Abstract
Throughout this research, a unique optical sensor for detecting one of the most dangerous heavy metal ions, Cu(II), was designed and developed. The (4-mercaptophenyl) iminomethylphenyl naphthalenyl carbamate (MNC) sensor probe was effectively prepared. The Schiff base of the sensor shows a “turn-off” state with excellent sensitivity to Cu(II) ions. This innovative fluorescent chemosensor possesses distinctive optical features with a substantial Stocks shift (about 114 nm). In addition, MNC has remarkable selectivity for Cu(II) relative to other cations. Density functional theory (DFT) and the time-dependent DFT (TDDFT) theoretical calculations were performed to examine Cu(II) chelation structures and associated electronic properties in solution, and the results indicate that the luminescence quenching in this complex is due to ICT. Chelation-quenched fluorescence is responsible for the internal charge transfer (ICT)-based selectivity of the MNC sensing molecule for Cu(II) ions. In a 1:9 (v/v) DMSO-HEPES buffer (20 mM, pH = 7.4) solution, Fluorescence and UV-Vis absorption of the MNC probe and Cu(II) ions were investigated. By utilizing a solution containing several metal ions, the interference of other metal ions was studied. This MNC molecule has outstanding selectivity and sensitivity, as well as a low LOD (1.45 nM). Consequently, these distinctive properties enable it to find the copper metal ions across an actual narrow dynamic range (0–1.2 M Cu(II)). The reversibility of the sensor was obtained by employing an EDTA as a powerful chelating agent.
1. Introduction
Copper Cu(II) ion is the third-most abundant metal ion needed by every living creature and is essential for several processes. In contrast, deficiencies or excesses of Cu(II) ions, as determined by cellular requirements, result in various diseases. Optical sensors with outstanding selectivity and sensitivity have been developed to detect Cu(II) ions. The designed sensor can detect luminescence in the refractive indices adsorption that arises from light and material interactions. They have garnered significant attention in recent years because of their simple and naked-eye identification, real-time recognition, cheap fee, excellent specificity against analytes, speedy response, and less equipment required in the investigation process. Copper (Cu(II)) ion is one of the essential metal ions. It plays a significant part in a variety of processes that are necessary to both nature and the human body [1,2].
As an enzymatic activator of oxidative enzymes involving tyrosinase, lysyl oxidase, cytochrome c oxidase, superoxide dismutase, and a number of metalloenzymes [3,4], it plays a vital function in the human body. It is an essential catalyst for iron absorption and ferroheme synthesis [5]. Under normal conditions, Cu(II) ion does not harm the human body; excess and deficiency can produce diverse effects. Although minimal levels of Cu(II) ion are necessary for normal physiological processes, exceeding the recommended daily consumption is harmful to human health [6,7]. The increase or decrease in copper ion concentration causes several disorders related to iron homeostasis, which is controlled by copper ions.
Excessive Cu(II) ion consumption can result in diarrhea, vomiting, vertigo, and abdominal pain [8]. Long-term exposure causes the accumulation of free Cu(II) ions in the body, which, owing to redox activity, produces reactive oxygen species that are harmful to fatty acids, DNA, and tissues. Oxygen radicals have the potential to be cytotoxic [9,10]. Several illnesses may emerge depending on the state of the body’s hemostasis. In addition to Wilson’s, Parkinson’s, Menkes, neurodegenerative, and Alzheimer’s illnesses [11,12], increasing the content of Cu(II) ion produces Huntington’s disease, and acute hepatic and renal failure [13,14]. Environmental Protection Agency (EPA) guidelines for Cu(II) ions in drinking water are 1.3 ppm [15,16]. The copper concentration in a healthy person’s blood serum should be 100–150 μg/dL [17,18]. Additionally, home, industrial, and agricultural operations emit Cu(II) ions into the environment [19]. Cu(II)-containing pesticides are often used to stimulate crop growth while also preventing illness. Since 1991, the EPA has limited the amount of Cu(II) ion in tap water to 20 μM [20]. In contrast, the World Health Organization (WHO) has set the limit at 30 μM [21]. The excessive use of these pesticides may contaminate different water resources.
Several techniques exist for detecting trace metals. Various advanced techniques are utilized in the sensing process of copper ions [22,23,24]. Nevertheless, these techniques have downsides. For example, many of these techniques are pricey and difficult, and important factors such as dependability, precision, and speed must be addressed [25,26]. It is imperative and urgent to enhance sensitive, simple, quick, repeatable, cheap, and analytical techniques for detecting Cu(II) ions in ecological and medicinal materials [27]. The detection mechanism relies on the unique binding of the specified analyte to the receptor. Based on the contact, the transducers may detect characteristics that include changes to the pH, electron, mass transfer, temperature, a change in optical qualities, and potential differences. This method turns the receptor’s response into a significant gesture exactly proportional to the presence or quantity of the analyte [28,29]. As a result of its low-priced, simple miniaturization, and rapid output of semi-quantitative data, sensor research has grown in popularity and importance in recent years. Because of this, sensors have become indispensable means for scientific ecological and dietary investigation, as well as the detection of chemical and biological contaminants [30,31,32,33].
The Schiff base is a magnificent class of target compounds owing to diverse applications in different fields that cover analytical, biological, and coordination chemistry. The Schiff base compound achieved enormous significance in the healthcare and medicinal industries and fascinated scientists to generate a novel construction outstanding variety of bioactivities involving antimicrobial [34], antibacterial [35], antifungal [36], anti-ulcer [37], anticonvulsant [38], antioxidant [39], anti-inflammatory [40], analgesic [41], and anti-tubercular [42]. As a part of biological activities, Schiff base emerged as a key intermediate to elaborate new structures that find multiple applications as optical [43], photosensitizes in dye-sensitized solar cells [44], catalyst [45], polymers stabilizers [46], semiconducting multicolumnar mesophases [47], pigments [48], dyes [49], sensors [50,51,52,53] and luminescence [54]. Carbamate derivatives are one of the prospective target molecules utilized in producing pharmaceuticals, cosmetics, pesticides, and polyurethanes [55]. Interestingly, naringenin carbamate and chalcone-O-carbamate have been studied as multifaceted Alzheimer’s disease treatments [56,57].
In this study, we describe a switch turn-OFF Schiff base sensor probe. Our group developed a practical, extremely selective, reversible, and susceptible chemosensor with a low detection limit and excellent sensitivity for copper metal ions. Moreover, the copper ion detecting process is based on the ICT mechanism. The Schiff base chelating ligand was produced following the scientific literature. The synthesized ligand is a unique optical sensor for detecting copper ions. The detection technique relies on measuring the fluorescence increase of the Schiff base sensing molecule throughout the complexation process based on Cu(II).
2. Experimental
2.1. Materials and Methods
All compounds were purchased from (Sigma-Aldrich, St. Louis, MO, USA) and utilized with no additional processing. The absorbance spectra (UV–vis) were collected using a 1 cm quartz cell and an EvolutionTM 200 series UV-Visible spectrophotometer. For emission and excitation spectrum investigations, a 1 cm quartz cell was used with a JASCOFP6300 spectrofluorometer to detect fluorescence spectra. The 20 mM HEBES buffer stock solution with a pH of 7.4 has been made. On a Bruker NMR operating at 800 and 125 MHz, the 1H and 13C-NMR spectra were acquired. The chemical shifts were calculated using DMSO-d6 as the solvent and TMS as the standard internal reference. The melting points were obtained using the Stuart SMP30 equipment. For analytical TLC, Silica Gel 60 F254 plates (40–60 m) were used from Sigma, and the chromatogram was produced using a 254 nm UV lamp. All reactions were conducted in a nitrogen-protected dry environment using oven-dried glassware without additional purification.
2.2. Procedure for MNC Synthesis
In a 100 mL round bottom flask, a solution of 4-mercaptoaniline (1.25 g, 10 mmol) in 15 mL of dichloromethane was added to a solution of salicylaldehyde (1.22 g, 10 mmol) in 40 mL of dichloromethane. After 2 h of refluxing, the mixture was allowed to cool to room temperature. A 30 mL dichloromethane solution of naphthyl isocyanate (1.69 g, 10 mmol) was added and agitated for 30 min. The mixture was then heated under reflux for 3 h. In order to monitor the reaction progress, diethyl ether/hexane (70/30) was utilized as an eluent. Following cooling, the mixture was filtered, and the crude material was recrystallized from ethanol to yield (3.46 g, 87.4%). M.p. = 215–217 °C. 1H NMR (850 MHz, DMSO d6): 12.88 (s, 1H, NH), 10.53 (s, 1H), 9.00 (s, 1H, CH=N-), 8.04 (d, 1H, J = 8.5 Hz, Harom), 7.97 (d, 1H, J = 7.68 Hz, Harom), 7.85 (d, 1H, J = 7.65 Hz, Harom), 7.68 (dd, 1H, J = 7.65 Hz, J = 1.7 Hz, Harom), 7.63–7.57 (m, 5H, Harom), 7.52 (t, 1H, J = 8.5 Hz, Harom); 7.47 (m, 2H, Harom), 7.44 (m, 1H, Harom), 7.02 (m, 2H, Harom);13C NMR (212.5 MHz, DMSO-d6) δ(ppm): 164.6 (C=O), 160.7 (HS-C), 149.3, 136.8, 134.2, 134.08, 134.09, 128.6, 127.0, 126.7, 126.0, 123.2, 122.4, 119.79, 119.72, 117.1. IR (cm−1) ν: 3245 (NH), 3051(CH), 1650 (C(O)NH), 1614(C=N); 1568(C=C), 1519, 1492, 1481, 1455, 1275, 1240, 1182, 1172. The NMR and IR spectra were displayed in the supporting information (Figures S1 and S2).
2.3. Optical Measurements
For the different kinds of examined metal ions, such as Gd(III), Ag(I), etc., stock solutions of metal nitrates were prepared. Fluorometric titration was conducted with a free ligand concentration of 1.25 M in a buffer solution containing 1:9 (v/v) DMSO-HEPES (pH = 7.4). In a 1.0 M aqueous solution, the UV-Vis spectra of free MNC and complexes (MNC-Cu(II)) were assessed. Throughout Absorbance titration, it is reasonable to suppose that the overall volume of free ligand MNC and metal ions is 2.0 mL since the volume of metal ions can be disregarded compared to that of free MNC. The length of the excitation and emission slits within every experiment was 5 nm.
3. Results
3.1. MNC Probe Synthesis
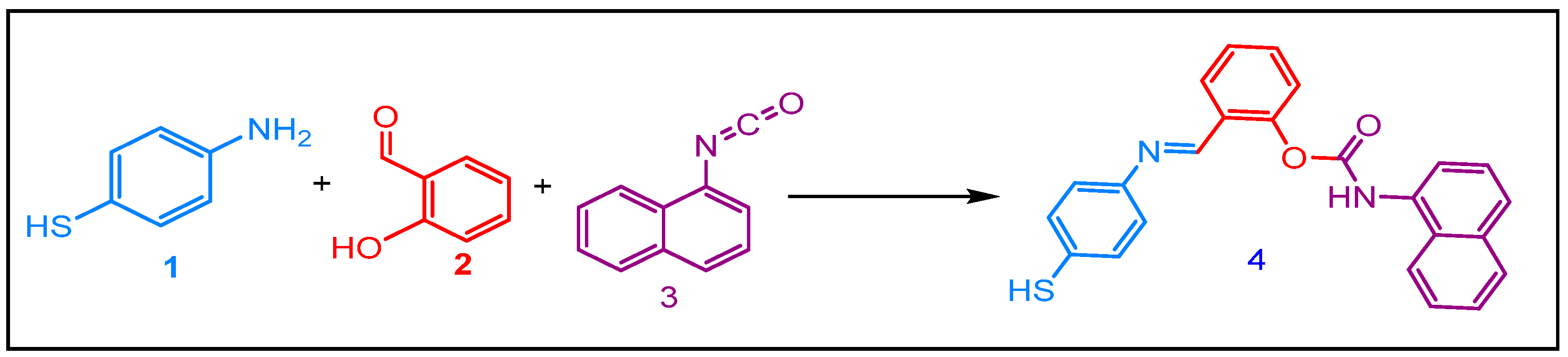
Our approach started with synthesizing Schiff base carbamate via the condensation of equimolar amount 4-mercaptoaniline 1 and salicylaldehyde 2 in anhydrous boiling dichloromethane. After 3 h of contact, one equivalent of naphtylisocyanate 3 was added. The reaction afforded the corresponding Schiff base carbamate 4 at 85 % yield (Scheme 1).
