Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications
Abstract
1. Introduction
2. Screen-Printing Electrodes (SPEs)
3. Carbon Nanomaterials-Based SPEs
3.1. Graphene-SPEs
3.1.1. Metals
3.1.2. Agrochemicals
3.1.3. Hormones
3.1.4. Dopamine, Ascorbic Acid, Uric Acid and Estriol
3.1.5. Glucose
3.1.6. Drugs
3.1.7. Hydrogen Peroxide
3.1.8. Food Dyes
| Analyte | Electrode * | Technique * | Linear Range (µmol L−1) | LOD (µmol L−1) | Samples | Reference |
|---|---|---|---|---|---|---|
| [Ni(dmgH2] | ERGO-AuNPCCAgPPE | SW-AdCSV | --- | 32.19 μg L−1 | Drinking water | [57] |
| 4-Cyanophenol | rGO/MFO-2/SPCE | DPV | 0.001–700 | 0.0012 | Tap water, industrial river water, and fish | [58] |
| 6-mercaptopurine and 6-thioguanine | RGO-Cu2O/Fe2O3/SPGE | DPV | 0.05–400 | 0.03 | Urine and tablets | [59] |
| 8-hydroxy-2′-deoxyguanosine | Ag-TiO2-rGO/SPE | DPV | 0.05–25 | 1.0 × 10−2 | Human urine | [60] |
| Ampicillin | ErGO-SPE/AuNPs | CV; DPV | 1.0 × 10−5–1 | 1.0 × 10−6 | Buffer and spiked milk | [46] |
| Arsenic ions | BTA-rGO/SPCE | DPV | 2.0 × 10−3–4.0 × 10−2 | 2.89 × 10−3 | HCl solution (0.1 mol L−1) | [61] |
| Atrazine | AIRGOC/SPE | SWV | --- | 4.0 × 10−4 | Complex aqueous matrices | [30] |
| Azathioprine | Mn2O3–rGO/SPCE | DPV | 9–5.73 × 105 | 4.0 × 10−3 | Human blood serum and urine | [62] |
| Beta-amyloid biomarkers | Graphene/rGOSPE/Pyr-NHS | DPV | 1.1 × 10−5–5.5 × 10−2 | 2.39 × 10−6 | PBS, Plasma | [63] |
| Bisphenol A, 8-hydroxy-2′-deoxyguanosine and hydroquinone | ERGO/MWCNTs/SPCEs | EIS; DPV | 0.5–25.0, 0.05–50.0 and 0.5–100.0 | 1.4 × 10−2, 3.0 × 10−3 and 2.8 × 10−2 | Human urine | [64] |
| Brucella | GO/Fe3O4/MB/Ab2/Ppy | CV; DPV | 1.6 × 102–1.6 × 108 CFU mL−1 | 2.2 × 102 CFU mL−1 | --- | [65] |
| Carbaryl | rGO/AuNP/Nafion | DPV | 0.5–250 | 0.2 | River and tap water | [66] |
| Cd(II) and Pb(II) | Nafion/rGO-MWCNTs-COOH/SPCE | SWASV | 8.9 × 10−4–2.8 × 10−2 and 4.98 × 10−4–1.5 × 10−2 | 3.6 × 10−4 and 9.7 × 10−5 | Tap water and lake water | [67] |
| Cd(II) and Pb(II) | Bi/LC-rGO/DSPE | LSV; DPV | 8.9 × 10−3–0.27 and 4.8 × 10−3–0.14 | 8.9 × 10−6 and 3.9 × 10−6 | Decorative materials | [68] |
| Cd(II), Pb(II) and As(III) | (BiO)2CO3-rGO-Nafion | ASV | 0–0.440–0.240–0.67 | 7.12 × 10−3; 5.79 × 10−6; 3.20 × 10−2 | Water | [27] |
| Chloramphenicol | SPE/rGO–NHS–AuNFs | CV | 0.05–100 | 1.0 × 10−3 | Blood serum, poultry feed, milk, eggs, honey, and powdered milk | [49] |
| Chloramphenicol | Sr-ZnO@rGO/SPCE | CV; LSV | 0.190–410.84 | 0.131 | Milk and powdered milk | [50] |
| Clonazepam | CoOOH-rGO/SPCE | DPV | 0–350 | 3.8 × 10−2 | Beverages | [47] |
| Cortisol | GO-AgNPs/SPCE | CV | --- | --- | --- | [33] |
| Dexorobucin | 2D-g-C3N4/SDS/GNPs/SPE | DPV | 0.03–13.5 | 0.01 | Human plasma and urine | [45] |
| Diclofenac | PtNFs/rGO | CV; DPV | 0.1–100 | 4.0 × 10−2 | Human urine | [51] |
| DNA | ErGO + AuNUs | DPV | 5.0 × 10−10–9.5 × 10−7 | 1.6 × 10−10 | Doxorubicin | [69] |
| Dopamine | rGO-500/SPCE and of rGO-600/SPCE | CV; DPV | 0.5–20 and 0.5–20 | 1.11 and 1.23 | --- | [70] |
| Dopamine | WO3/SPE | SWV | --- | 0.87 | Urine | [34] |
| Dopamine | tyrosinase/chitosan/rGO/SPCE | CV | 0.4–8 and 40–500 | 2.2 × 10−2 | Urine | [37] |
| Dopamine and Ascorbic acid | PDbS–rGO/SPCE | LSV; CV | 0.1–300 and 10–1100 | 0.134 and 0.88 | Ex vivo brain tissues | [35] |
| Dopamine, uric acid and estriol | RGO/AgNWs/AgNPs/SPCE | LSV; CV; EIS; DPV | 0.6–50; 1–100 and 1–90 | 0.16, 0.58 and 0.58 | Maternal urine | [36] |
| E-cadherin | SPCE/rGO/PVA/anti-5mC/BSA/DNA-probe-Fe3O4-CA nanoparticles | CV; EIS | 1 × 10−4–20 ng mL−1 | 9 × 10−5 ng mL−1 | --- | [71] |
| Escherichia coli | SPCE-PANI-AuNPs | CV | 8.9 × 103–8.9 × 109 CFU mL−1 | 2.84 × 103 CFU mL−1 | Milk | [72] |
| Estradiol | CdTe-GO/SPE | PEC | 4.0 × 10−8–1.0 × 10−5 | 1.0 × 10−8 | Royal jelly, milk powder and urine | [31] |
| Fenamiphos | ERGO-SPE | CV; SWV | 0.25–25.0 | 0.067 | Tomato | [29] |
| Fenitrothion | GO-CMF/SPCE | CV | --- | 8.0 × 10−3 | Water | [73] |
| Ferulic acid | SPE(a)/rGO-AuNPs | CV | 1.0 × 10−2–1 | 3.1 × 10−3 | Orange peels | [74] |
| Follicle-Stimulating Hormone | rGO/MWCNTs/Thi/AuNP | DPV; CV; EIS | 1 mI U mL−1–250 mI U mL−1 | 0.05 mI U mL−1 | Serum | [75] |
| Food Colorants | rGO-methionine/SPCE | DPV | 1–10 and 10–100 for amaranth, 1–10 and 10–85 for tartrazine | 5.74 × 10−2, 4.8 × 10−2, and 3.6 × 10−2 | Real | [54] |
| Ganoderma boninense infection | ZnO-NPs/rGO/SPCE | DPV | --- | 1.75 mg L−1 | Oil palm leaves | [76] |
| Glucose | rGO-Au-SPE | 3.3 × 103–2.77 × 104 | 1.0 × 102 | Whole blood | [39] | |
| Glucose | Co@MoS2/Rgo/SPE | CV; CA | --- | 3.0 × 10−2 | --- | [41] |
| Glucose | PANINS@rGO/SPCE | CV; CA | 1.0 × 103–1.0 × 104 | 3.0 × 10−2 | --- | [40] |
| Glucose | GOx/AuNP/PANI/rGO/NH2-MWCNTs | A | 1.0 × 103–1.0 × 10−4 | 64 | Human blood; serum | [42] |
| Glucose | rGO-PEDOT:PSS/SPCE | CV | --- | 86.8 | --- | [38] |
| Glucose and cholesterol | ChOx/Pt/rGO/P3ABA/SPCE | CV | 2.5 × 102–6.0 × 103 and 2.5 × 102–4.0 × 103 | 44.3 and 40.5 | Human serum | [43] |
| Glycoside toxins | GO/AuNPs/MPBA | CV; DPV | 10–1000 | 3.4 | Food | [77] |
| Glyphosate | rGO/DWCNTs/Oct-Fe3O4/Cs/SPAuE | CV; SWV | 5.9 × 10−7–5.9 × 10−3 | 4.7 × 10−7 | River water | [28] |
| GPC3 | GPC/GPC3apt/RGO-Hemin/Au NPs/SPE | CV; EIS; DPV | 0.001–10.0 µg mL−1 | 2.86 ng mL−1 | Spiked human plasma | [78] |
| H2O2 | g-C3N4/rGO/SPE | CV; LSV | --- | 0.09 | Water | [52] |
| H2O2 | MnFe2O4/Rgo/SPCE | CV; EIS | 100–4.0 × 103 | 5.28 × 10−4 | --- | [53] |
| Hg(II) | TTU-rGO/CSPE | DPV | 0.50–0.0 | 9.97 × 10−2 | River water | [79] |
| Hg(II) | P-rGO/SPCE | DPV | 2 × 10−7–2 × 10−6 | 5.57 × 10−8 | HCl solution (0.1 mol L−1) | [25] |
| HPV-18 | SPE/rGO, MWCNT, Au nanoparticle, L-cysteine | DPV | 1.0 × 10−11–1.0 × 10−5 | 5.0 × 10−11 | Extracted DNA from clinical | [80] |
| HTLV-1 | rGO-PPy-(L-Cys)-AuNPs/SPCE | DPV | 1.0 × 10−10–100 | 2.0 × 10−11 | 0.1 mol L−1 PBS (pH 6.5) containing 100 nmol L−1 oligonucleotides based on TAX gene HTLV-1 | [81] |
| Human T-Lymphotropic Virus-1 | rGO-PPy-AuNPs/SPCE | DPV | 10−9–10−1 | 4.0 × 10−11 | Peripheral Blood Mononuclear Cells (PBMC) | [82] |
| Hydrazine | ZnFe2O4/RGO/SPE | DPV | 0.03–610.0 | 0.01 | Drinking water; tap water and river water | [83] |
| Hydroxylamine | NiCo2O4/RGO/SPE | DPV | 0.007–385.0 | 2.0 × 10−3 | Water | [84] |
| IgG and Glucose | CuII-GO/SPCE | SWV | 1.0–500 pg mL−1 | 0.20 pg mL−1 | Serum | [44] |
| Levofloxacin | Ag/AgVO3/N-rGO/SPCE | DPV | 0.09–671 | 7.92 × 10−6 | Biological and river | [85] |
| Linagliptin | CuBi2O4/rGO@MoS2/SPCE | DPV | (0.07–0.5) × 10−3 | 5.7 × 10−5 | Human plasma, urine and tablet | [86] |
| L-tryptophan | SPE/rGO/AuNPs | CV; DPV | 0.5–500 | 0.39 | Human plasma, serum, and saliva | [87] |
| Lysozyme | SPCE-Amino-rGO/IL/Amino-MSNs | EIS; DPV | 1.0 × 10−8–2.0 × 10−1 and 2.0 × 10−8–5.0 × 10−2 | 2.1 × 10−9 and 4.2 × 10−9 | Serum, tears, urine, wine, and egg white | [88] |
| Metol | CoMn2O4RGO/SPCE | DPV | 0.010–137.67 | 0.050 | Lake water | [89] |
| Metronidazole | C60-rGO-NF/SPE | SWV | 2.5 × 10−1–34 | 2.1 × 10−1 | Urine and serum | [48] |
| Microcystin-LR | rGO/Au/Apt/BSA/Mxene/cDNA-MB | CV; SWV | 1.0 × 10−6–5.0 × 10−6 | 4.0 × 10−8 | Tap water and surface water | [90] |
| microRNA | rGO/Au/SPE | CV; DPV | 1.0 × 10−8–1.0 × 10−6 | 1.0 × 10−6 | Saliva | [91] |
| MMP-1 | AuNP/PEI/Rgo/SPE | DPV | 1–50 ng mL−1 | Urine, saliva, bovine serum, and cell culture mediums | [92] | |
| Mn(II) | Au/L-cys/Fe3O4/RGO | SW-CSV | 9.1 × 10−3–5.5 | --- | Soil | [26] |
| Mycobacterium tuberculosis | NH2-rGO/TEMPO-nanocellulose/SPE | DPV | 1.0 × 10−4–1.0 × 10−7 | 3.14 × 10−8 | M. tuberculosis. | [93] |
| Na+ | AgNPs/GO/SPE | CV | 0–1.0 × 10−5 | 9.34 × 103 | Fish sauce and seasoning powder of instant noodle | [94] |
| Nitrite | Au/NiO/rGO/SPCE | CV; DPV; CA | 1–500 | 0.2 | Water at different locations in Hainan Province | [95] |
| Nitrite | ERGO/β-CD/CdS/SPCE | CV | 0.05–447 | 2.1 × 10−2 | Water | [96] |
| Nitrite | Ag/rGO/β-CD/SPCE | CV | 1–2000 | 0.24 | (Spiked) pickles | [97] |
