4, 1754–1760. [Google Scholar] [CrossRef]
- Parvin, S.; Hashemi, P.; Afkhami, A.; Ghanei, M.; Bagheri, H. Simultaneous determination of bont/a and/e using an electrochemical sandwich immunoassay based on the nanomagnetic immunosensing platform. Chemosphere 2022, 298, 11. [Google Scholar] [CrossRef]
- Kumar, D.N.; Baider, Z.; Elad, D.; Blum, S.E.; Shtenberg, G. Botulinum neurotoxin-c detection using nanostructured porous silicon interferometer. Chemosensors 2021, 9, 228. [Google Scholar] [CrossRef]
- Wang, B.; Park, B.; Chen, J.; He, X.H. Rapid and label-free immunosensing of shiga toxin subtypes with surface plasmon resonance imaging. Toxins 2020, 12, 280. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.W.; Li, Z.; Xu, H.; Guo, L.; Wang, L.L.; Wang, Y.X.; Luo, L.L.; Wang, J.; Zhang, P.P.; et al. Label-free differentiation and quantification of ricin, abrin from their agglutinin biotoxins by surface plasmon resonance. Talanta 2022, 238, 8. [Google Scholar] [CrossRef]
- Stern, D.; Pauly, D.; Zydek, M.; Muller, C.; Avondet, M.A.; Worbs, S.; Lisdat, F.; Dorner, M.B.; Dorner, B.G. Simultaneous differentiation and quantification of ricin and agglutinin by an antibody-sandwich surface plasmon resonance sensor. Biosens. Bioelectron. 2016, 78, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cagnani, G.R.; Oliveira, T.D.; Mattioli, I.A.; Sedenho, G.C.; Castro, K.P.R.; Crespilho, F.N. From research to market: Correlation between publications, patent filings, and investments in development and production of technological innovations in biosensors. Anal. Bioanal. Chem. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Wang, S.M. Biosensor commercialization strategy—A theoretical approach. Front. Biosci. 2005, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
| Toxin | Type of Chemical Substance | Producing Organism | References |
|---|---|---|---|
| abrin | protein toxalbumin | plant Abrus pulchellus | [21] |
| aflatoxin | low molecular weight mycotoxins | molds Aspergillus | [22] |
| botulinum toxins | protein toxins | bacterium Clostridium botulinum | [23] |
| cholera toxin | protein toxins | bacterium Vibrio cholerae | [24] |
| Clostridium perfringens toxins | protein α, β1, β2, ε, ι toxins | bacterium Clostridium perfringens | [25] |
| conotoxins | neurotoxic peptides | marine cone snail, genus Conus | [26] |
| diacetoxyscirpenol | a low molecular weight mycotoxin from a group of trichothecenes | produced by fungi Fusarium | [27] |
| HT-2 toxin | a trichothecene mycotoxin | various fungi, mainly Fusarium species | [28] |
| microcystins | cyanotoxins, a group of organic compounds | various cyanobacteria | [29] |
| modeccin | a glycoprotein | plant Adenia digitata | |
| ricin | a carbohydrate-binding protein | plant Ricinus communis | [30] |
| saxitoxin | a cyanotoxin, organic compound | various cyanobacteria | [31] |
| Shiga toxins | a group of protein toxins | Shigella dysenteriae and some serotypes of Escherichia coli | [32] |
| T-2 toxin | a trichothecene mycotoxin | produced by various fungi, mainly Fusarium species | [33] |
| tetrodotoxin | an organic neurotoxic substance | bacteria like Pseudoalteromonas, Pseudomonas, and Vibrio, it can be transmitted to other water organisms | [34] |
| viscumin | toxic protein lectins | mistletoe plant Viscum album | [35] |
| volkensin | a toxic glycoprotein | Adenia volkensii plant | [36] |
| Name of Device | Manufacturer | Type of Immunosensor or Assay | Analytical Specifications | References |
|---|---|---|---|---|
| Raptor | Research International (Monroe, WA, USA) | automatic flow through fluorescence immunoassay | limits of detection up to 0.1 ng/mL for staphylococcal enterotoxin B, 5 ng/mL for ricin, and up to 1 ng/mL for botulinum toxin, assay time 15 min | [74,75,76,77,78,79,80] |
| Biosensor 220R | MSA (Pittsburgh, PA, USA) | fluorescence immunoassay based on magnetic separation | sensitivity for ricin and staphylococcal enterotoxin B < 1 ng, assay time 5 min | [81] |