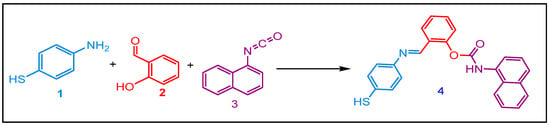
Scheme 1.
Synthesis of the Schiff Base carbamate.
3.2. Optical Characteristics of MNC
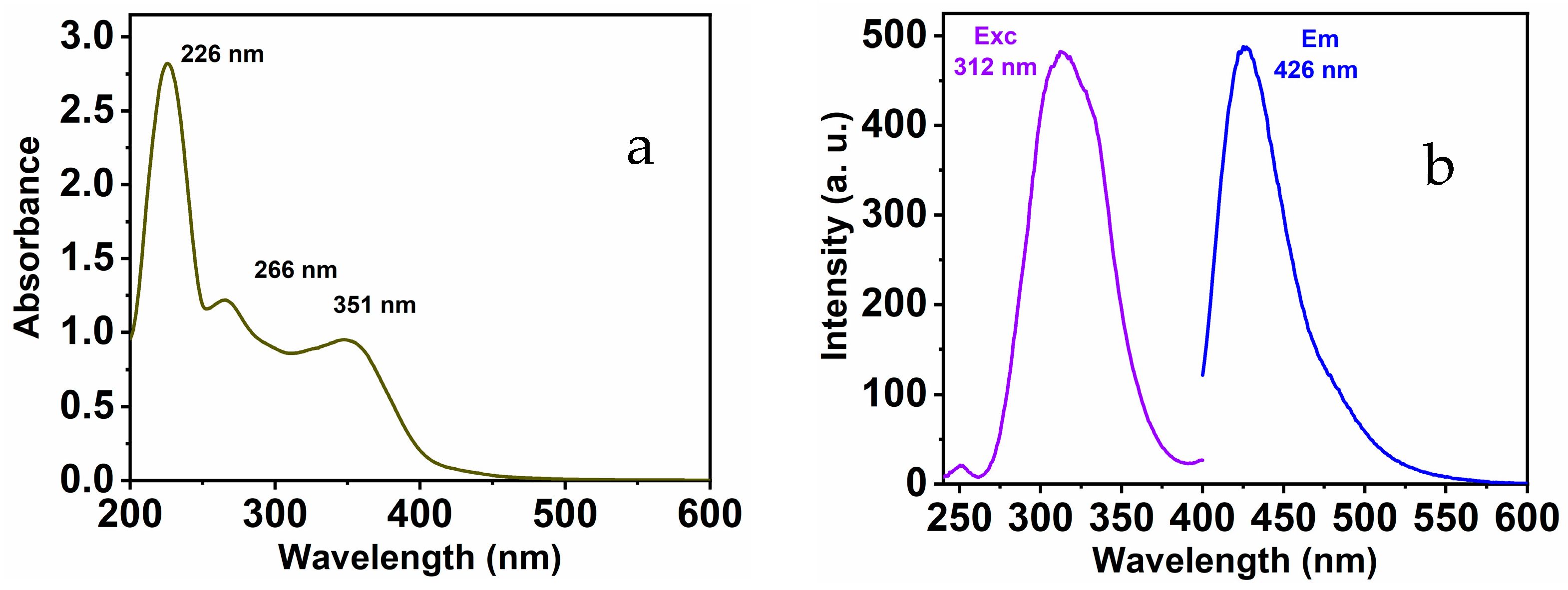
The as-prepared MNC chemical sensor shows distinguishing optical characteristics; these features were collected in a 1:9 (v/v) DMSO-HEPES buffer solution (20 mM, pH = 7.4) (see Figure 1). Under excitation with 312 nm, the free chemical sensor displays a distinct peak located at 426 nm. In addition, the absorbance spectra provide the existence of three maximums at 226, 266, and 351 nm correspondingly. These peaks might be ascribed to π-π* and n-π* transitions.
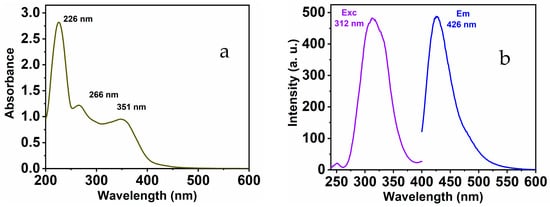
Figure 1.
MNC (a) UV-Vis; (b) emission and excitation spectra.
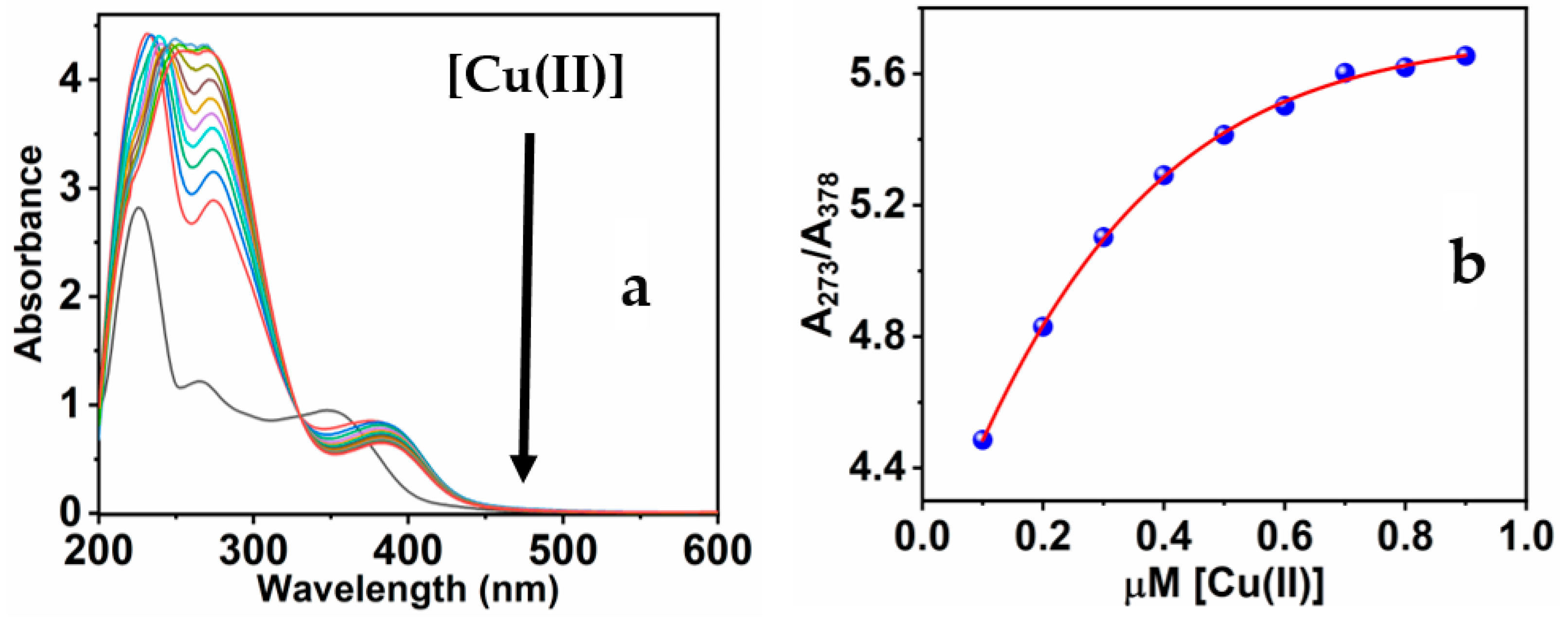
Absorbance and fluorescence methods were used to observe the fluorescence response of MNC to the copper ions. In addition to the two notable absorbance peaks of the free MNC probe, the addition of Cu(II) ions to the MNC molecules formed a novel absorbance peak at 378 nm [58]. The absorbance of the 378 nm peaks quenches when more copper ions are added. In contrast, the 266 absorption peak begins to intensify and shift to 273 nm, a redshift, when Cu(II) is added to the MNC solution. With the development of an isosbestic point at 329 nm, the synchronized behavior of the three absorption peaks continues (Figure 2a).
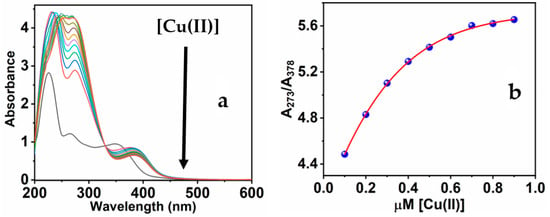
Figure 2.
(a) The UV-Vis spectra of MNC throughout the gradual adding Cu(II); (b) the relation of the band ratios at 273 and 378 nm (A273/A378) against [Cu(II)].
Intriguingly, the peculiar response of the MNC UV-Vis spectra verifies the binding procedure involving Cu(II) metal and the functioning groups, which include oxygen and nitrogen atoms of the sensitive chemical sensor [59]. The association between the absorption ratio at 273 and 378 nm of the chemical sensor and as a function of Cu(II) concentration throughout the range of 0.1 to 0.9 M is noteworthy (Figure 2b). Particularly, when the Cu(II) concentration reaches an equal molar ratio of 1:1 (MNC/Cu(II)), the absorbance starts to be constant even at higher molar ratios. The ratiometric detection approach demonstrates that the mechanism of Cu(II) and MNC interaction is a ratio of 1:1 stoichiometric.