| Ochratoxin A | SPCEs/GO/cDNA-aptamer/3D-rGO-AuNPs | CV; DPV | 2.5 × 10−8–2.5 × 10−3 | 1.2 × 10−8 | Rice and oat | [98] |
| Pb(II) | rGO/SPCE | DPV | 5.0 × 10−5–8.67 × 10−3 | 5.0 × 10−5 | 0.1 mol L−1 HCl | [99] |
| Pemetrexed | M-GRNs/SPCE | DPV | 0.05–2.2 | 9.7 × 10−3 | Human plasma | [9] |
| Pork | (SPC-RGO) | DPV | 0–10.0 µg mL−1 | 1.76 µg mL−1 | Pork, chicken, and beef | [100] |
| Progesterone | AuNPs/AMBI/rGO/SPCE | CV; EIS; SWV | 9.0 × 10−4–27 | 2.8 × 10−4 | Calf serum and milk | [32] |
| Ractopamine | Fe3O4/GO-MSPE | CV; DPV | 0.05–10 and 10–100 | 1.3 × 10−2 | Spiked real pork | [101] |
| ROS | rGO-CeO2@Cyt c hydrogel/SPE | CV; DPV | 5–30 | 0.166, 0.338 and 0.229 | PBS solutions (pH 7.4) | [102] |
| Staphylococcal Enterotoxin B | rGOAuNUs/SPCE | DPV; CV; EIS | 5.0 × 10−9–5.0 × 10−7 | 2.1 × 10−10 | --- | [103] |
| Sudan I and bisphenol A | CuO/GO/SPGE | DPV | 0.3–700.0 | 0.093 | Ketchup sauce, tomato paste chili powder and water | [56] |
| Sulfadiazine | AuNP-VS2-rGO/SPCEs | SWV | 1.0 × 10−2–3.45 × 10−1 | 4.4 × 10−4 | Contaminated water | [104] |
| Sulfite | rGO/PPy NTs-GSPE | LSV; CV; DPV; CA | 0.04–565.0 | 0.01 | Water and apple juice | [8] |
| Sunset yellow and tartrazine | rGO/NiBTC/SPCEs | DPV | 0.05–5.0 and 0.075–5.0 | 0.025 and 0.05 | Drinks | [55] |
| Tartrazine | Pt/CQDs@rGO/SPCE | DPV | 0.01–1.57 and 1.57–9.3 | 7.93 × 10−3 | Candy, soft drink, jelly powder and water | [105] |
| Tetracycline | AdTDPV-ERGO-SPEs | DPV | (2.11 ± 0.25) × 10−8–(2.09 ± 1.39) × 10−7 | 12 | Milk and water | [106] |
3.2. Carbon Nanotubes-SPEs
3.2.1. Environmental Analysis
3.2.2. Human Body Fluids Analysis

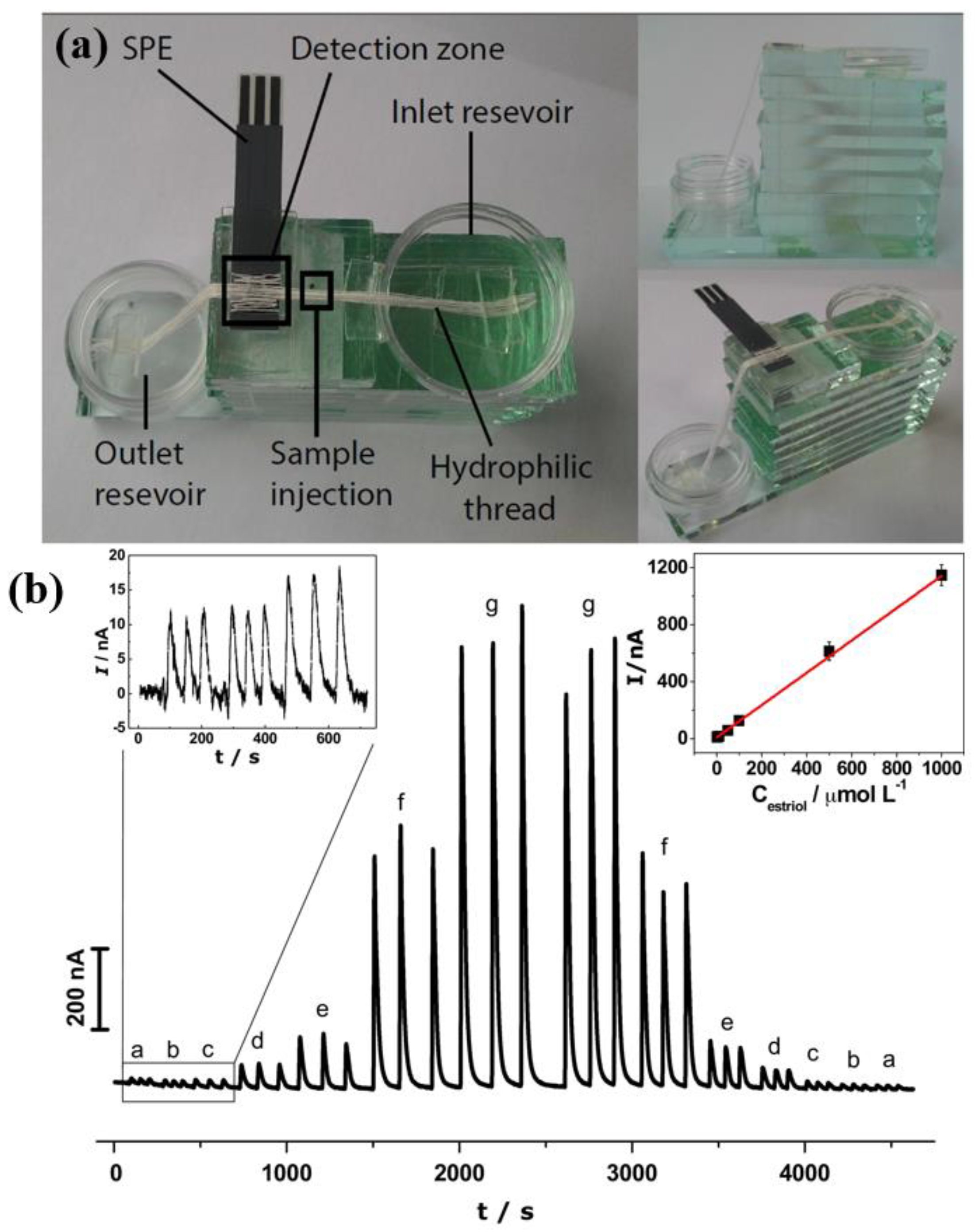
3.2.3. Food Products

| Analyte | Electrode * | Technique * | Linear Range (µmol L−1) | LOD (µmol L−1) | Samples | Reference |
|---|---|---|---|---|---|---|
| 8-hydroxyguanine | MWCNTs-COOH/SPCE | DPV | 3.0 × 10−1–12 | 5.7 × 10−1 | Electrochemical monitoring of stability of 8-hydroxyguanine | [125] |
| Antihistamine drug bilastine (BIL) | MWCNTs-SPCE | CV and LSV | 2.29 × 10−1–4.58 | 1.3 × 10−1 | Pharmaceutical formulations and urine | [119] |
| Bilirubin | MWCNTs-SPCE and GO-SPCE | CV | MWCNTs 0.5–500 and graphene 0.1–600 | MWCNTs (3.0 ± 0.22) × 10−4 and Graphene (1.0 ± 0.18) × 10−4 | Blood serum | [120] |
| Catechol | f-MWCNTs/SPCE | CV | 8.0 × 10−2–725 | 3.0 × 10−2 | Water | [113] |
| Caffeic acid | MWCNT/SPEs | DPV | 2.0–50.0 | 0.2 | Tea | [124] |
| Cd(II) | CuF/GCE and CuF/CN/SPE | ASV | 5 × 10−4–5 × 10−1 for CuF/GCE and 3 × 10−4–3 × 10−1 for CuF/CN/SPE | CuF/GCE: 1.7 × 10−4 and CuF/CN/SPE: 1.3 × 10−4 | Water | [126] |
| Cd(II) and Pb(II) | RGO-MWCNT-AuNP/SPE | SWSV | 8.90 × 10−3–7.12 × 10−1 for Cd(II) and 4.83 × 10−3–3.86 × 10−1 for Pb(II) | 6.23 × 10−3 for Cd(II) and 1.45 × 10−3 for Pb(II) | Soil | [114] |
| Diclofenac | SPCE/MWCNTs-COOH | DPAdSV | 1.0 × 10−4–1.0 × 10−2 | 2.8 × 10−5 | Water | [127] |
| Dopamine | mMWCNTs/SPE | CV | 5–8 | 4.3 × 10−1 | Spiked human blood serum | [121] |
| Dopamine | Modified GCE and SPCE with sodium bis[N-2-oxyphenyl-5-bromosalicylideneiminato-ONO] ruthenate(III), MWCNTs and Nafion | CV, DPV, and flow injection amperometry | up to 326 | (7.18 ± 2.61) × 10−1 | Ampoules of dopamine hydrochloride | [128] |
| Estriol | CNT-SPCE | Amperommetry; CV | 1–1.0 × 103 | 5.3 × 10−1 | Commercial pharmaceutical formulation | [123] |
| H2O2 | SPCE/PAKB NPs/CNTs | CV and amperometry | 20–6.48 × 103 | 2.7 | Milk and water | [129] |
| Imidacloprid | IL-SWCNT/SPC | LSV; CV | 11–5.75 | 8.2 × 10−1 | Spiked commercial honey | [130] |
| Indole | MWCNTs-CS/SPCE | CV; DPV | 4.27 × 10−2–8.54 × 10−1 | 4.27 × 10−3 | Plasma | [131] |
| K4FeCN6, H2O2 and nicotinamide adenine dinucleotide (NAD+/NADH) | MWCNT/GP/SPCE | CV | 10–1.0 × 103 | 3.1 for K4FeCN6, 7.1 × 106 for H2O2 and 3.6 for NADH | --- | [132] |
| Kojic acid | MWCNTs-CS/SPCE | DPV | 20–5.0 × 103 | 16 | Apple vinegar and Rice vinegar | [133] |
| Levothyroxine (LT4) | SPCEs, containing CNTs, graphene, and AuNPs individually | DPV | 5.0 × 10−4–3.0 × 10−3 (for CNT) | 1.5 × 10−1 | Diluted fetal bovine serum | [134] |
| Methyldopa | AuNPs/CNT/SPCE | Flow injection amperometry | 2.0 × 10−1–100 | 1.0 × 10−1 | Pharmaceutical and urine | [135] |
| Ochratoxin | SPE on PET and PDMS, coated with SWCNTs and immobilized with anti-OTA antibodies. | CV | 2.48 × 10−5–2.48 × 10−3 | PET 1.98 × 10−4 and PDMS 3.22 × 10−4 | Grape juice and wine | [136] |
| Paracetamol, Ibuprofen and Caffeine | SPCE, SPCNTE, SPCNFE, and SPGPHE | DPV | 1.32 × 10−2–6.62 × 10−1 for PA, 9.70 × 10−3–4.85 × 10−1 for IB and 1.03 × 10−2–5.15 × 10−1 for CF | 5.95 × 10−1 for PA, 10.7 for IB, and 1.03 for CF | Spikes tap water and hospital wastewater | [137] |
| Paraquat | SPCE-CNT/Nafion | CV; DPV | 5.4 × 10−1–4.3 | 1.7 × 10−1 | Natural water | [118] |
| Piperazine | CNTs-Nafion/GCE and SPCE | LSV; DPV | 4.0 × 10−1–12 | 1.1 × 10−1 | Human Plasma | [138] |
| Polyphenols content | GCE and SWCNTs-SPCE | SWV | --- | --- | Red wines | [139] |
| Secretion of electroactive metabolite(s) in the extracellular matrix | MWCNTs/SPE | CV | 1.0 × 105–1.1 × 106 OD600 | 1.0 × 105 OD600 | Bacterial cell suspensions | [140] |
| Stimulant modafinil | SPE-CNT | AdSWV | 7.5–300 | 2 | Saliva | [122] |
| Tl(I) | SPCE/MWCNTs/BiF | ASV | 1.0 × 10−2–1 | 2.8 × 10−3 | Spiked water from the Vistula River | [115] |
| Thrombin | SWCNTs/SPCE | CV | 1.0 × 10−1–1 | 2.0 × 10−5 | --- | [141] |
| U(VI) | MWCNTs/SPE (electrode III) and IL-MWCNTs/SPE (electrode VII) | Potentiometry | 10–1.0 × 105 for electrode III and 4.7 × 10−1–1.0 × 105 for electrode VII | 10 for electrode III and 4.7 × 10−1 for electrode VII | Water | [116] |
| Zn(II), Pb(II) and Cu(II) | SPCE/CNTs/AuNP | DPASV | Zn2+: 1.51 × 10−2–1.84; Pb2+: 4.78 × 10−2–5.79 × 10−1, and Cu2+: 1.56 × 10−2–1.89 × 10−1 | Zn2+: 1.53 × 10−2–5.35 × 10−2; Pb2+: 7.24 × 10−3–2.41 × 10−2; Cu2+: 1.57 × 10−3–5.19 × 10−3 for Cu2+ | --- | [117] |
3.3. Carbon Black SPEs
3.3.1. Drugs
3.3.2. Phenolic Compounds
3.3.3. Uric acid, Dopamine, Epinephrine, Paracetamol
3.3.4. Na+ Ion
3.3.5. Marine Toxins
3.3.6. Aflatoxin B1
3.3.7. SARS-CoV-2 Coronavirus
| Analyte | Electrode * | Technique * | Linear Range (µmol L−1) | LOD (µmol L−1) | Samples | Reference |
|---|---|---|---|---|---|---|
| Aflatoxin B1 | CB-SPE | DPV | 1.06 × 10−4–2.35 × 10−3 (buffer) and 1.82 × 10−4–4.99 × 10−3 (extract) | 4.16 × 10−5 (buffer) and 7.68 × 10−5 (extract) | Corn extract | [145] |
| Domoic acid (DOA) | CB-SPE | Amperometry | 1.61 × 10−2–1.99 × 10−2 (buffer) 1.61 × 10−2–1.86 × 10−1 (scallop extract) | 1.28 × 10−3 (buffer) 2.25 × 10−3 (buffer) | Buffer and Scallop extract | [142] |
| Dopamine (DA) and Epinephrine (EP) | aGO/CB-OMNiDIP-adduct/SPCE | DPV | 7.57 × 10−4–4.07 × 10−2 (DA) and 4.04 × 10−4–9.99 × 10−3 (EP) | 1.83 × 10−4–3.98 × 10−4 (DA) 9.28 × 10−5–1.09 × 10−4 (EP) | Aqueous, blood serum, urine and pharmaceutical | [151] |
| Dopamine (DA) Epinephrine (EP) and acetaminophen (ACP) | SPCE/CB-ERGO | SWV | 4.9–19 (DA) 9.9–95 (EP) 9.9–95 (ACP) | 4.1 × 10−1 (DA) 1.8 (EP) 1.5 (ACP) | Buffer | [150] |
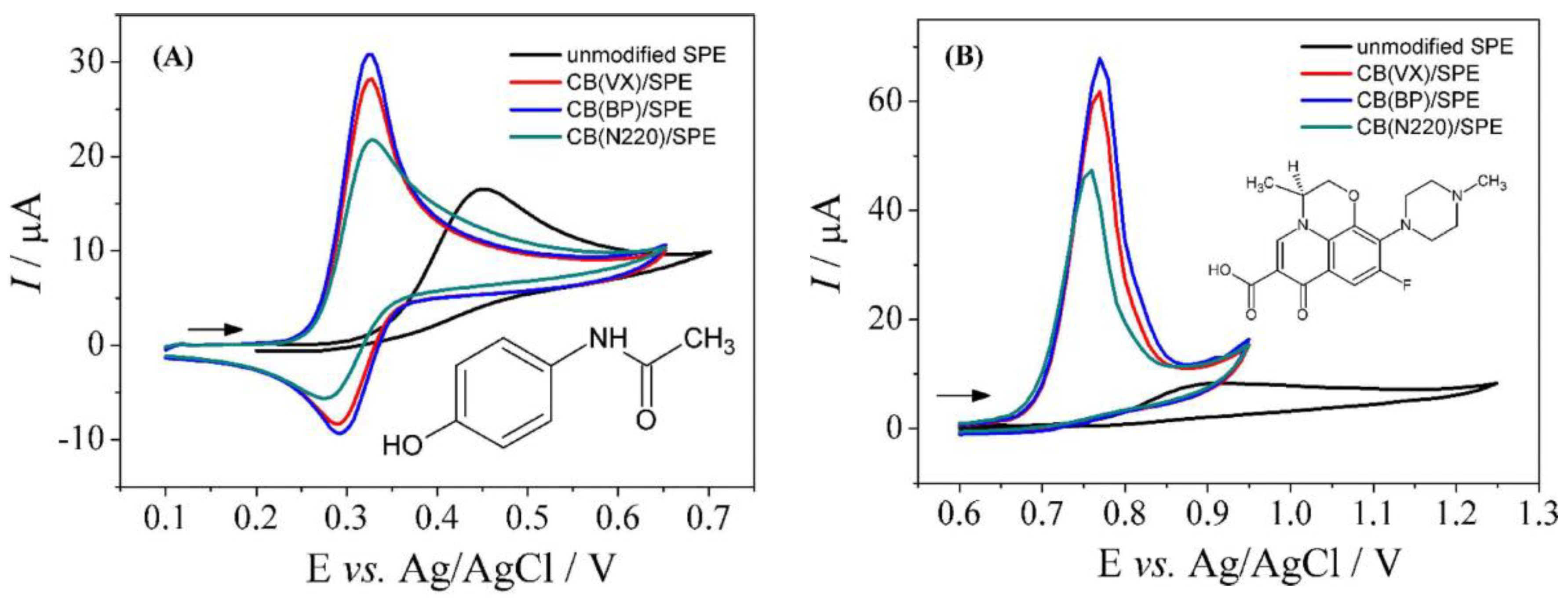
| Levofloxacin (LVF) and acetaminophen (ACP) | CB(BP4750)-SPE | SWV | 0.90–70.0 (LVF) 4.0–80.0 (ACP) | 0.42 (LVF) 2.6 (ACP) | River water | [147] |
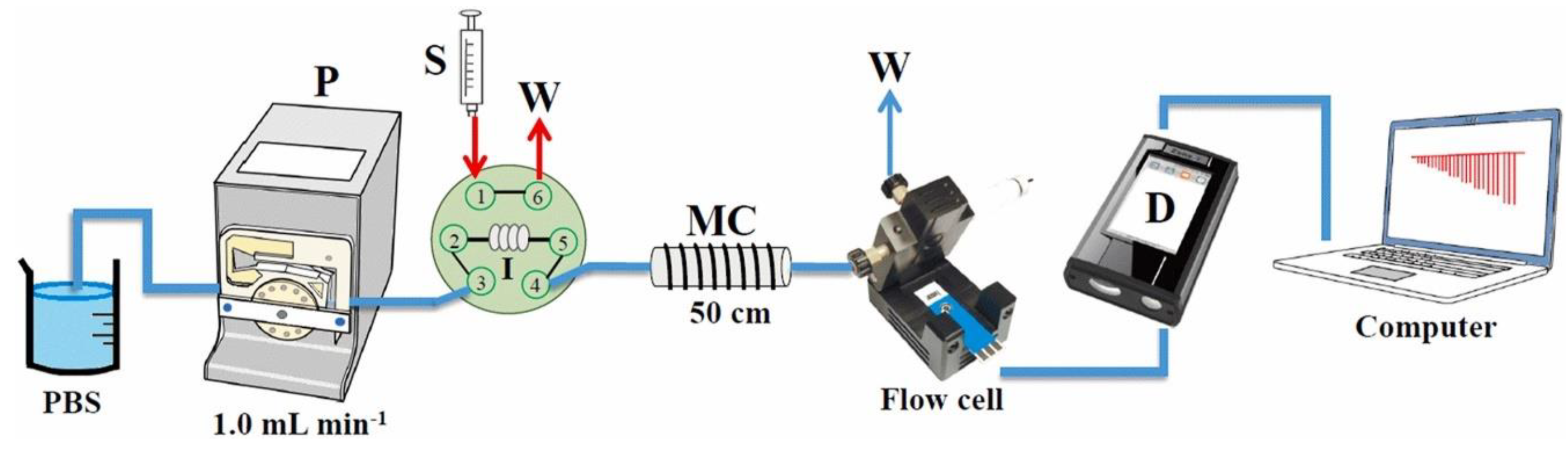
| Na+ ions | CB-SPE | DPV | 1.0 × 103 and 1.0 × 106 | 63 | Sweat | [144] |
| O-diphenols hydroxyty rosol (OLEU) and oleuropei (HYT) | CB-MoS2-SPE | DPV | 0.3–30 (OLEU) 2–100 (HYT) | 0.1 (OLEU) 1 (HYT) | Olive oil | [148] |
| Okadaic acid (OA) and domoic acid (DOA) | CB-SPE | DPV | 1.28 × 10−2–2.31 × 10−1 (DOA in buffer), 1.28 × 10−2–1.09 × 10−1 (DOA in mussel extract), 3.35 × 10−4–4.10 × 10−3 (OA in buffer) and 4.35 × 10−4–4.84 × 10−3 (OA in mussel extract) | 5.46 × 10−3 (DOA in buffer), 6.10 × 10−3 (DOA in mussel extract), 1.86 × 10−4 (OA in buffer), and 2.24 × 10−4 (OA in mussel extract) | Buffer and Mussel Extract | [143] |
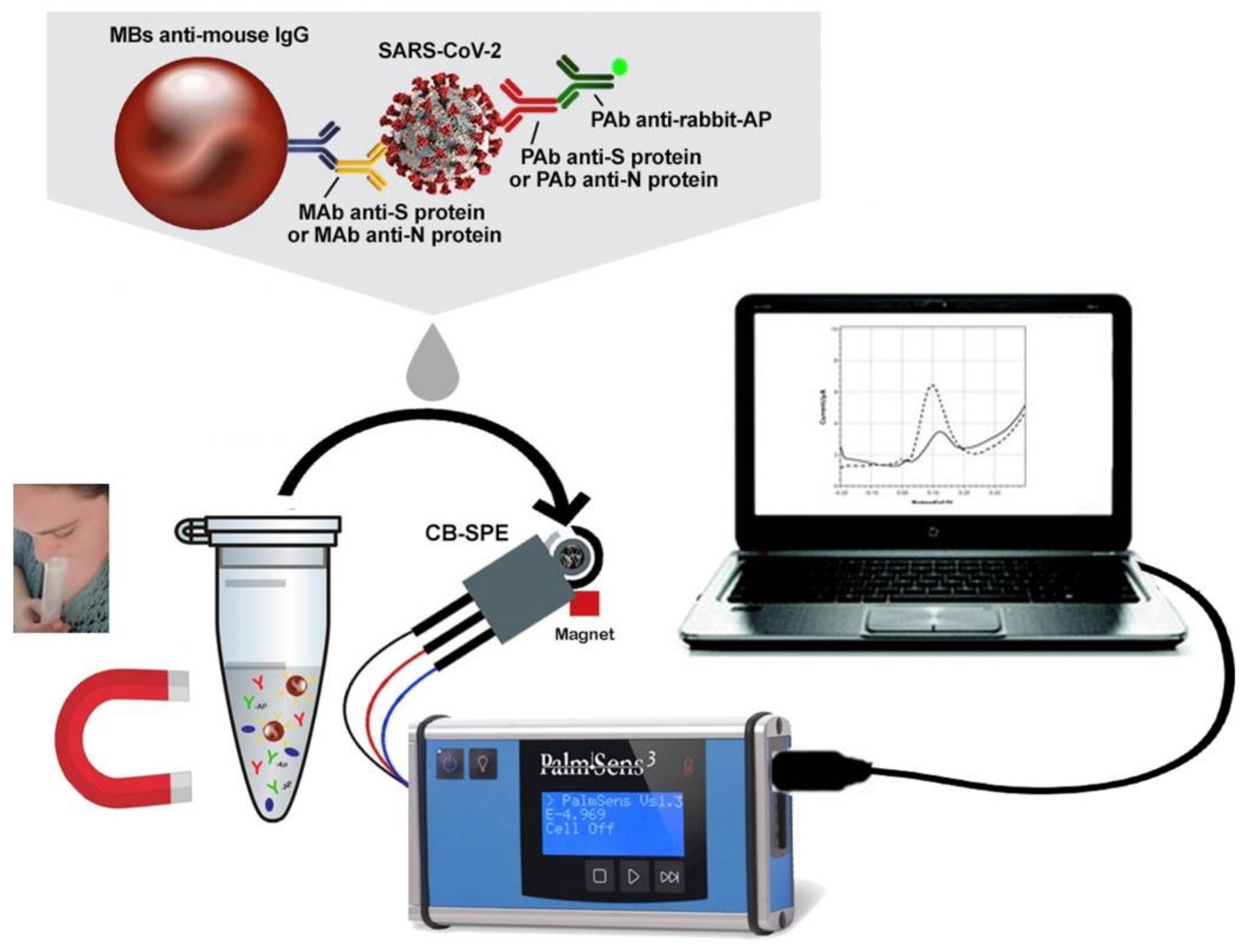
| Spike protein (S) and nucleocapsid protein (N) | CB-SPE | DPV | --- | 19 ng mL−1 (S) 8 ng mL−1 (N) | Saliva | [152] |