| BADD and Pro Strips-Rapid Screening System | Advent Biotechnologies | lateral flow test | limit of detection for ricin and staphylococcal enterotoxin B is 10 ng/mL, botulinum toxin variant A 33 ng/mL, botulinum toxin variant B 500 ng/mL, sample sized 0.2 mL, assay time 3 min, contemporary analyzed biological warfare agents: 1 or 5 | [89,90] |
| BioDetec, RAID 5, RAID 8, RAID 10 | Alexeter Technologies | lateral flow test | assay time 15 min, contemporary analyzed biological warfare agents: 1, 5, 8 or 10 | [89] |
| Type of Assay | Toxins | Analytical Specifications | References |
|---|---|---|---|
| Raman scattering-lateral flow immunoassay | ricin, botulinum toxin, and staphylococcal enterotoxin B | limit of detection 0.1 ng/mL for ricin and botulinum toxin A, and 0.05 ng/mL for staphylococcal enterotoxin B, assay time 15 min | [91] |
| voltametric immunoassay | vacuolating cytotoxin A from Helicobacter pylori | limit of detection 0.1 ng/mL, linear range of calibration between 0.1 and 12.8 ng/mL, assay time 10–15 min | [92] |
| electrochemiluminescence immunosensor with magnetic separation of immunocomplex on magnetic beads | ricin | limit of detection 5.5 pg/mL, linear assay range 0.01–100 ng/ml | [93] |
| magnetic nanoparticle-based fluorescent immunoassay | aflatoxin M1 | limit of detection 2.9 pg/mL, linear calibration range 3.0–100 pg/ml | [94] |
| non-competitive immunoassay, primary anti-aflatoxin antibody bound via streptavidin on magnetic beads, an immunocomplex is formed in the presence of aflatoxin B1 with a secondary Eu-labelled antibody | aflatoxin B1 | limit of detection 70 pg/mL, assay time 15 min | [95] |
| diffusivity measurement of sandwich immunocomplexes comprised of gold nanoparticles with antibodies, analyte, and antibodies on fluorescent probe particles | botulinum toxin | limit of detection 10 pg/mL, calibration range 0.01–500 ng/mL, assay time 2 min | [96] |
| voltametric immunosensor containing magnetic particles with antibodies forming a sandwich with analyte and other antibodies labeled with Ag or Cd nanoparticles | botulinum toxin A and E | dynamic range 0.1–1000 pg/mL and limit of detection 0.04 pg/mL (botulinum toxin A); dynamic range 0.5–1000 pg/mL and limit of detection 0.16 pg/mL (botulinum toxin E) | [97] |
| Fabry-Perot interferometric competitive immunoassay using primary and peroxidase-labeled secondary antibody, precipitation of 4-chloro-1-naphthol by peroxidase was responsible for the detected signal | toxoid form of botulinum toxin type C and D | linear response 10 pg/mL to 10 ng/mL, limit of detection 4.8 pg/mL, assay going in nearly real time | [98] |
| surface plasmon resonance imaging, antibody bound on gold film, signal improved by adding of gold nanoparticles with immobilized antibodies | Shiga toxin—tested on toxoid | limit of detection 50 ng/mL for label-free assay, 1 pg/mL when gold-immuno-nanoparticles are applied, assay time 20 min | [99] |
| surface plasmon resonance combined with magnetic separation | ricin and abrin | limit of detection 0.6 ng/ml | [100] |
| surface plasmon resonance with antibodies immobilized on chip and secondary antibody used for specific ricin assay and signal improvement | ricin, agglutinin | 3 ng/mL for ricin, 6 ng/mL for agglutinin, assay time including sample processing 30 min | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohanka, M. Immunosensors for Assay of Toxic Biological Warfare Agents. Biosensors 2023, 13, 402. https://doi.org/10.3390/bios13030402
Pohanka M. Immunosensors for Assay of Toxic Biological Warfare Agents. Biosensors. 2023; 13(3):402. https://doi.org/10.3390/bios13030402
Chicago/Turabian StylePohanka, Miroslav. 2023. "Immunosensors for Assay of Toxic Biological Warfare Agents" Biosensors 13, no. 3: 402. https://doi.org/10.3390/bios13030402
APA StylePohanka, M. (2023). Immunosensors for Assay of Toxic Biological Warfare Agents. Biosensors, 13(3), 402. https://doi.org/10.3390/bios13030402