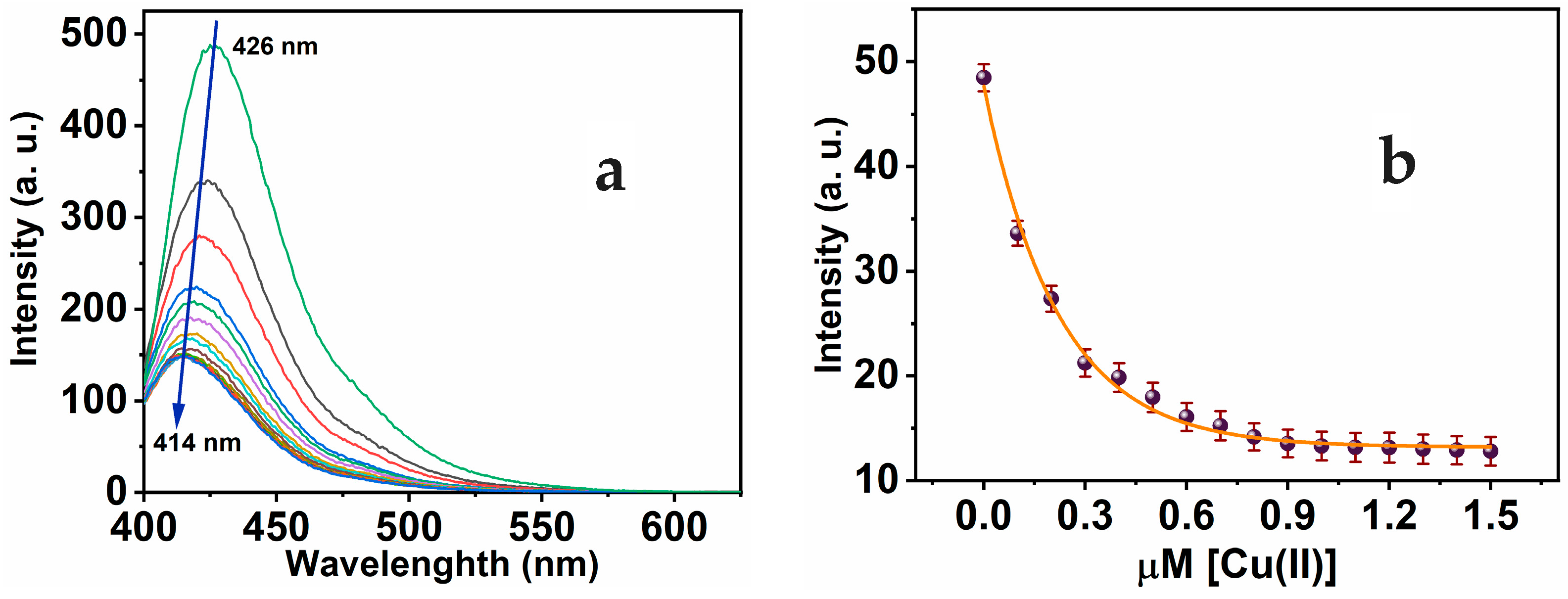
Furthermore, the MNC sensing molecule exhibits luminescent properties, through the dominant peak showing at 426 nm and λexc at 312 nm. The optical characteristics of MNC demonstrate the lack of overlap seen between absorbance and luminescence, and a considerable Stocks shift (Δλ = 114 nm) was observed. The substantial Stokes shift reduces self-quenching, which is necessary for potential implementation. With the stepwise addition of Cu(II) to MNC, the luminescence sensitivity of the prominent peak at 426 nm was substantially quenched. As seen in Figure 3a, the amplitude of signal attenuation reached about 74.43%. Moreover, the addition of Cu(II) ion to the MNC probe in a concentration range from 0 to 1 equivalent of Cu(II) causes the prominent emission peak to quench and shift to 414 nm. However, the fluorescence intensities stabilized following the addition of more than 1 equivalent of Cu(II) ions. The fluorescence titration demonstrated a nonlinear fit of the fluorescence emission titration curve. In accordance with absorption titration results, a 1:1 complex ratio was observed between the MNC chemical sensor and Cu(II).
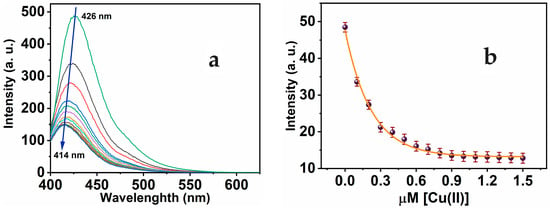
Figure 3.
(a) Luminescence of MNC 10 μM in a 1:9 (v/v) DMSO-HEPES buffer (20 mM, pH = 7.4) during gradual adding Cu(II) of 0–1.5 μM range, λexc 312 nm; (b) calibration curve of MNC intensity versus [Cu(II)].
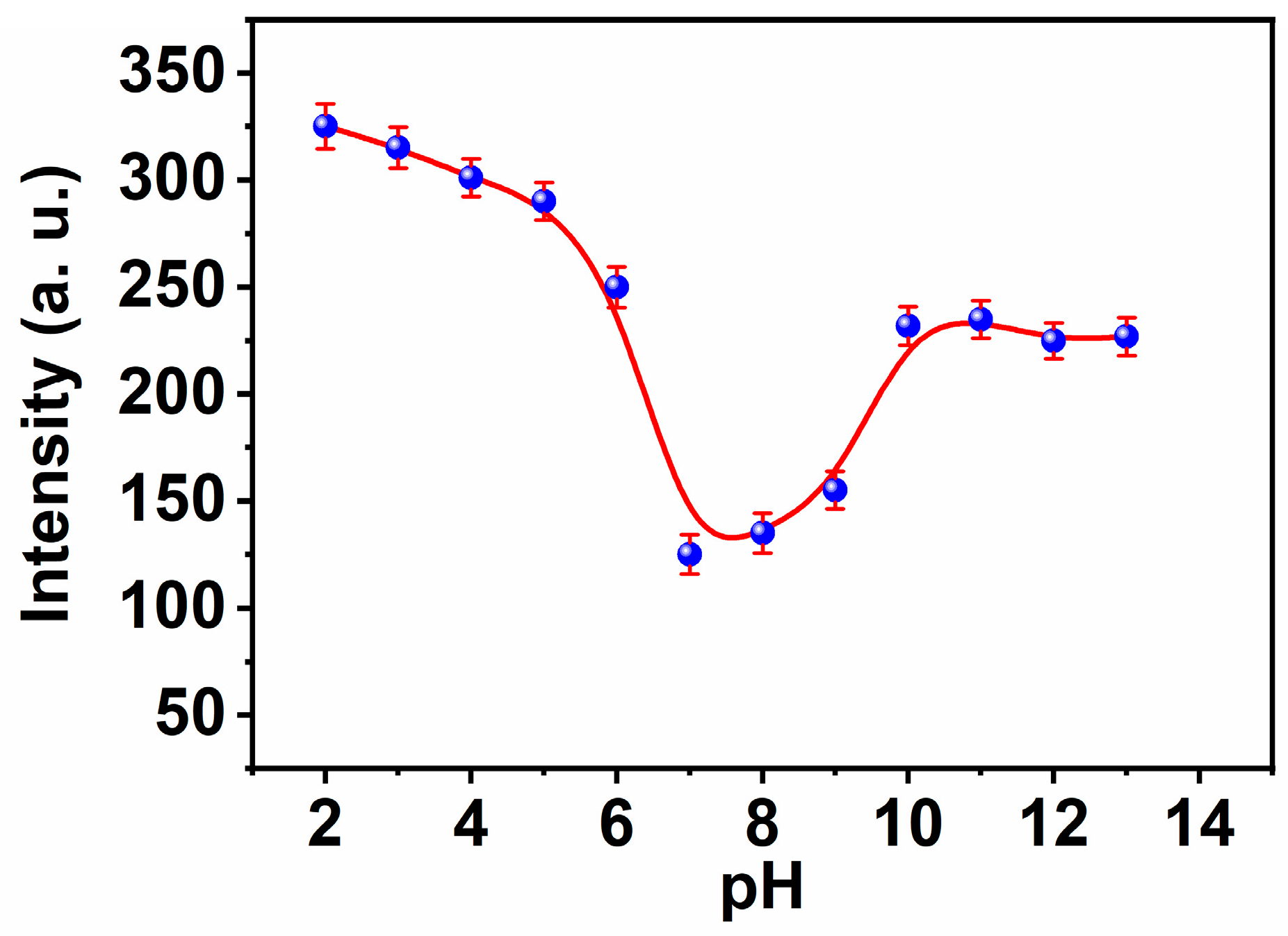
3.3. PH Influence on the MNC Luminescence
The majority of metal cations detection depends on optical sensor fluorescence modification coupled with hydrogen proton transfer. Changes in the pH of the ambient solution have an effect on this occurrence. In the pH range of 2 to 11, the effect of pH on the selectivity and responsiveness of the chemical sensor to copper ions was examined. By adjusting the pH of the aqueous solution with 20 M HEPES buffer solutions at a certain concentration of Cu(II) ion (1 μM) and excitation at 312 nm, we were able to determine the Cu(II) concentration (Figure 4). As seen, in the pH range of 2.0 to 7.0, the chemical sensor’s response is proportional to pH because the Schiff base exists in protonated form without complex formation. The binding of the proton to the nitrogen and oxygen atoms of the active groups inhibits the formation of complexes to a certain degree. However, the observed increase in MNC sensor fluorescence in response to alkaline pH or high pH > 7 is owing to the precipitation of Cu(II) hydroxide from the aqueous solution. In a variety of pH 7.4 buffered systems, including the HEBES buffer, the high reactivity and specificity of the MNC sensing molecule for copper ions were investigated.
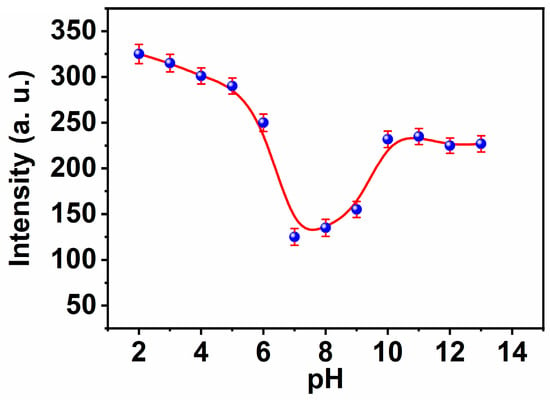
Figure 4.
The influence of the pH on the fluorescence intensities of MNC ligand under λexc 312 nm.
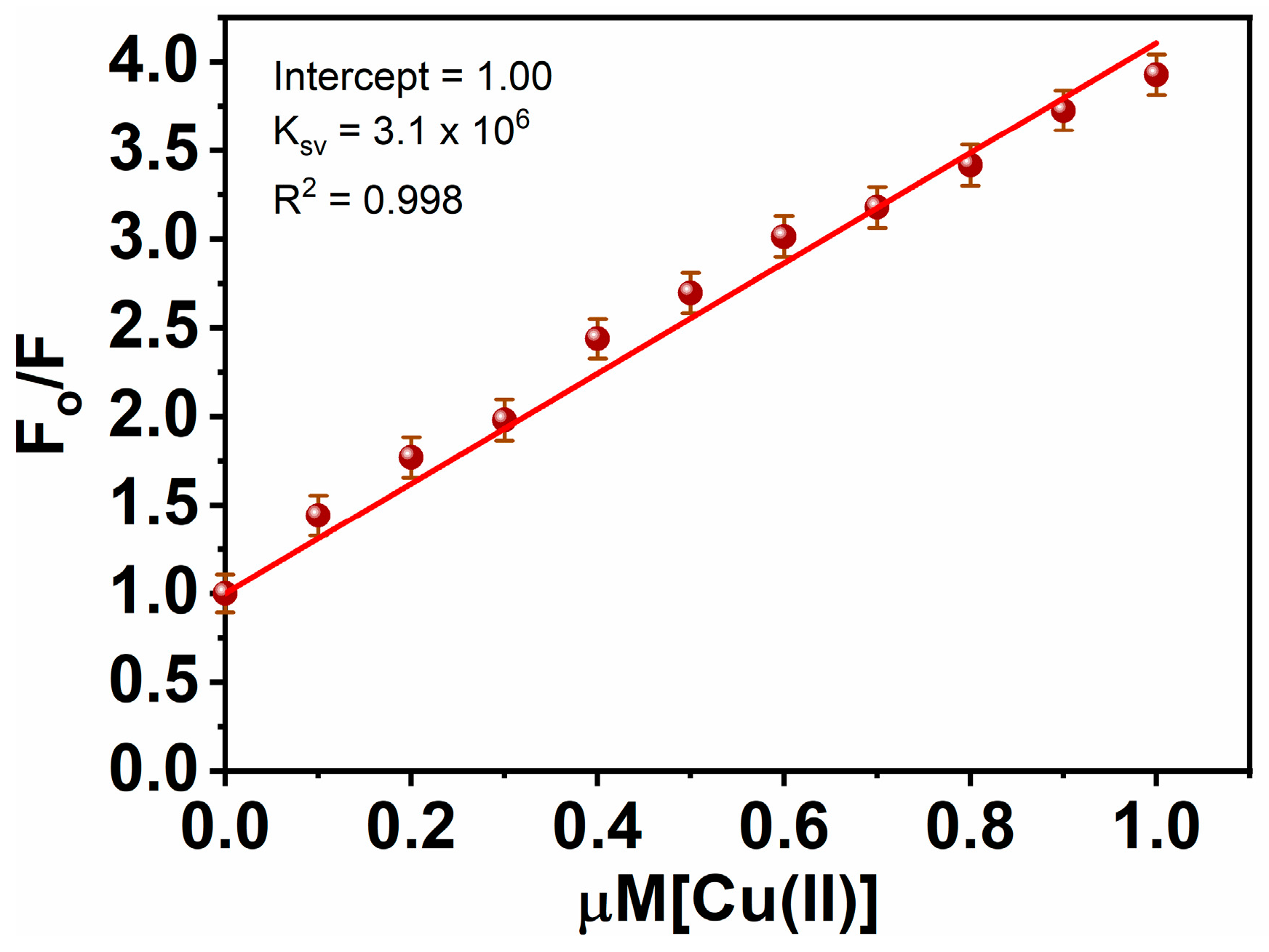
3.4. Quenching Phenomenon
Collisional quenching of fluorescence is described by the Stern-Volmer equation [60]:
F0 and F are the fluorescence intensities in the absence of a quencher and its presence, respectively, correspondingly, in this equation. Ksv is the quenching constant, and Q is the quencher concentration. Ksv denotes the Stern-Volmer quenching constant. Typically, data on quenching are displayed as graphs of F0/F vs. [Q] (see Figure 5). A linear Stern-Volmer curve demonstrates that collisional fluorescence quenching has occurred. Over a broad range from 0 to 1 M, R2 = 0.998% demonstrated an exceptional linear dependency. In order to assess the nanosensor’s sensitivity, the limit of detection (LOD) was estimated to be 1.45 nM, supposing that emission intensity can be assessed with 1% accuracy [61]. Notably, these LODs are far lower than the Cu2+ content (31.5 μM) recommended by the WHO for drinking water characteristic guidelines [62]. Furthermore, the LOD of the MNC is compared to some of the recently reported sensors (Table 1).