| Uric acid | CB-GO-SPCE | Flow injection amperometry | 0.05–2000 | 0.01 | Urine | [149] |
3.4. Carbon Quantum Dot SPEs
3.4.1. Carcinoembryonic Antigen
3.4.2. Food Additives
3.4.3. Glucose
3.4.4. Ifosfamide
3.4.5. Dopamine, Tyrosine, Theophylline, Ascorbic Acid, Uric Acid
3.4.6. Other Species of Interest
| Analyte | Electrode | Technique | Linear Range (µmol L−1) | LOD (µmol L−1) | Samples | Reference |
|---|---|---|---|---|---|---|
| AFP | CdS QDs | ASV differential pulse | 5–500 | 4.9 | Human blood serum | [166] |
| Antimicrobial resistance (RAM) GEMI | 1 to 7 | DPV | 1, 3 and 4: 10–1.0 × 104; 2: 1–1.0 × 103; 5, 6 and 7: 1–1.0 ×104 | 0.21 | Pharmaceutical formulation and water | [163] |
| Ascorbic acid, dopamine, and uric acid | GQDs/IL–SPCE | DPV | 25–400; 0.2–10 and 0.5–20 | 6.64; 0.06 and 0.03 | Vitamin C tablets, dopamine injection | [161] |
| Carcinoembryonic (CEA) | Bio AuNP/Pol/Cu2O–CD/SPE | DPV, EIS, CV and Chronoamperometry | 3.67 × 106–3.67 × 103 | 0.697 | Human blood serum and pharmaceutical formulations | [167] |
| CEA | CdS QDs | ASV differential pulse | 5–500 | 3.0 | Human blood serum | [166] |
| Chromium | PANI/GQD/SPCE | SWV | 0.05–5 | 0.005 | Water | [162] |
| Clozapine | Go/Fe3O4/SiO2 nanocomposite | DPV | 0.10–700 | 0.03 | Urine and clozapine tablet | [168] |
| Diethylstilbestrol (DES) | GQD/SPE | LSV | 0.05–7.5 | 8.8 × 10−3 | Synthetic urine, tap water | [169] |
| Dopamine | CS/N, GQDs@SPCE | CV DPV | 1–100 and 100–200 | 0.145 | Human urine | [155] |
| Dopamine and tyrosine | GQD/SPE | CV DPV | 0.1–1000 and 1.0–900 | 0.05 and 0.5 | Human urine | [154] |
| Ethinylestradiol | (mag@MIP)–GQDs–FG–NF/SPE | CV SWV | 1.0 × 10−2–2.5 | 2.6 × 10−3 | Water, serum, and urine | [156] |
| Glycose | PEDOT:PSS/Ti3C2/GQD | DPV | 0–500 | 65 | Human blood serum | [153] |
| ICG | CdS QDs | ASV differential pulse | 1–1.0 × 10−2 | 0.9 | Human blood serum | [166] |
| Ifosfamide | m–GQDs–MIP | DPASV | --- | 4.2 × 10−4 | Blood plasma, urine, and pharmaceutical formulations | [159] |
| NaDH | MagNP/C–dots/SPE | DPV | 0.2–5 | 0.15 | Serum | [164] |
| Progesterone | GQDs–NiO–AuNFs/f–MWCNTs (SPCE) | CV DPV | 1.0 × 10−4–1 | 1.86 | Human blood serum and pharmaceutical formulations | [170] |
| Solatol | MIP/AuNPs/GQD–SH/SPCE | DPV | 0.1–250 | 0.035 | Blood serum and tablets | [171] |
| Tartrazine dye (TRT) | Pt/CQDs@rGO/SPCE | DPV | 0.01–1.57 and 1.57–9.3 | 7.93 × 10−3 | Candy, soft drinks, jelly powder, and water | [105] |
| Theophylline | GQD/SPEs | CV DPV Chronoamperometry | 1–700 | 0.2 | Theophylline oral solution and urine | [160] |
| Tyrosine kinase | GQDs/SPCE | DPV | --- | 1.84 × 106 | Human blood serum | [165] |
| Vanillin | GQD@Nafion/AuNP–SPCE | LSV DPV | 0.66–33 | 3.2 | White-milk chocolate, custards, and sugar | [158] |
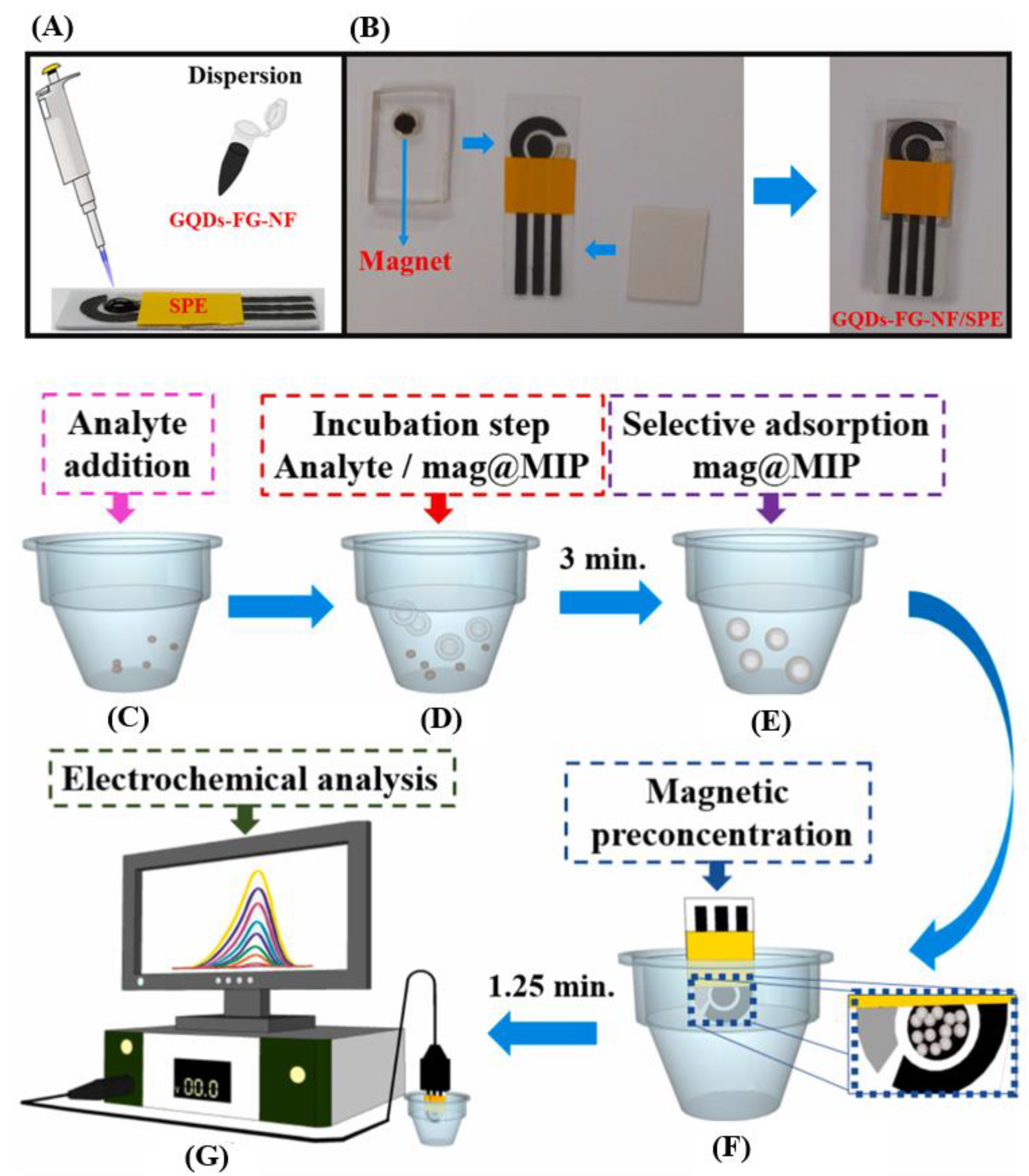
3.5. Other Materials
3.5.1. SPE Modified with Graphitic Carbon Nitride
3.5.2. SPE Modified with Nanospheres
3.5.3. SPE Modified with Biochar
3.6. Comparisons between Carbonaceous Nanomaterials
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simões, F.R.; Xavier, M.G. Electrochemical Sensors. In Nanoscience and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–178. [Google Scholar]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Z.; Liu, J.; Wang, X. Screen-printed electrode-based biosensors modified with functional nucleic acid probes and their applications in this pandemic age: A review. Anal. Methods 2022, 14, 2961–2975. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-X.; Lei, X.-W.; Natsuki, T. Review on Carbon Nanomaterials-Based Nano-Mass and Nano-Force Sensors by Theoretical Analysis of Vibration Behavior. Sensors 2021, 21, 1907. [Google Scholar] [CrossRef] [PubMed]
- Zittel, H.E.; Miller, F.J. A Glassy-Carbon Electrode for Voltammetry. Anal. Chem. 1965, 37, 200–203. [Google Scholar] [CrossRef]
- Zhou, K.-G.; Zhang, H.-L. Graphene: Synthesis, Characterization, and Applications. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–21. [Google Scholar]
- Jahani, P.M.; Beitollahi, H.; Tajik, S. Surface amplification of graphite screen printed electrode using reduced graphene oxide/polypyrrole nanotubes nanocomposite; a powerful electrochemical strategy for determination of sulfite in food samples. Food Chem. Toxicol. 2022, 167, 113274. [Google Scholar] [CrossRef]
- Er, E. A portable electrochemical platform based on graphene nanosheets by metal intercalation engineering for anticancer drug pemetrexed sensing. FlatChem 2022, 33, 100353. [Google Scholar] [CrossRef]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-iron selenides embedded in porous carbon nanofibers for simultaneous electrochemical detection of trace of hydroquinone, catechol and resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Lakhera, P.; Chaudhary, V.; Jha, A.; Singh, R.; Kush, P.; Kumar, P. Recent developments and fabrication of the different electrochemical biosensors based on modified screen printed and glassy carbon electrodes for the early diagnosis of diverse breast cancer biomarkers. Mater. Today Chem. 2022, 26, 101129. [Google Scholar] [CrossRef]
- Fava, E.L.; do Prado, T.M.; Garcia-Filho, A.; Silva, T.A.; Cincotto, F.H.; de Moraes, F.C.; Faria, R.C.; Fatibello-Filho, O. Non-enzymatic electrochemical determination of creatinine using a novel screen-printed microcell. Talanta 2020, 207, 120277. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Li, T.; Yu, Y.; Yong, J.; Bahk, J.-H.; Skafidas, E. Recent advances in printable thermoelectric devices: Materials, printing techniques, and applications. RSC Adv. 2020, 10, 8421–8434. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.-W.; Xiu, G.; Long, Y.-T. Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Fonseca, W.T.; de Oliveira Setti, G.; Faria, R.C. Fast and flexible strategy to produce electrochemical paper-based analytical devices using a craft cutter printer to create wax barrier and screen-printed electrodes. Talanta 2019, 195, 480–489. [Google Scholar] [CrossRef]
- Sher, M.; Faheem, A.; Asghar, W.; Cinti, S. Nano-engineered screen-printed electrodes: A dynamic tool for detection of viruses. TrAC Trends Anal. Chem. 2021, 143, 116374. [Google Scholar] [CrossRef] [PubMed]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-printing inks for the fabrication of solid oxide fuel cell films: A review. Renew. Sustain. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Imran, M.; Ahmed, S.; Abdullah, A.Z.; Hakami, J.; Chaudhary, A.A.; Rudayni, H.A.; Khan, S.-U.-D.; Khan, A.; Basher, N.S. Nanostructured material-based optical and electrochemical detection of amoxicillin antibiotic. Luminescence 2022, 36, 1–23. [Google Scholar] [CrossRef]
- Chu, Z.; Peng, J.; Jin, W. Advanced nanomaterial inks for screen-printed chemical sensors. Sens. Actuators B Chem. 2017, 243, 919–926. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, S.; Patel, S.S.; Singh, S.P.; Kumar, P.; Khan, M.A.; Awasthi, H.; Singh, S. Carbon nanomaterials for the detection of pesticide residues in food: A review. Environ. Pollut. 2022, 310, 119804. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Beitollahi, H.; Kumar, P.S.; Tajik, S.; Jahani, P.M.; Karimi, F.; Karaman, C.; Vasseghian, Y.; Baghayeri, M.; Rouhi, J.; et al. Recent advances in carbon nanomaterials-based electrochemical sensors for food azo dyes detection. Food Chem. Toxicol. 2022, 164, 112961. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Gumpu, M.B.; Kumar, R.G.; Lakshmanakumar, M.; Nesakumar, N. Amperometric Detection of Mercury Ions Using Piperazine-Functionalized Reduced Graphene Oxide as an Efficient Sensing Platform. ChemistrySelect 2022, 7, e202103601. [Google Scholar] [CrossRef]
- Mc Eleney, C.; Alves, S.; Mc Crudden, D. Novel magneto-electrochemical determination of Mn(II). J. Electroanal. Chem. 2021, 900, 115734. [Google Scholar] [CrossRef]