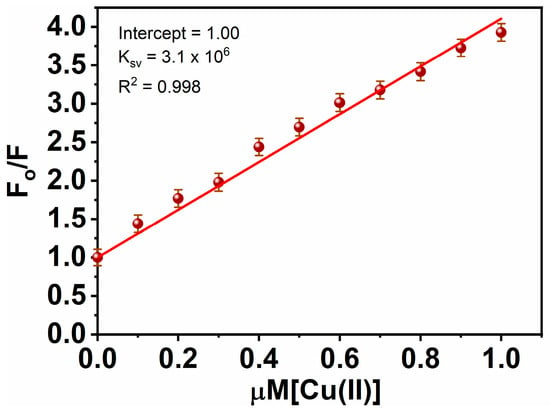
Figure 5.
Stern Volmer plot for Cu(II)-MNC.

Table 1.
Comparison of Cu(II) optical sensors found in the literature with the investigated work.
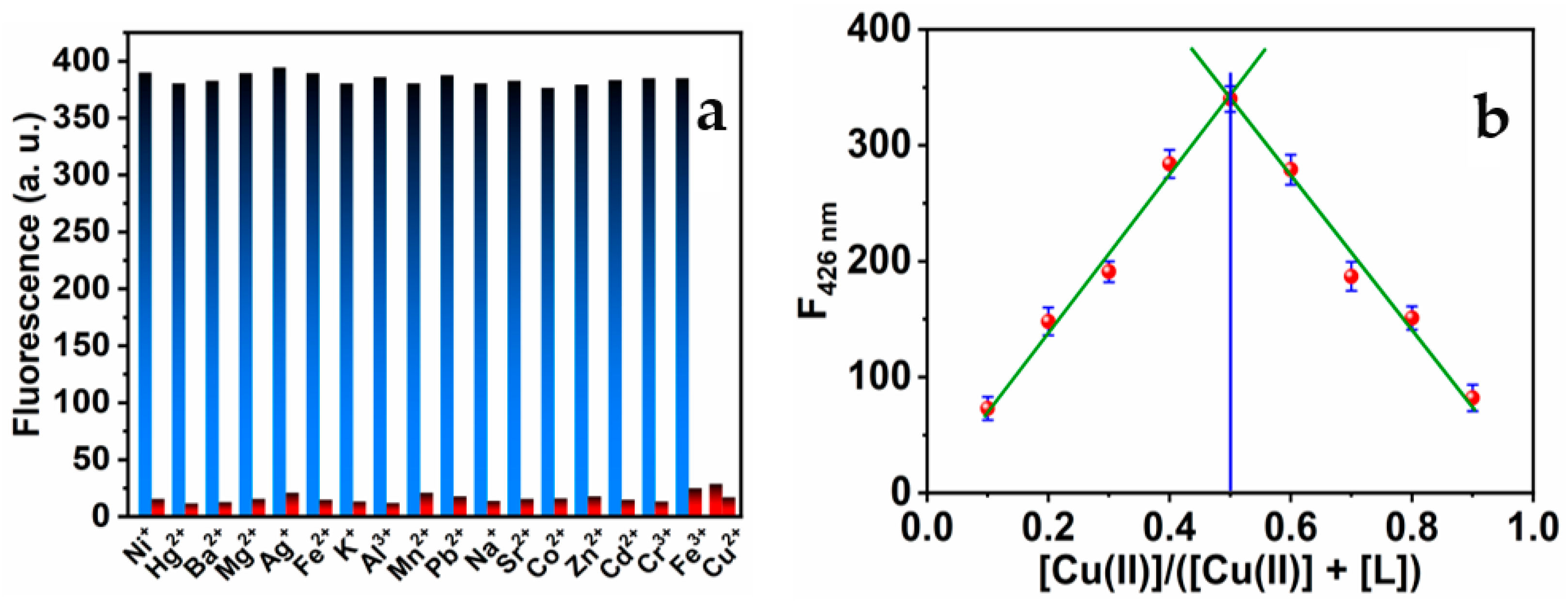
Under the optimum conditions, the interaction between MNC molecules and Cu(II) was investigated in the absence and the presence of various metal ions of a 1 μM concentration. There were minor variations in the MNC probe’s emission intensities. As seen in Figure 6a, none of the studied interfering salt ions had any discernible effect on the chemical detection of Cu(II) ions. Consequently, the chemical sensor might be used as a highly selective sensor for Cu(II). The MNC:Cu(II) complex stoichiometric ratio was established using Job’s plot-based luminescence analyses [69,70]. The Job’s plot (see Figure 7b) was generated by varying the copper ion concentration within a range of up to 1 M. The greatest fluorescence intensity quenching value was observed when the molar ratio of Cu(II) was around 0.5, indicating that MNC and Cu(II) ions formed a 1:1 complex. In addition, this is consistent with the Absorbance titration.
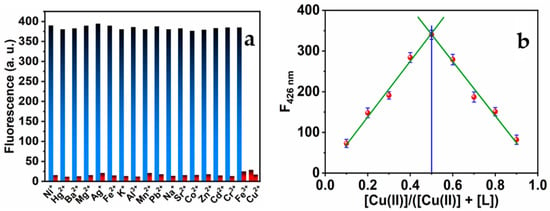
Figure 6.
(a) Maximum luminescence intensities of the MNC sensing molecule (I) in the presence of various metal ions (blue bar) and (II) in the presence of the same metal ions and Cu(II) ions (red bar). The ratio of (MNC: Cu(II): M(II) has a 1:1:1 ratio (λex = 312 nm); (b) Job’s plot of MNC with Cu(II) ions.
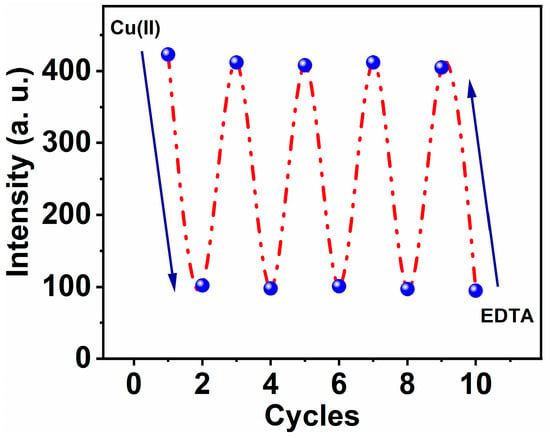
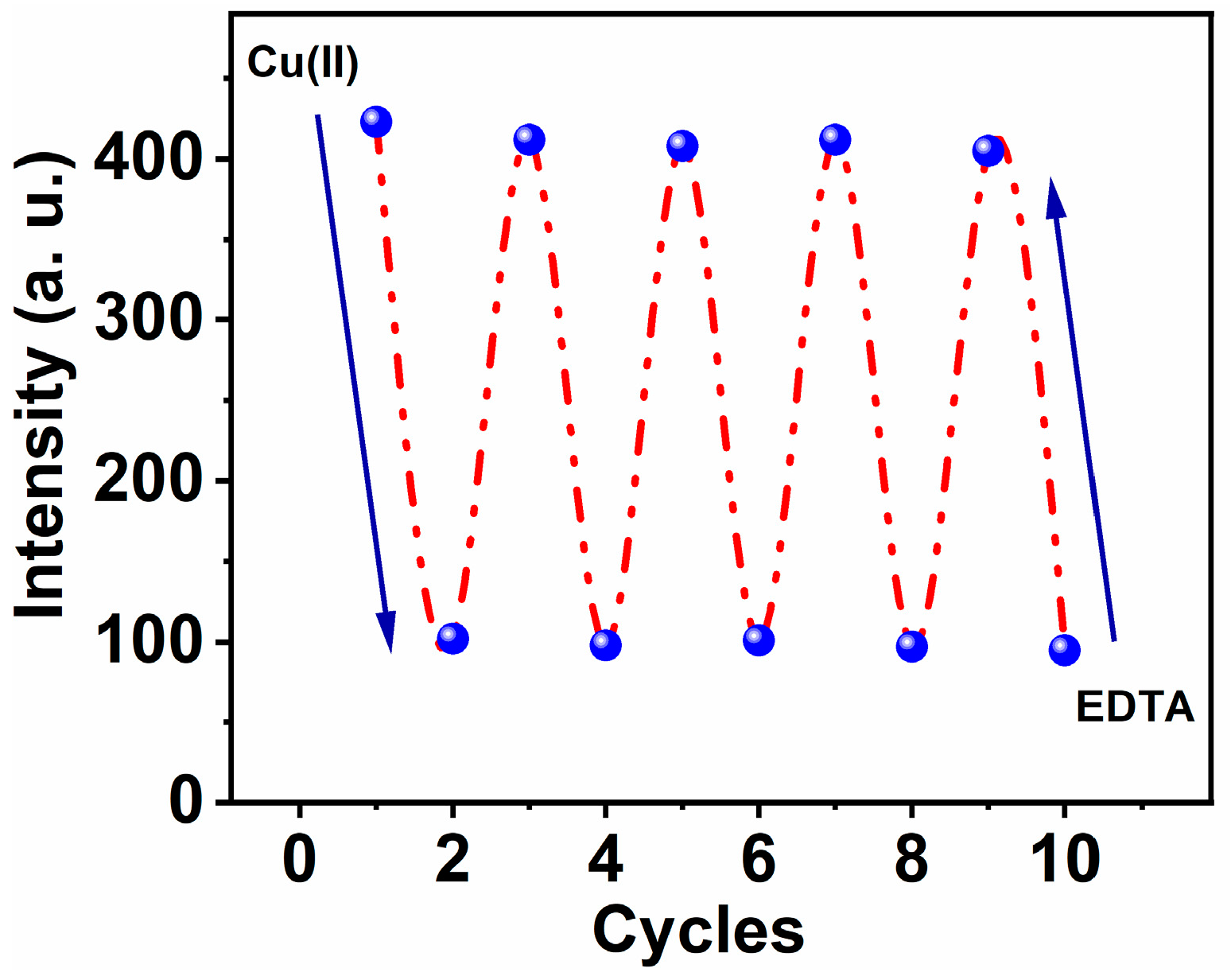
Figure 7.