- Zhao, G.; Tran, T.-T.; Modha, S.; Sedki, M.; Myung, N.V.; Jassby, D.; Mulchandani, A. Multiplexed Anodic Stripping Voltammetry Detection of Heavy Metals in Water Using Nanocomposites Modified Screen-Printed Electrodes Integrated with a 3D-Printed Flow Cell. Front. Chem. 2022, 10, 60. [Google Scholar] [CrossRef]
- Thanh, C.T.; Binh, N.H.; Duoc, P.N.D.; Thu, V.T.; Van Trinh, P.; Anh, N.N.; Van Tu, N.; Tuyen, N.V.; Van Quynh, N.; Tu, V.C.; et al. Electrochemical Sensor Based on Reduced Graphene Oxide/Double-Walled Carbon Nanotubes/Octahedral Fe3O4/Chitosan Composite for Glyphosate Detection. Bull. Environ. Contam. Toxicol. 2021, 106, 1017–1023. [Google Scholar] [CrossRef]
- Gevaerd, A.; Watanabe, E.Y.; Fernandes, K.; Papi, M.A.P.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Electrochemically Reduced Graphene Oxide as Screen-printed Electrode Modifier for Fenamiphos Determination. Electroanalysis 2020, 32, 1689–1695. [Google Scholar] [CrossRef]
- Ahmed, S.; Shaikh, H.; Solangi, A.; Barek, J.; Sirajuddin; Denizli, A.; Agheem, M.H. A composite of imprinted polypyrrole beads and reduced graphene oxide for specific electrochemical sensing of atrazine in complex matrices. Mon. Für Chem.-Chem. Mon. 2020, 151, 1271–1282. [Google Scholar] [CrossRef]
- Hao, X.; Guan, Y.; Liu, F.; Zhang, Y.; Zhai, Y.; Niu, L. Ultrasensitive detection and application of estradiol based on nucleic acid aptamer and circulating amplification technology. J. Electroanal. Chem. 2022, 913, 116284. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, L.; Yan, Y.; Cao, R.; Zhang, J. An electrocatalytic active AuNPs/5-Amino-2-mercaptobenzimidazole/rGO/SPCE composite electrode for ultrasensitive detection of progesterone. J. Electroanal. Chem. 2021, 882, 115023. [Google Scholar] [CrossRef]
- Sonawane, A.; Mujawar, M.A.; Bhansali, S. Atmospheric Plasma Treatment Enhances the Biosensing Properties of Graphene Oxide-Silver Nanoparticle Composite. J. Electrochem. Soc. 2019, 166, B3084. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Design and fabrication of WO3/SPE for dopamine sensing application. Mater. Chem. Phys. 2022, 287, 126298. [Google Scholar] [CrossRef]
- Thirumalai, D.; Lee, S.; Kwon, M.; Paik, H.; Lee, J.; Chang, S.-C. Disposable Voltammetric Sensor Modified with Block Copolymer-Dispersed Graphene for Simultaneous Determination of Dopamine and Ascorbic Acid in Ex Vivo Mouse Brain Tissue. Biosensors 2021, 11, 368. [Google Scholar] [CrossRef]
- Zhao, Q.; Faraj, Y.; Liu, L.-Y.; Wang, W.; Xie, R.; Liu, Z.; Ju, X.-J.; Wei, J.; Chu, L.-Y. Simultaneous determination of dopamine, uric acid and estriol in maternal urine samples based on the synergetic effect of reduced graphene oxide, silver nanowires and silver nanoparticles in their ternary 3D nanocomposite. Microchem. J. 2020, 158, 105185. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chou, Y.-C.; Tsai, J.-H.; Huang, T.-M.; Chen, J.-Z.; Yeh, Y.-C. Tyrosinase/Chitosan/Reduced Graphene Oxide Modified Screen-Printed Carbon Electrode for Sensitive and Interference-Free Detection of Dopamine. Appl. Sci. 2019, 9, 622. [Google Scholar] [CrossRef]
- Abd-Wahab, F.; Guthoos, H.A.; Salim, W.W. Solid-State rGO-PEDOT:PSS Transducing Material for Cost-Effective Enzymatic Sensing. Biosensors 2019, 9, 36. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khoshfetrat, S.M.; Kabiri, S.; Fotouhi, L.; Dorraji, P.S.; Omidfar, K. Impedimetric Paper-Based Enzymatic Biosensor Using Electrospun Cellulose Acetate Nanofiber and Reduced Graphene Oxide for Detection of Glucose From Whole Blood. IEEE Sens. J. 2021, 21, 9210–9217. [Google Scholar] [CrossRef]
- Kailasa, S.; Reddy, R.K.K.; Reddy, M.S.B.; Rani, B.G.; Maseed, H.; Sathyavathi, R.; Rao, K.V. High sensitive polyaniline nanosheets (PANINS) @rGO as non-enzymatic glucose sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 2926–2937. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Hu, Y.; Xu, J.; Sun, D.; Hu, T.; Ni, Z. Screen-printed electrochemical biosensor based on a ternary Co@MoS2/rGO functionalized electrode for high-performance non-enzymatic glucose sensing. Biomed. Microdevices 2020, 22, 17. [Google Scholar] [CrossRef]
- Maity, D.; Minitha, C.R.; RT, R.K. Glucose oxidase immobilized amine terminated multiwall carbon nanotubes/reduced graphene oxide/polyaniline/gold nanoparticles modified screen-printed carbon electrode for highly sensitive amperometric glucose detection. Mater. Sci. Eng. C 2019, 105, 110075. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly(3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef] [PubMed]
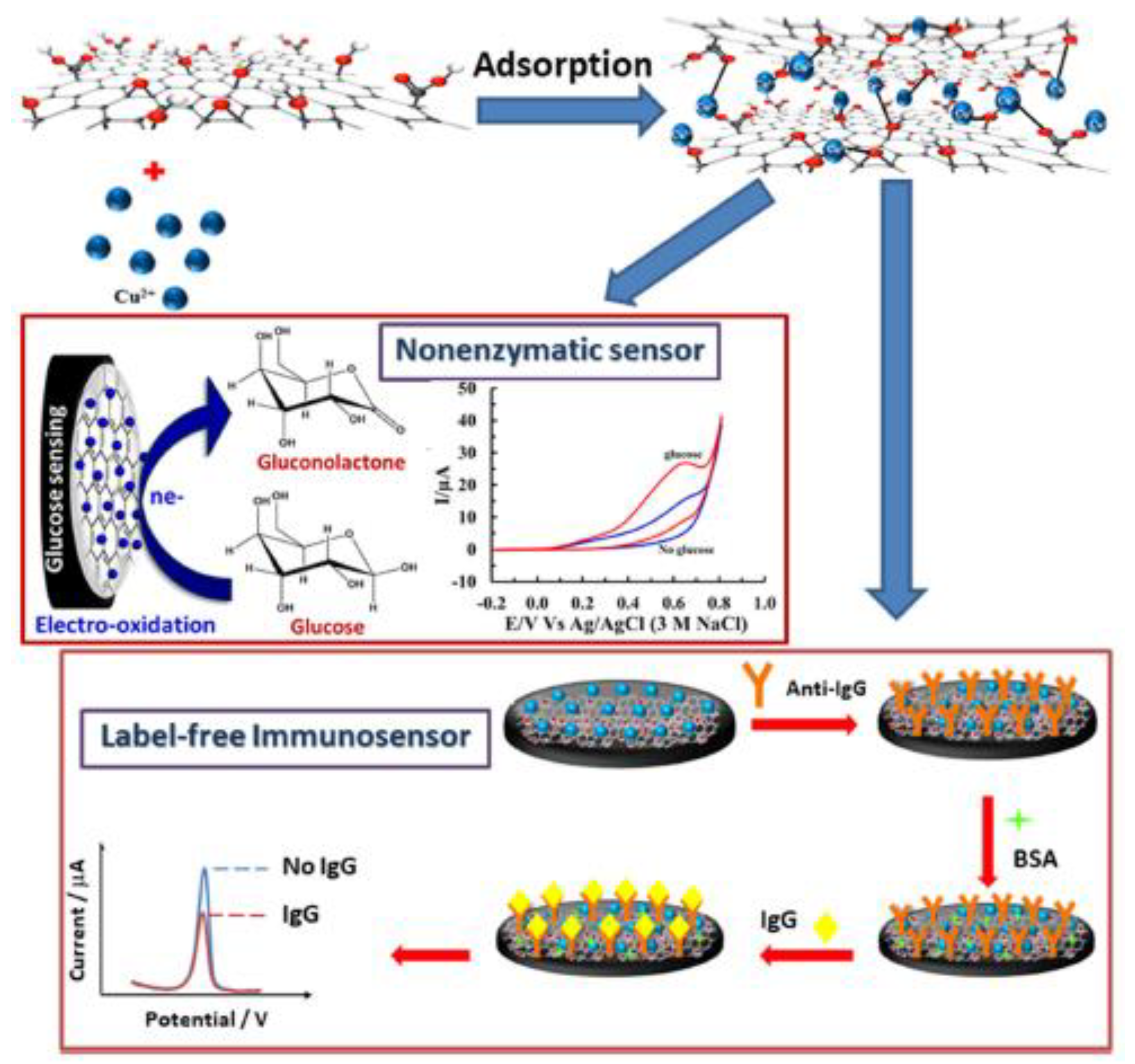
- Phetsang, S.; Khwannimit, D.; Rattanakit, P.; Chanlek, N.; Kidkhunthod, P.; Mungkornasawakul, P.; Jakmunee, J.; Ounnunkad, K. A Redox Cu(II)-Graphene Oxide Modified Screen Printed Carbon Electrode as a Cost-Effective and Versatile Sensing Platform for Electrochemical Label-Free Immunosensor and Non-enzymatic Glucose Sensor. Front. Chem. 2021, 9, 671173. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Khoshnavaz, Y.; Karimi, F.; Çakar, S.; Özacar, M.; Erk, N. A novel 2-dimensional nanocomposite as a mediator for the determination of doxorubicin in biological samples. Environ. Res. 2022, 213, 113590. [Google Scholar] [CrossRef]
- Guan, J.; He, K.; Gunasekaran, S. Self-assembled tetrahedral DNA nanostructures-based ultrasensitive label-free detection of ampicillin. Talanta 2022, 243, 123292. [Google Scholar] [CrossRef] [PubMed]
- Garima; Sachdev, A.; Matai, I. An electrochemical sensor based on cobalt oxyhydroxide nanoflakes/reduced graphene oxide nanocomposite for detection of illicit drug-clonazepam. J. Electroanal. Chem. 2022, 919, 116537. [Google Scholar] [CrossRef]
- Materón, E.M.; Wong, A.; Freitas, T.A.; Faria, R.C.; Oliveira, O.N. A sensitive electrochemical detection of metronidazole in synthetic serum and urine samples using low-cost screen-printed electrodes modified with reduced graphene oxide and C60. J. Pharm. Anal. 2021, 11, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Bacchu, M.S.; Al-Mamun, M.R.; Ahommed, M.S.; Aly, M.A.S.; Khan, M.Z.H. N-Hydroxysuccinimide crosslinked graphene oxide–gold nanoflower modified SPE electrode for sensitive detection of chloramphenicol antibiotic. RSC Adv. 2021, 11, 15565–15572. [Google Scholar] [CrossRef]
- Selvi, S.V.; Nataraj, N.; Chen, S. The electro-catalytic activity of nanosphere strontium doped zinc oxide with rGO layers screen-printed carbon electrode for the sensing of chloramphenicol. Microchem. J. 2020, 159, 105580. [Google Scholar] [CrossRef]
- Kimuam, K.; Rodthongkum, N.; Ngamrojanavanich, N.; Chailapakul, O.; Ruecha, N. Single step preparation of platinum nanoflowers/reduced graphene oxide electrode as a novel platform for diclofenac sensor. Microchem. J. 2020, 155, 104744. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Design and preparation of g-C3N4/rGO modified screen printed electrode for hydrogen peroxide sensing application. Synth. Met. 2022, 286, 117047. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Chen, C.; Xie, L.; Zhu, Z.; Zhao, H.; Lan, M. MnFe2O4 nanoparticles-decorated graphene nanosheets used as an efficient peroxidase minic enable the electrochemical detection of hydrogen peroxide with a low detection limit. Microchem. J. 2021, 166, 106240. [Google Scholar] [CrossRef]
- Akkapinyo, C.; Subannajui, K.; Poo-arporn, Y.; Poo-arporn, R.P. Disposable Electrochemical Sensor for Food Colorants Detection by Reduced Graphene Oxide and Methionine Film Modified Screen Printed Carbon Electrode. Molecules 2021, 26, 2312. [Google Scholar] [CrossRef]
- Wu, J.-H.; Lee, H.-L. Determination of sunset yellow and tartrazine in drinks using screen-printed carbon electrodes modified with reduced graphene oxide and NiBTC frameworks. Microchem. J. 2020, 158, 105133. [Google Scholar] [CrossRef]
- Ebrahimi-Tazangi, F.; Beitollahi, H.; Hekmatara, H.; Seyed-Yazdi, J. Design of a new electrochemical sensor based on the CuO/GO nanocomposites: Simultaneous determination of Sudan I and bisphenol A. J. Iran. Chem. Soc. 2021, 18, 191–199. [Google Scholar] [CrossRef]
- Pokpas, K.; Jahed, N.; McDonald, E.; Bezuidenhout, P.; Smith, S.; Land, K.; Iwuoha, E. Graphene-AuNP Enhanced Inkjet-printed Silver Nanoparticle Paper Electrodes for the Detection of Nickel(II)-Dimethylglyoxime [Ni(dmgH2)] Complexes by Adsorptive Cathodic Stripping Voltammetry (AdCSV). Electroanalysis 2020, 32, 3017–3031. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Ganguly, A.; Tata, S.K.S. Influence of temperature variation on spinel-structure MgFe2O4 anchored on reduced graphene oxide for electrochemical detection of 4-cyanophenol. Microchim. Acta 2020, 187, 633. [Google Scholar] [CrossRef]
- Irannezhad, F.; Seyed-Yazdi, J.; Hekmatara, S.H. Electrochemical sensing platform for simultaneous detection of 6-mercaptopurine and 6-thioguanine using RGO-Cu2O/Fe2O3 modified screen-printed graphite electrode. J. Electrochem. Sci. Eng. 2021, 12, 47–57. [Google Scholar] [CrossRef]
- Dhulkefl, A.J.; Atacan, K.; Bas, S.Z.; Ozmen, M. An Ag-TiO2-reduced graphene oxide hybrid film for electrochemical detection of 8-hydroxy-2′-deoxyguanosine as an oxidative DNA damage biomarker. Anal. Methods 2020, 12, 499–506. [Google Scholar] [CrossRef]
- Gunasekaran, B.M.; Rayappan, J.B.B.; Rajendran, G.K.; Gopalakrishnan, G.; Nesakumar, N.; Muthiah, S.; Sivanesan, J.R. Electrochemical Sensing of Arsenic Ions Using a Covalently Functionalized Benzotriazole-Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode. ChemistrySelect 2022, 7, e202201169. [Google Scholar] [CrossRef]
- Selvi, S.V.; Nataraj, N.; Chen, S.-M.; Prasannan, A. An electrochemical platform for the selective detection of azathioprine utilizing a screen-printed carbon electrode modified with manganese oxide/reduced graphene oxide. New J. Chem. 2021, 45, 3640–3651. [Google Scholar] [CrossRef]
- Sethi, J.; Van Bulck, M.; Suhail, A.; Safarzadeh, M.; Perez-Castillo, A.; Pan, G. A label-free biosensor based on graphene and reduced graphene oxide dual-layer for electrochemical determination of beta-amyloid biomarkers. Microchim. Acta 2020, 187, 288. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-S.; Li, Y.; Yu, M.-J.; Lee, H.-L. Multiwalled carbon nanotubes/reduced graphene oxide nanocomposite electrode for electroanalytical determination of bisphenol A, 8-hydroxy-2′-deoxyguanosine and hydroquinone in urine. Int. J. Environ. Anal. Chem. 2020, 100, 774–788. [Google Scholar] [CrossRef]
- Chen, H.; Cui, C.; Ma, X.; Yang, W.; Zuo, Y. Amperometric Biosensor for Brucella Testing through Molecular Orientation Technology in Combination with Signal Amplification Technology. ChemElectroChem 2020, 7, 2672–2679. [Google Scholar] [CrossRef]
- Albalawi, I.; Alatawi, H.; Alsefri, S.; Moore, E. Electrochemical Synthesis of Reduced Graphene Oxide/Gold Nanoparticles in a Single Step for Carbaryl Detection in Water. Sensors 2022, 22, 5251. [Google Scholar] [CrossRef]
- Zhou, J.; Pan, K.; Qu, G.; Ji, W.; Ning, P.; Tang, H.; Xie, R. rGO/MWCNTs-COOH 3D hybrid network as a high-performance electrochemical sensing platform of screen-printed carbon electrodes with an ultra-wide detection range of Cd(II) and Pb(II). Chem. Eng. J. 2022, 449, 137853. [Google Scholar] [CrossRef]
- Hou, X.; Xiong, B.; Wang, Y.; Wang, L.; Wang, H. Determination of Trace Lead and Cadmium in Decorative Material Using Disposable Screen-Printed Electrode Electrically Modified with Reduced Graphene Oxide/L-Cysteine/Bi-Film. Sensors 2020, 20, 1322. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Aghili, Z.; Jannat, B.; Jafari, S.; Tafti, S.R.; Nasirizadeh, N. Nanocomposite of electrochemically reduced graphene oxide and gold nanourchins for electrochemical DNA detection. IET Nanobiotechnol. 2022, 16, 190–198. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, G.; Sharma, V.; Nagraik, R.; Shandilya, M. Increasing the efficiency of reduced graphene oxide obtained via high temperature electrospun calcination process for the electrochemical detection of dopamine. J. Electroanal. Chem. 2022, 904, 115904. [Google Scholar] [CrossRef]
- Khodaei, R.; Ahmady, A.; Khoshfetrat, S.M.; Kashanian, S.; Tavangar, S.M.; Omidfar, K. Voltammetric immunosensor for E-cadherin promoter DNA methylation using a Fe3O4-citric acid nanocomposite and a screen-printed carbon electrode modified with poly(vinyl alcohol) and reduced graphene oxide. Microchim. Acta 2019, 186, 170. [Google Scholar] [CrossRef]
- Mo, X.; Wu, Z.; Huang, J.; Zhao, G.; Dou, W. A sensitive and regenerative electrochemical immunosensor for quantitative detection of Escherichia coli O157:H7 based on stable polyaniline coated screen-printed carbon electrode and rGO-NR-Au@Pt. Anal. Methods 2019, 11, 1475–1482. [Google Scholar] [CrossRef]
- Velusamy, V.; Palanisamy, S.; Chen, S.-W.; Balu, S.; Yang, T.C.K.; Banks, C.E. Novel electrochemical synthesis of cellulose microfiber entrapped reduced graphene oxide: A sensitive electrochemical assay for detection of fenitrothion organophosphorus pesticide. Talanta 2019, 192, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Buffon, E.; Stradiotto, N.R. A molecularly imprinted polymer on reduced graphene oxide-gold nanoparticles modified screen-printed electrode for selective determination of ferulic acid in orange peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Fan, Y.; Guo, Y.; Shi, S.; Ma, J. An electrochemical immunosensor based on reduced graphene oxide/multiwalled carbon nanotubes/thionine/gold nanoparticle nanocomposites for the sensitive testing of follicle-stimulating hormone. Anal. Methods 2021, 13, 3821–3828. [Google Scholar] [CrossRef]
- Rahmat, N.; Yusof, N.A.; Isha, A.; Mui-Yun, W.; Hushiarian, R.; Akanbi, F.S. Detection of Stress Induced by Ganoderma boninense Infection in Oil Palm Leaves Using Reduced Graphene Oxide and Zinc Oxide Nanoparticles Screen Printed Carbon Electrode. IEEE Sens. J. 2020, 20, 13253–13261. [Google Scholar] [CrossRef]
- Shi, Z.; Lu, Y.; Chen, Z.; Cheng, C.; Xu, J.; Zhang, Q.; Yan, Z.; Luo, Z.; Liu, Q. Electrochemical non-enzymatic sensing of glycoside toxins by boronic acid functionalized nano-composites on screen-printed electrode. Sens. Actuators B Chem. 2021, 329, 129197. [Google Scholar] [CrossRef]
- Li, G.; Feng, H.; Shi, X.; Chen, M.; Liang, J.; Zhou, Z. Highly sensitive electrochemical aptasensor for Glypican-3 based on reduced graphene oxide-hemin nanocomposites modified on screen-printed electrode surface. Bioelectrochemistry 2021, 138, 107696. [Google Scholar] [CrossRef] [PubMed]
- Sapari, S.; Razak, N.H.A.; Hasbullah, S.A.; Heng, L.Y.; Chong, K.F.; Tan, L.L. A regenerable screen-printed voltammetric Hg(II) ion sensor based on tris-thiourea organic chelating ligand grafted graphene nanomaterial. J. Electroanal. Chem. 2020, 878, 114670. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Rezayi, M.; Rasouli, E.; Avan, A.; Gholami, M.; Mobarhan, M.G.; Karimi, E.; Alias, Y. Early-stage cervical cancer diagnosis based on an ultra-sensitive electrochemical DNA nanobiosensor for HPV-18 detection in real samples. J. Nanobiotechnol. 2020, 18, 11. [Google Scholar] [CrossRef]
- Fani, M.; Rezayi, M.; Pourianfar, H.R.; Meshkat, Z.; Makvandi, M.; Gholami, M.; Rezaee, S.A. Rapid and label-free electrochemical DNA biosensor based on a facile one-step electrochemical synthesis of rGO–PPy–(L-Cys)–AuNPs nanocomposite for the HTLV-1 oligonucleotide detection. Biotechnol. Appl. Biochem. 2021, 68, 626–635. [Google Scholar] [CrossRef]
- Fani, M.; Rezayi, M.; Meshkat, Z.; Rezaee, S.A.; Makvandi, M.; Angali, K.A. A Novel Electrochemical DNA Biosensor Based on a Gold Nanoparticles Reduced Graphene Oxide Polypyrrole Nanocomposite to Detect Human T-Lymphotropic Virus-1. IEEE Sens. J. 2020, 20, 10625–10632. [Google Scholar] [CrossRef]