Reversibility of MNC-Cu(II) and EDTA mutually, λex = 312 nm and λem = 426 nm, respectively.
3.5. Reversibility
Ethylenediaminetetraacetate (EDTA), a very effective binding reagent for especially Cu(II) ions, was used to investigate the reversibility of the MNC-based optical chemosensor. Cu(II)-MNC complex solution, with a molar ratio of (1:1) Cu(II) to MNC and a concentration of 10 μM, was exposed and mixed with an equal molar concentration of EDTA solution. Upon addition of EDTA, their molecules begin to swap MNC molecules, and an immediate Cu-EDTA complex formed. Intriguingly, the exchange chelation procedure was followed by an increase in fluorescence caused by the freed chemical probe MNC. Consequently, the fluorescence intensity increased until it reached roughly 75% of the intensity of the free form (see Figure 7).
3.6. Quantum Yield
The quantum yield (QY) estimate for MNC was presented as 0.22. The QY was detected depending on a standard and reference chromophore of quinine sulfate [71]. The aqueous solution of the reference chromophore in sulfuric acid was prepared to give a 55% QY. The ultimate deviation for such QY was predicted to be (±6%).
While
- X and R point to the MNC and reference solutions
- n is a refractive index at room temperature
- I is the integrated area under the peak
- A is the maximum absorbance peak.
3.7. Binding Constant Kb
To further comprehend the metal ligand binding mechanism, variations in the fluorescence spectra of the MNC probe in the presence of various Cu(II) concentrations were examined to calculate the binding constant. On the basis of a 1:1 stoichiometry, the binding constant was calculated using the modified Stern-Volmer equation,
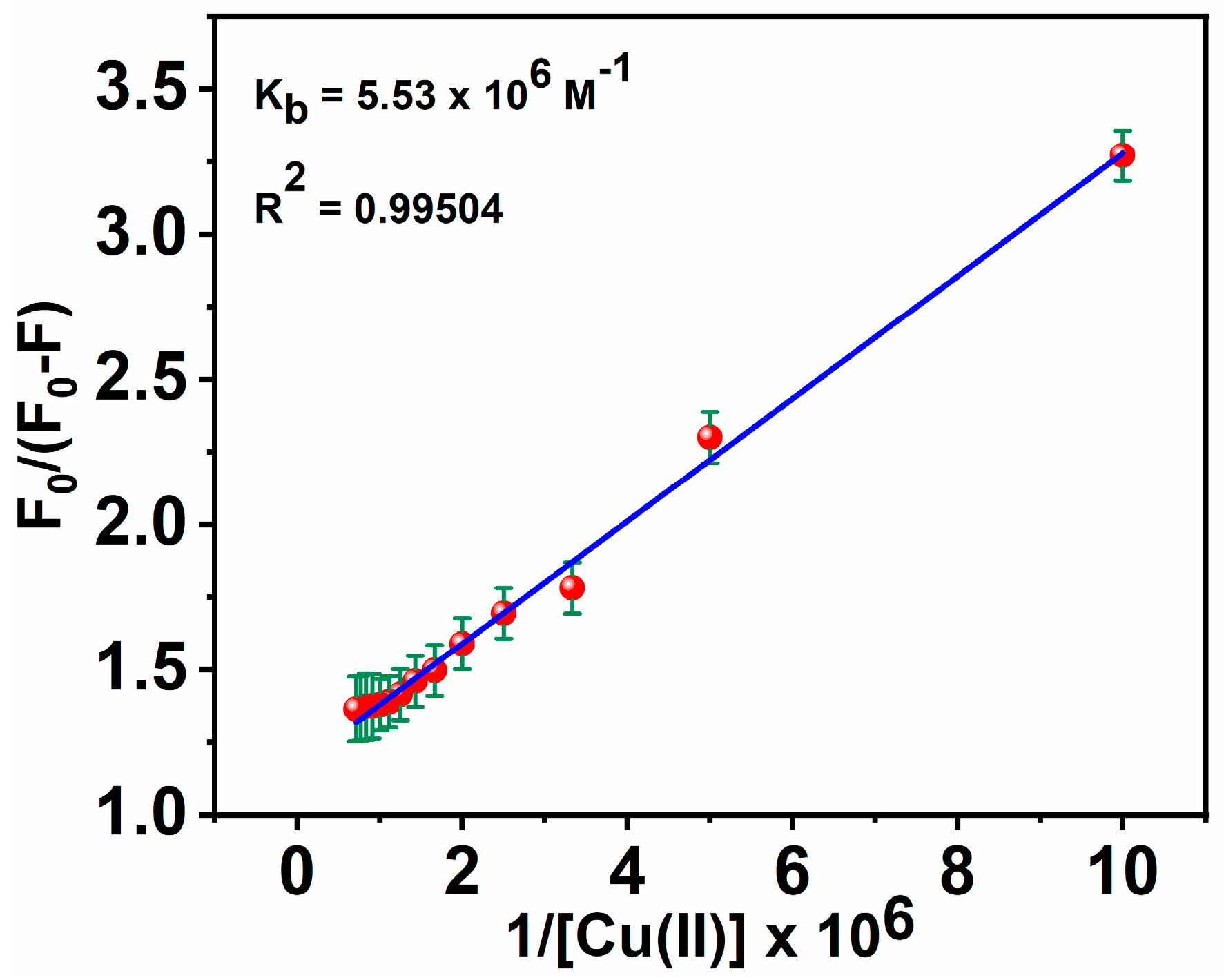
where F0 is the fluorescence intensity of the free ligand MNC, F is the fluorescence intensity of the MNC-Cu(II) complex, Q is [Cu(II)], A is a constant, and Kb is a binding constant [72]. When F0/(F0 − F) was plotted as a function of the 1/[Q] concentration, a linear relationship was obtained: (y = α + βx), y = F0/(F0 − F), α (intercept) = 1/A, β (slope) = 1/A.Kb, x = 1/[Q], and K was estimated from α/β (Figure 8). Inferred from the fluorescence titration curves of the MNC probe with Cu(II), Kb, the binding association constant, was calculated to be 5.53 × 106 M−1.
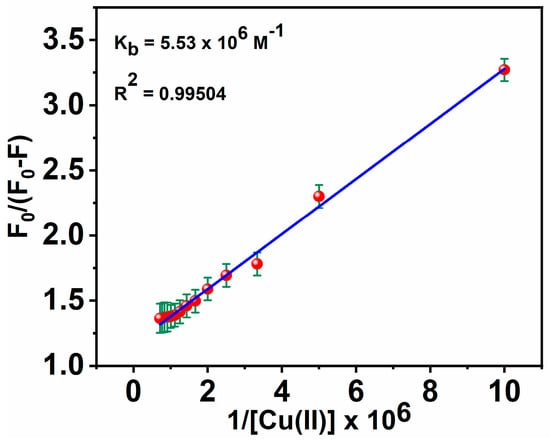
Figure 8.
Stern-Volmer plot for calculating Kb of MNC-Cu(II).
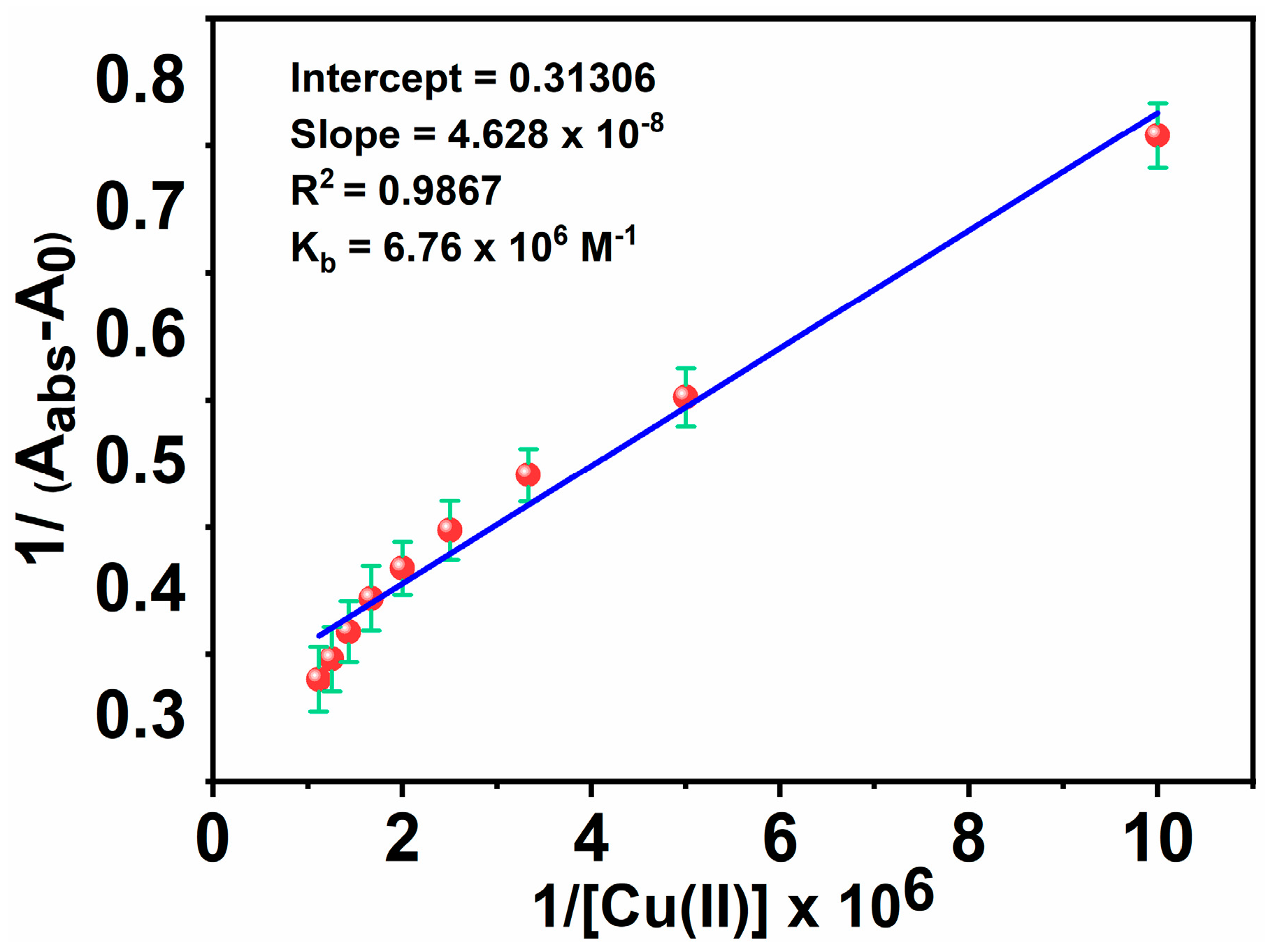
Also, the extent of the binding of MNC to Cu(II) ions is determined based on the absorbance enhancement at 266 nm using an experimental plot of absorption data based on the Benesi-Hildebrand relation [73]:
where A0, Aabs, and Ac are the absorption values in the absence of, at the intermediate, and at the saturation of Cu(II) ion interaction, respectively, and [Cu(II)] indicates the concentration of Cu(II) ion added. By linearly fitting an absorbance titration curve, the binding constant (Kb) was calculated (Figure 9). The greater value of the binding constant (6.76 × 106 M−1) suggests that the interaction between MNC and Cu(II) ion is strong.
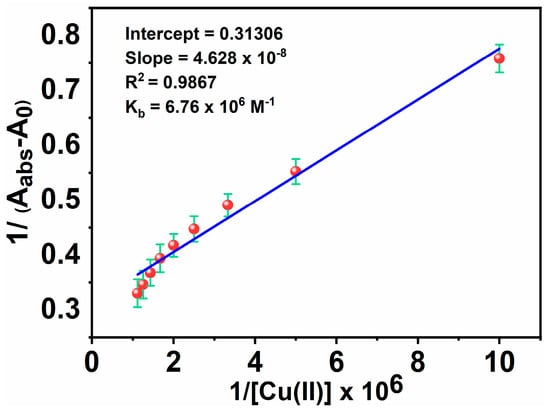
Figure 9.
Benesi-Hildebrand plots for Kb of MNC- Cu(II).
3.8. Theoretical Study
Software Gaussian09 conducted mathematical methods that rely on the density functional theory (DFT) in conjunction with the time-dependent DFT (TDDFT) [74]. The purpose is to clarify the complex structure of Cu2+ and its electronic properties, and It may lead to fluorescence increase or decrease. The structures’ geometry was developed using the module CAM-B3LYP, and the basis set was 6–31G(d)/Lanl2dz. The LANL2DZ basis set treated Cu, and we used a 6–31G(d) basis set for all the other atoms. At the same level of theory, vibrational frequencies were calculated to confirm that all the structures are stable. As the experiment is realized in methanol, TDDFT with SMD solvation model and methanol was used on the compounds optimized by CAM-B3LYP at the PBE1PBE/Lanl2DZ/6–31 + G(d) level of theory.
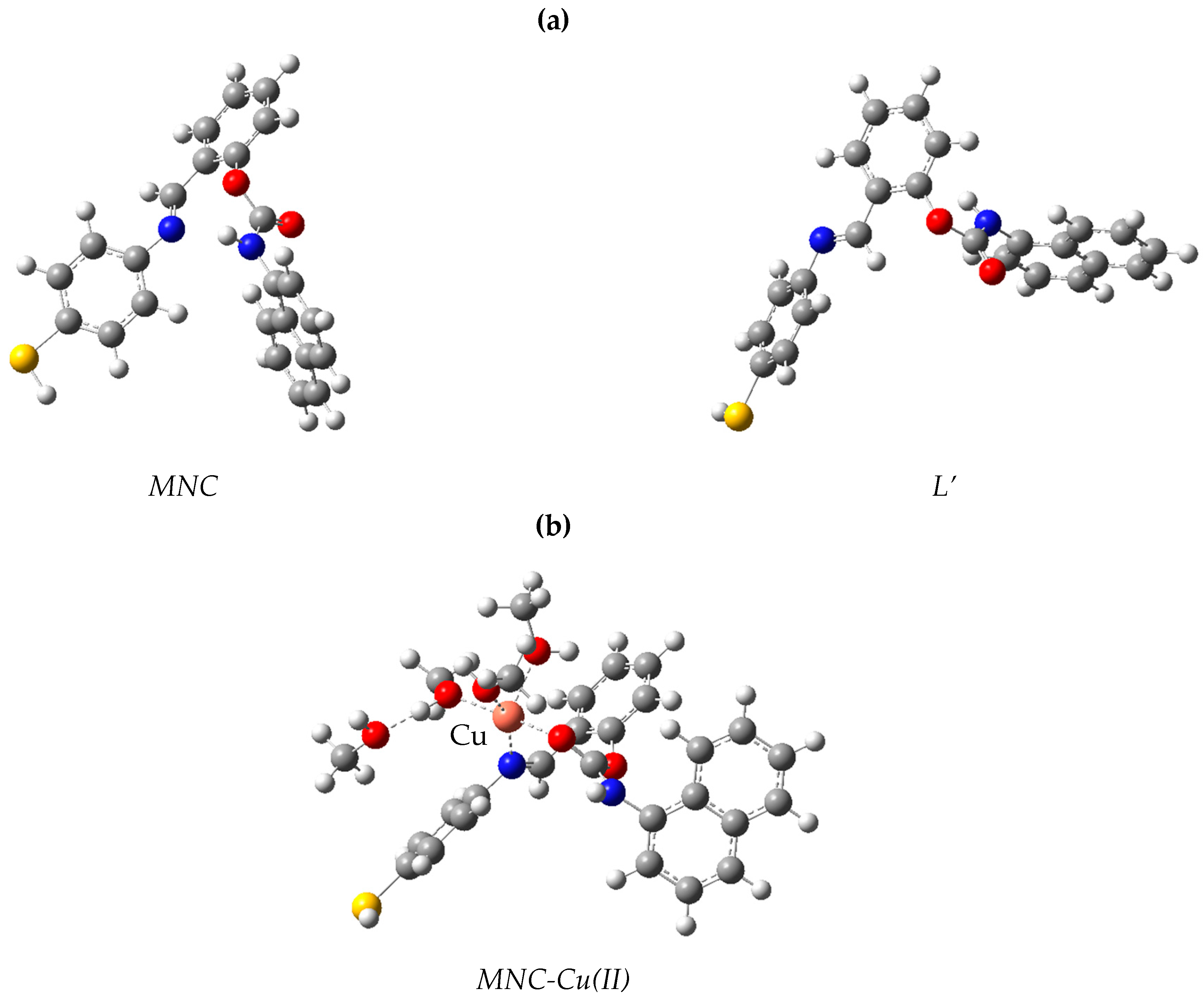
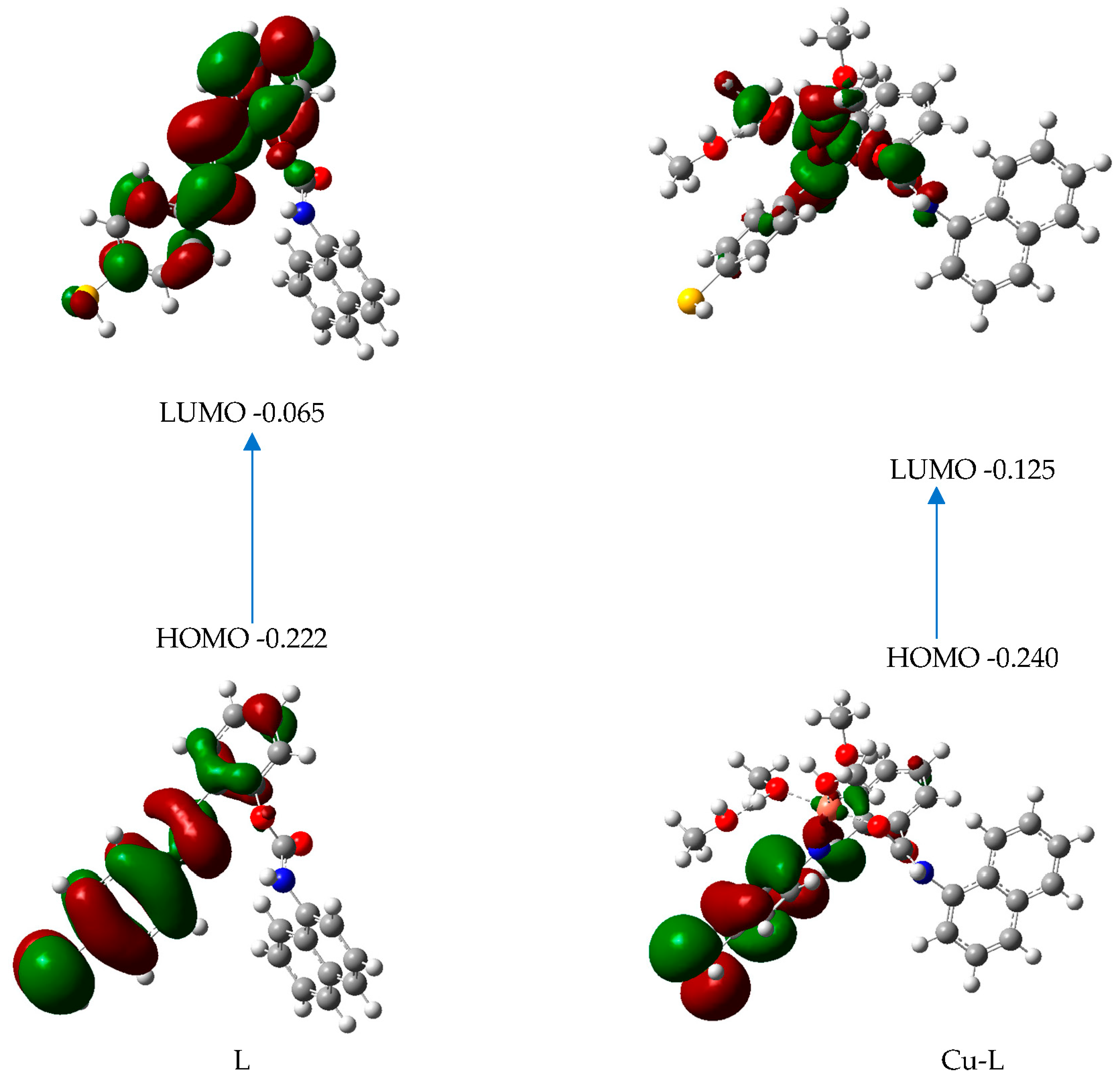
Ligand conformers for L and L’ are presented in Figure 10a. The L conformer is more stable than the L’ structure by 3.31 kcal/mol (Table 2). The theoretical study of the ligand’s electronic structure alone will focus on the most stable conformer, L. The copper complex optimized structure (Cu-L) is represented in Figure 10b. In this figure, we see that the copper center in the complex is five coordinated to the ligand L through one nitrogen atom and one oxygen atom. The bond length of Cu-N is 2.032 Å, and the bond length of Cu-O is 1.95 Å in the optimized structure. Thus, the ligand L can accommodate the cation Cu2+ by forming bonds with one nitrogen and one oxygen. The likely transitions in such chromophores were identified using time-dependent DFT Numerical simulations. Figure 11 depicts the border molecular orbitals of MNC and Cu-LMNC-Cu(II). The data of electronic transitions, wavelength, and oscillator strength from TDDFT calculations are found in Table 3.
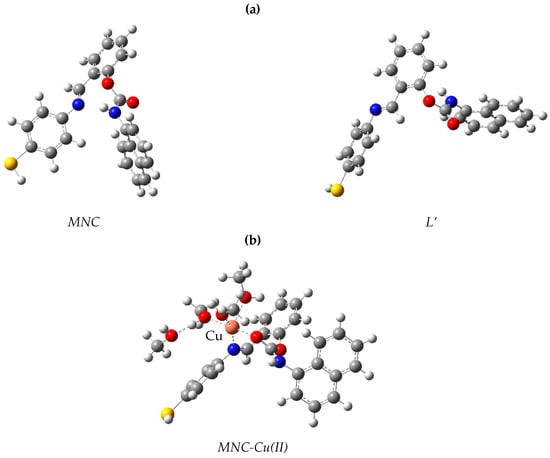
Figure 10.