- Tajik, S.; Askari, M.B.; Ahmadi, S.A.; Nejad, F.G.; Dourandish, Z.; Razavi, R.; Beitollahi, H.; Di Bartolomeo, A. Electrochemical Sensor Based on ZnFe2O4/RGO Nanocomposite for Ultrasensitive Detection of Hydrazine in Real Samples. Nanomaterials 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Beitollahi, H.; Ahmadi, S.A.; Askari, M.B.; Di Bartolomeo, A. Screen-Printed Electrode Surface Modification with NiCo2O4/RGO Nanocomposite for Hydroxylamine Detection. Nanomaterials 2021, 11, 3208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.S.K.; Hwa, K.-Y. Facile Synthesis of Ag/AgVO3/N-rGO Hybrid Nanocomposites for Electrochemical Detection of Levofloxacin for Complex Biological Samples Using Screen-Printed Carbon Paste Electrodes. Inorg. Chem. 2021, 60, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Mehmandoust, M.; Erk, N.; Karaman, C.; Karaman, O. An electrochemical molecularly imprinted sensor based on CuBi2O4/rGO@MoS2 nanocomposite and its utilization for highly selective and sensitive for linagliptin assay. Chemosphere 2022, 291, 132807. [Google Scholar] [CrossRef] [PubMed]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- Jamei, H.R.; Rezaei, B.; Ensafi, A.A. An ultrasensitive electrochemical anti-lysozyme aptasensor with biorecognition surface based on aptamer/amino-rGO/ionic liquid/amino-mesosilica nanoparticles. Colloids Surf. B Biointerfaces 2019, 181, 16–24. [Google Scholar] [CrossRef]
- Venkatesh, K.; Muthukutty, B.; Chen, S.-M.; Karuppasamy, P.; Haidyrah, A.S.; Karuppiah, C.; Yang, C.-C.; Ramaraj, S.K. Spinel CoMn2O4 nano-/micro-spheres embedded RGO nanosheets modified disposable electrode for the highly sensitive electrochemical detection of metol. J. Ind. Eng. Chem. 2022, 106, 287–296. [Google Scholar] [CrossRef]
- Fan, L.; Huang, J.J.; Liao, J. Competitive smartphone-based portable electrochemical aptasensor system based on an MXene/cDNA-MB probe for the determination of Microcystin-LR. Sens. Actuators B Chem. 2022, 369, 132164. [Google Scholar] [CrossRef]
- Low, S.S.; Pan, Y.; Ji, D.; Li, Y.; Lu, Y.; He, Y.; Chen, Q.; Liu, Q. Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sens. Actuators B Chem. 2020, 308, 127718. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.-Y.; Tseng, F.-Y.; Tan, Y.-C.; Li, J.; Feng, L.; Song, L.; Lai, C.-F.; Li, X.; He, J.-H.; et al. Label-free electrochemical immunosensor based on gold nanoparticle/polyethyleneimine/reduced graphene oxide nanocomposites for the ultrasensitive detection of cancer biomarker matrix metalloproteinase-1. Analyst 2021, 146, 4066–4079. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Abdullah, J.; Yusof, N.A.; Wasoh, H.; Sulaiman, Y.; Noh, M.F.M.; Issa, R. Reduced Graphene Oxide/TEMPO-Nanocellulose Nanohybrid-Based Electrochemical Biosensor for the Determination of Mycobacterium tuberculosis. J. Sens. 2020, 2020, 4051474. [Google Scholar] [CrossRef]
- Traiwatcharanon, P.; Siriwatcharapiboon, W.; Wongchoosuk, C. Electrochemical Sodium Ion Sensor Based on Silver Nanoparticles/Graphene Oxide Nanocomposite for Food Application. Chemosensors 2020, 8, 58. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Q.; Zhao, Y.; Ge, C.; Lin, S.; Liao, J. Cost-effective, wireless, and portable smartphone-based electrochemical system for on-site monitoring and spatial mapping of the nitrite contamination in water. Sens. Actuators B Chem. 2020, 319, 128221. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Velmurugan, M.; Chen, S.M.; Chen, T.W.; Ye, Y.T. A Single-Step Electrochemical Preparation of Cadmium Sulfide Anchored erGO/β-CD Modified Screen-Printed Carbon Electrode for Sensitive and Selective Detection of Nitrite. J. Electrochem. Soc. 2019, 166, B690. [Google Scholar] [CrossRef]
- Zhe, T.; Sun, X.; Wang, Q.; Liu, Y.; Li, R.; Li, F.; Wang, L. A screen printed carbon electrode modified with a lamellar nanocomposite containing dendritic silver nanostructures, reduced graphene oxide, and β-cyclodextrin for voltammetric sensing of nitrite. Microchim. Acta 2019, 186, 319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, H.; Hu, J.; Yang, D. Disposable Electrochemical Aptasensor Based on Graphene Oxide-DNA Complex as Signal Amplifier towards Ultrasensitive Detection of Ochratoxin A. Micromachines 2022, 13, 834. [Google Scholar] [CrossRef]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Setiawati, T.A.; Sofyatin, T.; Fitrilawati, F.; Anggraeni, A.; Gaffar, S. Electrochemical DNA biosensor for detection of pork (Sus scrof) using screen printed carbon-reduced graphene oxide electrode. ScienceAsia 2020, 46, 72. [Google Scholar] [CrossRef]
- Poo-Arporn, Y.; Pakapongpan, S.; Chanlek, N.; Poo-Arporn, R.P. The development of disposable electrochemical sensor based on Fe3O4-doped reduced graphene oxide modified magnetic screen-printed electrode for ractopamine determination in pork sample. Sens. Actuators B Chem. 2019, 284, 164–171. [Google Scholar] [CrossRef]
- Kumar, V.; Sachdev, A.; Matai, I. Self-assembled reduced graphene oxide–cerium oxide nanocomposite@cytochrome c hydrogel as a solid electrochemical reactive oxygen species detection platform. New J. Chem. 2020, 44, 11248–11255. [Google Scholar] [CrossRef]
- Nodoushan, S.M.; Nasirizadeh, N.; Amani, J.; Halabian, R.; Fooladi, A.A.I. An electrochemical aptasensor for staphylococcal enterotoxin B detection based on reduced graphene oxide and gold nano-urchins. Biosens. Bioelectron. 2019, 127, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Hwang, S.-K.; Lee, M.J.; Park, B.; Huh, Y.S.; Han, Y.-K. Gold nanoparticle decorated patronite on rGO for the quantification of sulfadiazine at nanomolar levels in contaminated water. Chem. Eng. J. 2022, 439, 135782. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, N.; Karaman, O.; Karimi, F.; Bijad, M.; Karaman, C. Three-dimensional porous reduced graphene oxide decorated with carbon quantum dots and platinum nanoparticles for highly selective determination of azo dye compound tartrazine. Food Chem. Toxicol. 2021, 158, 112698. [Google Scholar] [CrossRef]
- Lorenzetti, A.S.; Sierra, T.; Domini, C.E.; Lista, A.G.; Crevillen, A.G.; Escarpa, A. Electrochemically Reduced Graphene Oxide-Based Screen-Printed Electrodes for Total Tetracycline Determination by Adsorptive Transfer Stripping Differential Pulse Voltammetry. Sensors 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized Carbon Nanotubes in Drug Design and Discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar] [CrossRef]
- Grady, B.P. Recent Developments Concerning the Dispersion of Carbon Nanotubes in Polymers. Macromol. Rapid Commun. 2010, 31, 247–257. [Google Scholar] [CrossRef]
- Gooding, J.J. Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochim. Acta 2005, 50, 3049–3060. [Google Scholar] [CrossRef]
- Pumera, M. The Electrochemistry of Carbon Nanotubes: Fundamentals and Applications. Chem. Eur. J. 2009, 15, 4970–4978. [Google Scholar] [CrossRef]
- Rao, M.M. Electrochemical Determination of Catechol Using Functionalized Multiwalled Carbon Nanotubes modified Screen Printed Carbon Electrode. Int. J. Electrochem. Sci. 2018, 13, 6126–6134. [Google Scholar] [CrossRef]
- Wang, H.; Yin, Y.; Zhao, G.; Bienvenido, F.; Flores-Parrad, I.M.; Wang, Z.Q.; Liu, G. Graphene oxide/multi-walled carbon nanotubes/gold nanoparticle hybridfunctionalized disposable screen-printed carbon electrode to determine Cd(II) and Pb(II) in soil. Int. J. Agric. Biol. Eng. 2019, 3, 194–200. [Google Scholar] [CrossRef]
- Kozak, J.; Tyszczuk-Rotko, K.; Rotko, M. Voltammetric screen-printed carbon sensor modified with multiwalled carbon nanotubes and bismuth film for trace analysis of thallium(I). Physicochem. Probl. Miner. Process. 2019, 55, 1422–1428. [Google Scholar] [CrossRef]
- Ali, T.A.; Akl, Z.F. Ionic liquid-multi-walled carbon nanotubes modified screen-printed electrodes for sensitive electrochemical sensing of uranium. J. Radioanal. Nucl. Chem. 2021, 328, 267–276. [Google Scholar] [CrossRef]
- GAMBOA, J.C.M. Screen Printed Electrode of Carbon Nanotubes Modified with Gold Nanoparticles for Simultaneous Determination of Zinc, Lead and Copper. J. Chil. Chem. Soc. 2020, 65, 4842–4844. [Google Scholar] [CrossRef]
- Chuntib, P.; Themsirimongkon, S.; Saipanya, S.; Jakmunee, J. Sequential injection differential pulse voltammetric method based on screen printed carbon electrode modified with carbon nanotube/Nafion for sensitive determination of paraquat. Talanta 2017, 170, 1–8. [Google Scholar] [CrossRef]