Model structures for (a) MNC and (b) MNC-Cu(II).

Table 2.
Calculated Relative Energies of the ligands.
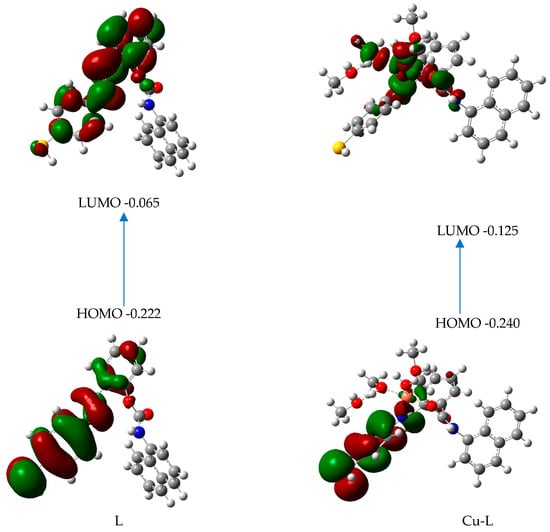
Figure 11.
Frontier orbitals and their energies for L and Cu-L.

Table 3.
TDDFT outcomes of certain transitions, including associated absorbance energies and oscillator strengths.
The electron density for the frontier orbitals in the ligand alone is almost located on the sulfur atom and the two aromatic rings linked by a CN double bond. In L-Cu, the delocalization of electrons between the HOMO and LUMO transition levels is due to ICT: the electron density in HOMO is almost on the sulfur atom and the two aromatic rings linked by a CN double bond, but in the LUMO, it is almost located on the metal and the atoms of the ligand linked to the metal. We might speculate that the intensity quenching in this molecule is the consequence of MNC-Cu(II) binding. The weak value of the oscillator strength for this transition in the complex, which is equal to 0.0156 (Table 2), and the relatively large oscillator strength for the transition in MNC, which is equal to 0.4950 (Table 3), support these findings.
4. Conclusions
In summary, an extremely sensitive and innovative turn-off chemical sensor probe MNC was developed being prepared for the sensing of Cu(II) ions depending on a chelation approach to avoid interfering with different types of metal ions in a 1:9 (v/v) DMSO-HEPES buffer (20 mM, pH = 7.4) solution. The colorimetric and absorption MNC sensor revealed a considerable Stocks shift of 114 nm; thus, the proposed chemosensor provides a novel approach for creating a chemical probe with substantial luminescence changes. The MNC sensor can readily detect Cu(II) ions with high selectivity, exceptional sensitivity, and a detection limit (LOD) of 1.45 nM. Copper ions and MNC in nano concentrations exhibited an excellent linear relationship that could be used for quantitatively measuring Cu(II) ions. Job’s experiments demonstrated that MNC chelates Cu(II) ions in a 1:1 metal/ligand ratio. The studied chemical sensor depends on the binding of Cu(II) to the MNC synthetic chromophore and the formation of the MNC-Cu(II) complex molecule for its mechanism. DFT and TDDFT demonstrate that the quenching of emission intensities in this synthetic MNC molecule results from ICT throughout MNC to the binding metal ions. This chemical sensor based on an MNC sensor might provide a unique method for detecting Cu(II) in the environment and for research purposes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bios13030359/s1, Figure S1: FTIR spectra of the MNC probe; Figure S2: NMR spectra of the MNC probe.
Author Contributions
Conceptualization, S.M.S. and R.A.; methodology, S.M.S. and R.A.; software, S.M. and A.E.A.E.A.; validation, S.M.S., S.M., L.M.A. and F.M.A.; formal analysis, L.M.A. and S.M.; investigation, R.A. and L.M.A.; resources, S.M.S. and R.A.; data curation, S.M.S. and R.A.; writing-original draft preparation, S.M.S., R.A. and L.M.A.; writing-review and editing, S.M.S. and R.A.; visualization, A.E.A.E.A. and L.M.A.; supervision, S.M.S. and R.A.; project administration, S.M.S. and R.A.; funding acquisition, S.M.S. and F.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pietrini, F.; Carnevale, M.; Beni, C.; Zacchini, M.; Gallucci, F.; Santangelo, E. Effect of different copper levels on growth and morpho-physiological parameters in giant reed (Arundo donax L.) in semi-hydroponic mesocosm experiment. Water 2019, 11, 1837. [Google Scholar] [CrossRef]
- Linder, M.C. Copper homeostasis in mammals, with emphasis on secretion and excretion. A review. Int. J. Mol. Sci. 2020, 21, 4932. [Google Scholar] [CrossRef]
- Oliveri, V. Biomedical applications of copper ionophores. Coord. Chem. Rev. 2020, 422, 213474. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Luo, P.; He, B.; Cao, X.; Lu, T. Iron sites on defective BiOBr nanosheets: Tailoring the molecular oxygen activation for enhanced photocatalytic organic synthesis. Nano Res. 2022, 15, 1509–1516. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Alminderej, F.; Ali, I.A. Ultrasensitive optical chemosensor for Cu (II) detection. Int. J. Anal. Chem. 2019, 2019, 7381046. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Ali, I.A. A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu (II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 225–231. [Google Scholar] [CrossRef]
- Barber, R.G.; Grenier, Z.A.; Burkhead, J.L. Copper toxicity is not just oxidative damage: Zinc systems and insight from Wilson disease. Biomedicines 2021, 9, 316. [Google Scholar] [CrossRef]
- Sakunkaewkasem, S.; Petdum, A.; Panchan, W.; Sirirak, J.; Charoenpanich, A.; Sooksimuang, T.; Wanichacheva, N. Dual-analyte fluorescent sensor based on [5] helicene derivative with super large stokes shift for the selective determinations of Cu2+ or Zn2+ in buffer solutions and its application in a living cell. ACS Sens. 2018, 3, 1016–1023. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Salam, A.A.; Nassory, N.S. Ion imprinted polymer based electrochemical sensor for environmental monitoring of copper (II). Int. J. Eelectrochem. Sci. 2015, 10, 6780. [Google Scholar]
- Nadimetla, D.N.; Bhosale, S.V. Tetraphenylethylene AIEgen bearing thiophenylbipyridine receptor for selective detection of copper (II) ion. New J. Chem. 2021, 45, 7614–7621. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, C.; Wang, S.; Li, M.; Guo, W. MOF-derived porous ZnO-Co3O4 nanocages as peroxidase mimics for colorimetric detection of copper (ii) ions in serum. Analyst 2021, 146, 605–611. [Google Scholar] [CrossRef]
- Kuras, M.J.; Więckowska, E. Synthesis and characterization of a new copper (II) ion-imprinted polymer. Polym. Bull. 2015, 72, 3227–3240. [Google Scholar] [CrossRef]
- Bagheri, N.; Mazzaracchio, V.; Cinti, S.; Colozza, N.; Di Natale, C.; Netti, P.A.; Saraji, M.; Roggero, S.; Moscone, D.; Arduini, F. Electroanalytical sensor based on gold-nanoparticle-decorated paper for sensitive detection of copper ions in sweat and serum. Anal. Chem. 2021, 93, 5225–5233. [Google Scholar] [CrossRef]
- Saleh, S.M.; Almotiri, M.K.; Ali, R. Green synthesis of highly luminescent gold nanoclusters and their application in sensing Cu(II) and Hg(II). J. Photochem. Photobiol. A 2022, 426, 113719. [Google Scholar] [CrossRef]
- Ali, R.; Alfeneekh, B.; Chigurupati, S.; Saleh, S.M. Green synthesis of pregabalin-stabilized gold nanoclusters and their applications in sensing and drug release. Arch. Pharm. 2022, 355, 2100426. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef]
- Frag, E.Y.; Mohamed, M.E.B.; Fahim, E.M. Application of carbon sensors for potentiometric determination of copper (II) in water and biological fluids of Wilson disease patients. Studying the surface reaction using SEM, EDX, IR and DFT. Biosens. Bioelectron. 2018, 118, 122–128. [Google Scholar] [CrossRef]
- Kirk, K.A.; Andreescu, S. Easy-to-use sensors for field monitoring of copper contamination in water and pesticide-sprayed plants. Anal. Chem. 2019, 91, 13892–13899. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency Office of Pesticide Programs. Copper Facts; US Environmental Protection Agency: New York, NY, USA, 2008.