- Teixeira, J.G.; Oliveira, J. Voltammetric Study of the Antihistamine Drug Bilastine: Anodic Characterization and Quantification Using a Reusable MWCNTs Modified Screen Printed Carbon Electrode. Electroanalysis 2021, 33, 891–899. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Gabriel, W.; Santschi, C.; Martin, O. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Xu, P.-L.; Zeng, Q.; Liu, Y.-M.; Liao, X.; Hou, M.-F. Magnetism-assisted modification of screen printed electrode with magnetic multi-walled carbon nanotubes for electrochemical determination of dopamine. Mater. Sci. Eng. C 2017, 74, 62–69. [Google Scholar] [CrossRef]
- dos Santos, W.T.P.; Compton, R.G. A simple method to detect the stimulant modafinil in authentic saliva using a carbon-nanotube screen-printed electrode with adsorptive stripping voltammetry. Sens. Actuators B Chem. 2019, 285, 137–144. [Google Scholar] [CrossRef]
- Ochiai, L.M.; Agustini, D.; Figueiredo-Filho, L.C.S.; Banks, C.E.; Marcolino-Junior, L.H.; Bergamini, M.F. Electroanalytical thread-device for estriol determination using screen-printed carbon electrodes modified with carbon nanotubes. Sens. Actuators B Chem. 2017, 241, 978–984. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A lab-made screen-printed electrode as a platform to study the effect of the size and functionalization of carbon nanotubes on the voltammetric determination of caffeic acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Jeličová, M.; Metelka, R.; Pejchal, J.; Lierová, A.; Šinkorová, Z. Electrochemical detection of 8-hydroxyguanine using screen-printed carbon electrodes modified with carboxy-functionalized multi-walled carbon nanotubes. Mon. Für Chem.-Chem. Mon. 2019, 150, 1187–1193. [Google Scholar] [CrossRef]
- Wasąg, J.; Grabarczyk, M. Copper Film Modified Glassy Carbon Electrode and Copper Film with Carbon Nanotubes Modified Screen-Printed Electrode for the Cd(II) Determination. Materials 2021, 14, 5148. [Google Scholar] [CrossRef] [PubMed]
- Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials 2020, 13, 781. [Google Scholar] [CrossRef]
- Redžić, S.; Kahrović, E.; Zahirović, A.; Turkušić, E. Electrochemical Determination of Dopamine with Ruthenium(III)-Modified Glassy Carbon and Screen-Printed Electrodes. Anal. Lett. 2017, 50, 1602–1619. [Google Scholar] [CrossRef]
- Huang, X.; Xu, S.; Zhao, W.; Xu, M.; Wei, W.; Luo, J.; Li, X.; Liu, X. Screen-Printed Carbon Electrodes Modified with Polymeric Nanoparticle-Carbon Nanotube Composites for Enzymatic Biosensing. ACS Appl. Nano Mater. 2020, 3, 9158–9166. [Google Scholar] [CrossRef]
- River-Guzman, K. Electochemical Detection of Imidacloprid Using a Screen Printed Single Walled Carbon Nanotubes Coated with and Ionic Liquids. Int. J. Electrochem. Sci. 2018, 13, 5775–5787. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, X.; Zhen, Q.; He, Y.; Chen, X.; Lyu, W.; Han, R.; Ding, M. An electrochemical sensor for indole in plasma based on MWCNTs-chitosan modified screen-printed carbon electrode. Biosens. Bioelectron. 2017, 98, 392–397. [Google Scholar] [CrossRef]
- Pasakon, P.; Mensing, J.P.; Phokaratkul, D.; Karuwan, C.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. A high-performance, disposable screen-printed carbon electrode modified with multi-walled carbon nanotubes/graphene for ultratrace level electrochemical sensors. J. Appl. Electrochem. 2019, 49, 217–227. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Tache, F.; Moldovan, Z.; Stamatin, I.; Mihailciuc, C.; Ciucu, A.A. Rapid voltammetric detection of kojic acid at a multi-walled carbon nanotubes screen-printed electrode. Sens. Actuators B Chem. 2017, 241, 406–412. [Google Scholar] [CrossRef]
- David, M.; Şerban, A.; Enache, T.A.; Florescu, M. Electrochemical quantification of levothyroxine at disposable screen-printed electrodes. J. Electroanal. Chem. 2022, 911, 116240. [Google Scholar] [CrossRef]
- Upan, J.; Themsirimongkon, S.; Saipanya, S.; Jakmunee, J. Gold Nanoparticles Decorated on Carbon Nanotube Modified Screen-printed Electrode for Flow Injection Amperometric Determination of Methyldopa. Chiang Mai J. Sci. 2019, 3, 537–546. [Google Scholar]
- Abera, B.D.; Shkodra, B.; Douaki, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Single-Walled Carbon Nanotube-Coated Flexible and Soft Screen-Printed Electrochemical Biosensor for Ochratoxin a Detection. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), Seville, Spain, 12–14 October 2020; pp. 1–5. [Google Scholar]
- Serrano, N.; Castilla, Ò.; Ariño, C.; Diaz-Cruz, M.; Díaz-Cruz, J. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors 2019, 19, 4039. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Ahmadvand, E.; Ehsani, A. Electroanalytical Sensing of Piperazine at Carbon Nanotubes/Nafion Composite-modified Glassy Carbon and Screen-printed Carbon Electrodes in Human Plasma. J. Anal. Chem. 2020, 75, 238–245. [Google Scholar] [CrossRef]
- Newair, E.F.; Kilmartin, P.A.; Garcia, F. Square wave voltammetric analysis of polyphenol content and antioxidant capacity of red wines using glassy carbon and disposable carbon nanotubes modified screen-printed electrodes. Eur. Food Res. Technol. 2018, 244, 1225–1237. [Google Scholar] [CrossRef]
- Hassan, R.Y.A.; Wollenberger, U. Direct Determination of Bacterial Cell Viability Using Carbon Nanotubes Modified Screen-printed Electrodes. Electroanalysis 2019, 31, 1112–1117. [Google Scholar] [CrossRef]
- Park, K. Impedance Technique-Based Label-Free Electrochemical Aptasensor for Thrombin Using Single-Walled Carbon Nanotubes-Casted Screen-Printed Carbon Electrode. Sensors 2022, 22, 2699. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Migliorelli, D.; Jafari, S.; Generelli, S.; Lou-Franco, J.; Salvador, J.P.; Marco, M.P.; Cao, C.; Elliott, C.T.; Campbell, K. The benefits of carbon black, gold and magnetic nanomaterials for point-of-harvest electrochemical quantification of domoic acid. Microchim. Acta 2020, 187, 164. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Migliorelli, D.; Mühlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Serani, A.; Fiore, L.; Moscone, D.; Arduini, F. All-solid state ion-selective carbon black-modified printed electrode for sodium detection in sweat. Electrochim. Acta 2021, 394, 139050. [Google Scholar] [CrossRef]
- Jafari, S.; Burr, L.; Migliorelli, D.; Galve, R.; Marco, M.-P.; Campbell, K.; Elliott, C.; Suman, M.; Sturla, S.J.; Generelli, S. Smartphone-based magneto-immunosensor on carbon black modified screen-printed electrodes for point-of-need detection of aflatoxin B1 in cereals. Anal. Chim. Acta 2022, 1221, 340118. [Google Scholar] [CrossRef] [PubMed]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Magnetically induced enzymatic cascades—Advancing towards multi-fuel direct/mediated bioelectrocatalysis. Nanoscale Adv. 2019, 1, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Effect of Different Carbon Blacks on the Simultaneous Electroanalysis of Drugs as Water Contaminants Based on Screen-printed Sensors. Electroanalysis 2019, 31, 2145–2154. [Google Scholar] [CrossRef]
- Rojas, D.; Della Pelle, F.; Del Carlo, M.; Fratini, E.; Escarpa, A.; Compagnone, D. Nanohybrid carbon black-molybdenum disulfide transducers for preconcentration-free voltammetric detection of the olive oil o-diphenols hydroxytyrosol and oleuropein. Microchim. Acta 2019, 186, 363. [Google Scholar] [CrossRef] [PubMed]
- Reanpang, P.; Mool-am-kha, P.; Upan, J.; Jakmunee, J. A novel flow injection amperometric sensor based on carbon black and graphene oxide modified screen-printed carbon electrode for highly sensitive determination of uric acid. Talanta 2021, 232, 122493. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Wilson, D.; Gonçalves, D.; Oliveira, O.N. Low-cost screen-printed electrodes based on electrochemically reduced graphene oxide-carbon black nanocomposites for dopamine, epinephrine and paracetamol detection. J. Colloid Interface Sci. 2018, 515, 101–108. [Google Scholar] [CrossRef]