- Chereddy, N.R.; Janakipriya, S.; Korrapati, P.S.; Thennarasu, S.; Mandal, A.B. Solvent-assisted selective detection of sub-micromolar levels of Cu2+ ions in aqueous samples and live-cells. Analyst 2013, 138, 1130–1136. [Google Scholar] [CrossRef]
- Ali, R.; Alminderej, F.M.; Messaoudi, S.; Saleh, S.M. Ratiometric ultrasensitive optical chemisensor film based antibiotic drug for Al (III) and Cu (II) detection. Talanta 2021, 221, 121412. [Google Scholar] [CrossRef]
- Losev, V.N.; Buyko, O.V.; Trofimchuk, A.K.; Zuy, O.N. Silica sequentially modified with polyhexamethylene guanidine and Arsenazo I for preconcentration and ICP–OES determination of metals in natural waters. Microchem. J. 2015, 123, 84–89. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Fu, Q.; Du, W.; Zhu, R.; Ge, X.; Wang, C.; Li, Q.; Su, L.; Yang, H.; Song, J. Quantitative assessment of copper (II) in Wilson’s disease based on photoacoustic imaging and ratiometric surface-enhanced raman scattering. ACS Nano 2021, 15, 3402–3414. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Deng, P.; Li, J.; Tang, S. Fluorescent ion-imprinted sensor for selective and sensitive detection of copper (II) ions. Sens. Actuators B Chem. 2018, 255, 2095–2104. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Hegazy, M.E.F.; Alminderej, F.M.; Mohamed, T.A. The natural compound chrysosplenol-D is a novel, ultrasensitive optical sensor for detection of Cu (II). J. Mol. Liq. 2020, 302, 112558. [Google Scholar] [CrossRef]
- Ali, R.; Ghannay, S.; Messaoudi, S.; Alminderej, F.M.; Aouadi, K.; Saleh, S.M. A Reversible Optical Sensor Film for Mercury Ions Discrimination Based on Isoxazolidine Derivative and Exhibiting pH Sensing. Biosensors 2022, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; El-Sayed, W.A.; El-Manawaty, M.A.; Gassoumi, M.; Ali, R. Microwave-Assisted Rapid Synthesis of Luminescent Tryptophan-Stabilized Silver Nanoclusters for Ultra-Sensitive Detection of Fe (III), and Their Application in a Test Strip. Biosensors 2022, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Alminderej, F.M.; Saleh, S.M. A simple, quantitative method for spectroscopic detection of metformin using gold nanoclusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118744. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Elshaarawy, R.F.; Saleh, S.M. Turn-on ratiometric fluorescence sensor film for ammonia based on salicylaldehyde-ionic liquid. J. Environ. Chem. Eng. 2017, 5, 4813–4818. [Google Scholar] [CrossRef]
- Ali, R.; Saleh, S.M.; Aly, S.M. Fluorescent gold nanoclusters as pH sensors for the pH 5 to 9 range and for imaging of blood cell pH values. Microchim. Acta 2017, 184, 3309–3315. [Google Scholar] [CrossRef]
- Saleh, S.M.; Alminderej, F.M.; Ali, R.; Abdallah, O.I. Optical sensor film for metribuzin pesticide detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117971. [Google Scholar] [CrossRef] [PubMed]
- Matar, S.A.; Talib, W.H.; Mustafa, M.S.; Mubarak, M.S.; AlDamen, M.A. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3,3′-diaminodipropylamine. Arab. J. Chem. 2015, 8, 850–857. [Google Scholar] [CrossRef]
- Alpaslan, G.; Boyacioglu, B.; Demir, N.; Tümer, Y.; Yapar, G.; Yıldırım, N.; Yıldız, M.; Ünver, H. Synthesis, characterization, biological activity and theoretical studies of a 2-amino-6-methoxybenzothiazole-based fluorescent Schiff base. J. Mol. Struct. 2019, 1180, 170–178. [Google Scholar] [CrossRef]
- Magalhães, T.F.F.; da Silva, C.M.; Dos Santos, L.B.F.; Santos, D.A.; Silva, L.M.; Fuchs, B.B.; Mylonakis, E.; Martins, C.V.B.; de Resende-Stoianoff, M.A.; de Fátima, Â. Cinnamyl Schiff bases: Synthesis, cytotoxic effects and antifungal activity of clinical interest. Lett. Appl. Microbiol. 2020, 71, 490–497. [Google Scholar] [CrossRef]
- Gwaram, N.S.; Musalam, L.; Ali, H.M.; Abdulla, M.A. Synthesis of 2’-(5-Chloro-2-Hydroxybenzylidene) benzenesulfanohydrazide Schiff base and its anti-ulcer activity in ethanol-induced gastric mucosal lesions in rats. Trop. J. Pharm. Res. 2020, 11, 251–257. [Google Scholar] [CrossRef]
- Nilkanth, P.R.; Ghorai, S.K.; Sathiyanarayanan, A.; Dhawale, K.; Ahamad, T.; Gawande, M.B.; Shelke, S.N. Synthesis and Evaluation of Anticonvulsant Activity of Some Schiff Bases of 7-Amino-1, 3-dihydro-2H-1, 4-benzodiazepin-2-one. Chem. Biodivers. 2020, 17, e2000342. [Google Scholar]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Di Meo, F.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.F.; et al. Antioxidant properties of phenolic Schiff bases: Structure-activity relationship and mechanism of action. J. Comput. Aided Mol. Des. 2013, 27, 951–964. [Google Scholar] [CrossRef]
- Alam, M.S.; Choi, J.H.; Lee, D.U. Synthesis of novel Schiff base analogues of 4-amino-1, 5-dimethyl-2-phenylpyrazol-3-one and their evaluation for antioxidant and anti-inflammatory activity. Bioorg. Med. Chem. 2012, 20, 4103–4108. [Google Scholar] [CrossRef]
- Sakthivel, A.; Jeyasubramanian, K.; Thangagiri, B.; Raja, J.D. Recent advances in Schiff base metal complexes derived from 4-aminoantipyrine derivatives and their potential applications. J. Mol. Struct. 2020, 1222, 128885. [Google Scholar] [CrossRef]
- Pandey, A.; Dewangan, D.; Verma, S.; Mishra, A.; Dubey, R.D. Synthesis of schiff bases of 2-amino-5-aryl-1,3,4-thiadiazole and its analgesic, anti-inflammatory, anti-bacterial and antitubercular activity. Int. J. Chemtech Res. 2011, 3, 178–184. [Google Scholar]
- Arroudj, S.; Bouchouit, M.; Bouchouit, K.; Bouraiou, A.; Messaadia, L.; Kulyk, B.; Figa, V.; Bouacida, S.; Sofiani, Z.; Taboukhat, S. Synthesis, spectral, optical properties and theoretical calculations on schiff bases ligands containing o-tolidine. Opt. Mater. 2016, 56, 116–120. [Google Scholar] [CrossRef]
- Mahadevi, P.; Sumathi, S. Mini review on the performance of Schiff base and their metal complexes as photosensitizers in dye-sensitized solar cells. Synth. Commun. 2020, 50, 2237–2249. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Adam, M.S.S.; Hamdan, S.K. Some new nanosized mononuclear Cu(II) Schiff base complexes: Design, characterization, molecular modeling and catalytic potentials in benzyl alcohol oxidation. Catal. Lett. 2016, 146, 1373–1396. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, H.; Wang, Y.; Luo, Y.; Yao, Y. Lanthanum complexes stabilized by a pentadentate Schiff-base ligand: Synthesis, characterization, and reactivity in statistical copolymerization of ε-caprolactone and L-lactide. Dalton Trans. 2020, 49, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- De Domingo, E.; Folcia, C.L.; Ortega, J.; Etxebarria, J.; Termine, R.; Golemme, A.; Coco, S.; Espinet, P. Striking Increase in Hole Mobility upon Metal Coordination to Triphenylene Schiff Base Semiconducting Multicolumnar Mesophases. Inorg. Chem. 2020, 59, 10482–10491. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.; Gao, H.; Cao, H.; Zhao, Y.; Miao, Z.; Yang, Z.; He, W. Photoacoustic effect and controlled release of azo and Schiff base derivatives modified by click reagents under the NIR light. Pigment. Resin Technol. 2020, 49, 331–338. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, A.; Huang, G.; Yang, M.; Li, D.; Teng, M.; Han, D. Enhanced efficiency with CDCA co-adsorption for dye-sensitized solar cells based on metallosalophen complexes. Sol. Energy 2020, 209, 316–324. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, Y.; Nie, J.; Wei, J.; Miao, B.; Zhao, Y.; Zhang, L.; Ni, Z. Multifunctional AIE-ESIPT dual mechanism tetraphenylethene-based Schiff base for inkless rewritable paper and a colorimetric/fluorescent dual-channel Zn2+ sensor. Mater. Chem. Front. 2021, 5, 347–354. [Google Scholar] [CrossRef]
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-containing schiff bases on thiacalixarene: Synthesis, copper (II) recognition, and formation of organic-inorganic copper-based materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.Y.; Zhang, Q.L.; Yang, X.J.; Huang, Y.L.; Redshaw, C.; Xu, H. A simple turn-off Schiff base fluorescent sensor for copper (II) ion and its application in water analysis. Molecules 2021, 26, 1233. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, L.; Liu, J.; Liu, T.; Li, G.; Wu, W.; Ren, T. Arylpyrazole Schiff base: Synthesis and selective recognition of Cu2+. J. Mol. Struct. 2022, 1263, 133119. [Google Scholar] [CrossRef]
- Maeda, C.; Nomoto, S.; Takaishi, K.; Ema, T. Aggregation-Induced Circularly Polarized Luminescence from Boron Complexes with a Carbazolyl Schiff Base. Chem. Eur. J. 2020, 26, 13016–13021. [Google Scholar] [CrossRef] [PubMed]
- Kreye, O.; Mutlu, H.; Meier, M.A. Sustainable routes to polyurethane precursors. Green Chem. 2013, 15, 1431–1455. [Google Scholar] [CrossRef]
- Wu, J.; Kou, X.; Ju, H.; Zhang, H.; Yang, A.; Shen, R. Design, synthesis, and biological evaluation of naringenin carbamate derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2021, 49, 128316. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Wang, K.; Shi, J.; Liu, W.; Tan, Z. Design, synthesis, in-silico and biological evaluation of novel chalcone-O-carbamate derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 178, 726–739. [Google Scholar] [CrossRef]
- Chandra, R.; Ghorai, A.; Patra, G.K. A simple benzildihydrazone derived colorimetric and fluorescent ‘on–off-on’ sensor for sequential detection of copper (II) and cyanide ions in aqueous solution. Sens. Actuators B Chem. 2018, 255, 701–711. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Wolfbeis, O.S. Quenching of the luminescence of upconverting luminescent nanoparticles by heavy metal ions. Chem. Eur. J. 2011, 17, 14611–14617. [Google Scholar] [CrossRef]
- Bahaffi, S.O.; Aziz, A.A.A.; El-Naggar, M.M. Synthesis, spectral characterization, DNA binding ability and antibacterial screening of copper (II) complexes of symmetrical NOON tetradentate Schiff bases bearing different bridges. J. Mol. Struct. 2012, 1020, 188–196. [Google Scholar] [CrossRef]
- Ali, R.; Lang, T.; Saleh, S.M.; Meier, R.J.; Wolfbeis, O.S. Optical sensing scheme for carbon dioxide using a solvatochromic probe. Anal. Chem. 2011, 83, 2846–2851. [Google Scholar] [CrossRef]
- You, G.R.; Park, G.J.; Lee, J.J.; Kim, C. A colorimetric sensor for the sequential detection of Cu2+ and CN− in fully aqueous media: Practical performance of Cu2+. Dalton Trans. 2015, 44, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Torawane, P.; Sahoo, S.K.; Borse, A.; Kuwar, A. A new Schiff base as a turn-off fluorescent sensor for Cu2+ and its photophysical properties. J. Lumin. 2017, 32, 1426–1430. [Google Scholar] [CrossRef]
- Tang, L.; Zheng, Z.; Bian, Y. AN-(2-hydroxyethyl) piperazine dangled 2, 5-diphenyl-1, 3, 4-oxadiazole-based fluorescent sensor for selective relay recognition of Cu2+ and sulfide in water. J. Lumin. 2016, 31, 1456–1460. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, Z.L.; Yu, G.Q.; He, S.L.; Hu, Q.H.; Wu, Q.; Jiang, B.; Wei, G. Single Chemosensor for Double Analytes: Spectrophotometric Sensing of Cu2+ and Fluorogenic Sensing of Al3+ Under Aqueous Conditions. J. Fluoresc. 2016, 26, 43–51. [Google Scholar] [CrossRef]
- Chou, C.Y.; Liu, S.R.; Wu, S.P. A highly selective turn-on fluorescent sensor for Cu (II) based on an NSe 2 chelating moiety and its application in living cell imaging. Analyst 2013, 138, 3264–3270. [Google Scholar] [CrossRef]
- Park, G.J.; You, G.R.; Choi, Y.W.; Kim, C. A naked-eye chemosensor for simultaneous detection of iron and copper ions and its copper complex for colorimetric/fluorescent sensing of cyanide. Sens. Actuators B Chem. 2016, 229, 257–271. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganguly, A.; Uddin, M.R.; Mandal, S.; Alam, M.A.; Guchhait, N. Dual mode selective chemosensor for copper and fluoride ions: A fluorometric, colorimetric and theoretical investigation. Dalton Trans. 2016, 45, 11042–11051. [Google Scholar] [CrossRef]
- More, P.A.; Shankarling, G.S. Reversible ‘turn off’fluorescence response of Cu2+ ions towards 2-pyridyl quinoline based chemosensor with visible colour change. Sens. Actuators B Chem. 2017, 241, 552–559. [Google Scholar] [CrossRef]
- Roy, N.; Dutta, A.; Mondal, P.; Paul, P.C.; Sanjoy Singh, T. Coumarin based fluorescent probe for colorimetric detection of Fe3+ and fluorescence turn on-off response of Zn2+ and Cu2+. J. Fluoresc. 2017, 27, 1307–1321. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.; Ali, R.; Saleh, S.M.; Janiak, C. A novel water-soluble highly selective “switch-on” ionic liquid-based fluorescent chemi-sensor for Ca (II). J. Mol. Liq. 2017, 241, 308–315. [Google Scholar] [CrossRef]
- Karak, D.; Banerjee, A.; Sahana, A.; Guha, S.; Lohar, S.; Adhikari1, S.S.; Das, D. 9-Acridone-4-carboxylic acid as an efficient Cr(III) fluorescent sensor: Trace level detection, estimation and speciation studies. J. Hazard. Mater. 2011, 188, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Mariam, J.; Dongre, P.M.; Kothari, D.C. Study of interaction of silver nanoparticles with bovine serum albumin using fluorescence spectroscopy. J. Fluoresc. 2011, 21, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).