- Fatma, S.; Prasad, B.B.; Jaiswal, S.; Singh, R.; Singh, K. Electrochemical simultaneous analysis of dopamine and epinephrine using double imprinted One MoNomer acryloylated graphene oxide-carbon black composite polymer. Biosens. Bioelectron. 2019, 135, 36–44. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Nashruddin, S.N.A.; Abdullah, J.; Haniff, M.A.S.M.; Zaid, M.H.M.; Choon, O.P.; Wee, M.F.M.R. Label free glucose electrochemical biosensor based on poly(3,4-ethylenedioxy thiophene):Polystyrene sulfonate/titanium carbide/graphene quantum dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Dourandish, Z.; Ganjali, M.R.; Shakeri, S. Voltammetric determination of dopamine in the presence of tyrosine using graphite screen-printed electrode modified with graphene quantum dots. Ionics 2018, 24, 4023–4031. [Google Scholar] [CrossRef]
- Ben Aoun, S. Nanostructured carbon electrode modified with N-doped graphene quantum dots–chitosan nanocomposite: A sensitive electrochemical dopamine sensor. R. Soc. Open Sci. 2017, 4, 171199. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Wong, A.; Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.P.T.; Moraes, F.C. Voltammetric determination of ethinylestradiol using screen-printed electrode modified with functionalized graphene, graphene quantum dots and magnetic nanoparticles coated with molecularly imprinted polymers. Talanta 2021, 224, 121804. [Google Scholar] [CrossRef]
- Naik, J.P.; Sutradhar, P.; Saha, M. Molecular scale rapid synthesis of graphene quantum dots (GQDs). J. Nanostruct. Chem. 2017, 7, 85–89. [Google Scholar] [CrossRef]
- Durán, G.M.; Llorent-Martínez, E.J.; Contento, A.M.; Ríos, Á. Determination of vanillin by using gold nanoparticle-modified screen-printed carbon electrode modified with graphene quantum dots and Nafion. Microchim. Acta 2018, 185, 204. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Kumar, A.; Singh, R. Synthesis of novel monomeric graphene quantum dots and corresponding nanocomposite with molecularly imprinted polymer for electrochemical detection of an anticancerous ifosfamide drug. Biosens. Bioelectron. 2017, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.R.; Dourandish, Z.; Beitollahi, H.; Tajik, S.; Hajiaghababaei, L.; Larijani, B. Highly sensitive determination of theophylline based on graphene quantum dots modified electrode. Int. J. Electrochem. Sci. 2018, 13, 2448–2461. [Google Scholar] [CrossRef]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid, dopamine, and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
- Punrat, E.; Maksuk, C.; Chuanuwatanakul, S.; Wonsawat, W.; Chailapakul, O. Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr(VI) using stopped-flow analysis coupled with voltammetric technique. Talanta 2016, 150, 198–205. [Google Scholar] [CrossRef]
- Ayad, M.F.; Trabik, Y.A.; Abdelrahman, M.H.; Fares, N.V.; Magdy, N. Potentiometric carbon quantum dots-based screen-printed arrays for nano-tracing gemifloxacin as a model fluoroquinolone implicated in antimicrobial resistance. Chemosensors 2021, 9, 8. [Google Scholar] [CrossRef]
- Canevari, T.C.; Cincotto, F.H.; Gomes, D.; Landers, R.; Toma, H.E. Magnetite Nanoparticles Bonded Carbon Quantum Dots Magnetically Confined onto Screen Printed Carbon Electrodes and their Performance as Electrochemical Sensor for NADH. Electroanalysis 2017, 29, 1968–1975. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Serafín, V.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Asadpour-Zeynali, K. Ultrasensitive determination of receptor tyrosine kinase with a label-free electrochemical immunosensor using graphene quantum dots-modified screen-printed electrodes. Anal. Chim. Acta 2018, 1011, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, L.; Xie, Q. Sensitive bioanalysis based on in-situ droplet anodic stripping voltammetric detection of cds quantum dots label after enhanced cathodic preconcentration. Sensors 2016, 16, 1342. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Barazesh, B.; Moshtaghioun, S.M. An Aptasensor based on electrosynthesized conducting polymers, Cu2O-carbon dots and biosynthesized gold nanoparticles, for monitoring carcinoembryonic antigen. J. Nanostruct. 2019, 9, 659–668. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Aflatoonian, M.R.; Nejad, F.G.; Zhang, K.; Asl, M.S.; Van Le, Q.; Cha, J.H.; Shokouhimehr, M.; Peng, W. A novel screen-printed electrode modified by graphene nanocomposite for detecting clozapine. Int. J. Electrochem. Sci. 2020, 15, 9271–9281. [Google Scholar] [CrossRef]
- Gevaerd, A.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Graphene Quantum Dots Modified Screen-printed Electrodes as Electroanalytical Sensing Platform for Diethylstilbestrol. Electroanalysis 2019, 31, 838–843. [Google Scholar] [CrossRef]
- Samie, H.A.; Arvand, M. Label-free electrochemical aptasensor for progesterone detection in biological fluids. Bioelectrochemistry 2020, 133, 107489. [Google Scholar] [CrossRef]
- Roushani, M.; Jalilian, Z.; Nezhadali, A. Screen printed carbon electrode sensor with thiol graphene quantum dots and gold nanoparticles for voltammetric determination of solatol. Heliyon 2019, 5, e01984. [Google Scholar] [CrossRef]
- Karimi, M.A.; Aghaei, V.H.; Nezhadali, A.; Ajami, N. Graphitic Carbon Nitride as a New Sensitive Material for Electrochemical Determination of Trace Amounts of Tartrazine in Food Samples. Food Anal. Methods 2018, 11, 2907–2915. [Google Scholar] [CrossRef]
- Jahani, P.M.; Aflatoonian, M.R.; Rayeni, R.A.; Di Bartolomeo, A.; Mohammadi, S.Z. Graphite carbon nitride-modified screen-printed electrode as a highly sensitive and selective sensor for detection of amaranth. Food Chem. Toxicol. 2022, 163, 112962. [Google Scholar] [CrossRef]
- Nataraj, N.; Chen, T.-W.; Akilarasan, M.; Chen, S.M.; Al-Ghamdi, A.A.; Elshikh, M.S. Se substituted 2D-gC3N4 modified disposable screen-printed carbon electrode substrate: A bifunctional nano-catalyst for electrochemical and absorption study of hazardous fungicide. Chemosphere 2022, 302, 134765. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, L.; Deng, C.; Nie, Z.; Yang, M.; Si, S. Simple and sensitive aptasensor based on quantum dot-coated silica nanospheres and the gold screen-printed electrode. Talanta 2012, 99, 637–642. [Google Scholar] [CrossRef]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from Brewers’ Spent Grain: A Green and Low-Cost Smart Material to Modify Screen-Printed Electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Zarbin, A.J.G.; Oliveira, M.M. Carbon nanostructures (nanotubes and graphene): Quo Vadis? Quim. Nova 2013, 36, 1533–1539. [Google Scholar] [CrossRef]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-Printed Electrodes Modified with Carbon Nanomaterials: A Comparison among Carbon Black, Carbon Nanotubes and Graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Mechlińska, A.; Namieśnik, J. Green analytical chemistry—Theory and practice. Chem. Soc. Rev. 2010, 39, 2869–2878. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. https://doi.org/10.3390/bios13040453
Silva RM, da Silva AD, Camargo JR, de Castro BS, Meireles LM, Silva PS, Janegitz BC, Silva TA. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors. 2023; 13(4):453. https://doi.org/10.3390/bios13040453
Chicago/Turabian StyleSilva, Rafael Matias, Alexsandra Dias da Silva, Jéssica Rocha Camargo, Bruna Santos de Castro, Laís Muniz Meireles, Patrícia Soares Silva, Bruno Campos Janegitz, and Tiago Almeida Silva. 2023. "Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications" Biosensors 13, no. 4: 453. https://doi.org/10.3390/bios13040453
APA StyleSilva, R. M., da Silva, A. D., Camargo, J. R., de Castro, B. S., Meireles, L. M., Silva, P. S., Janegitz, B. C., & Silva, T. A. (2023). Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors, 13(4), 453. https://doi.org/10.3390/bios13040453







