Improving the Efficiency of Electrocatalysis of Cytochrome P450 3A4 by Modifying the Electrode with Membrane Protein Streptolysin O for Studying the Metabolic Transformations of Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.3. Electrochemical Measurements
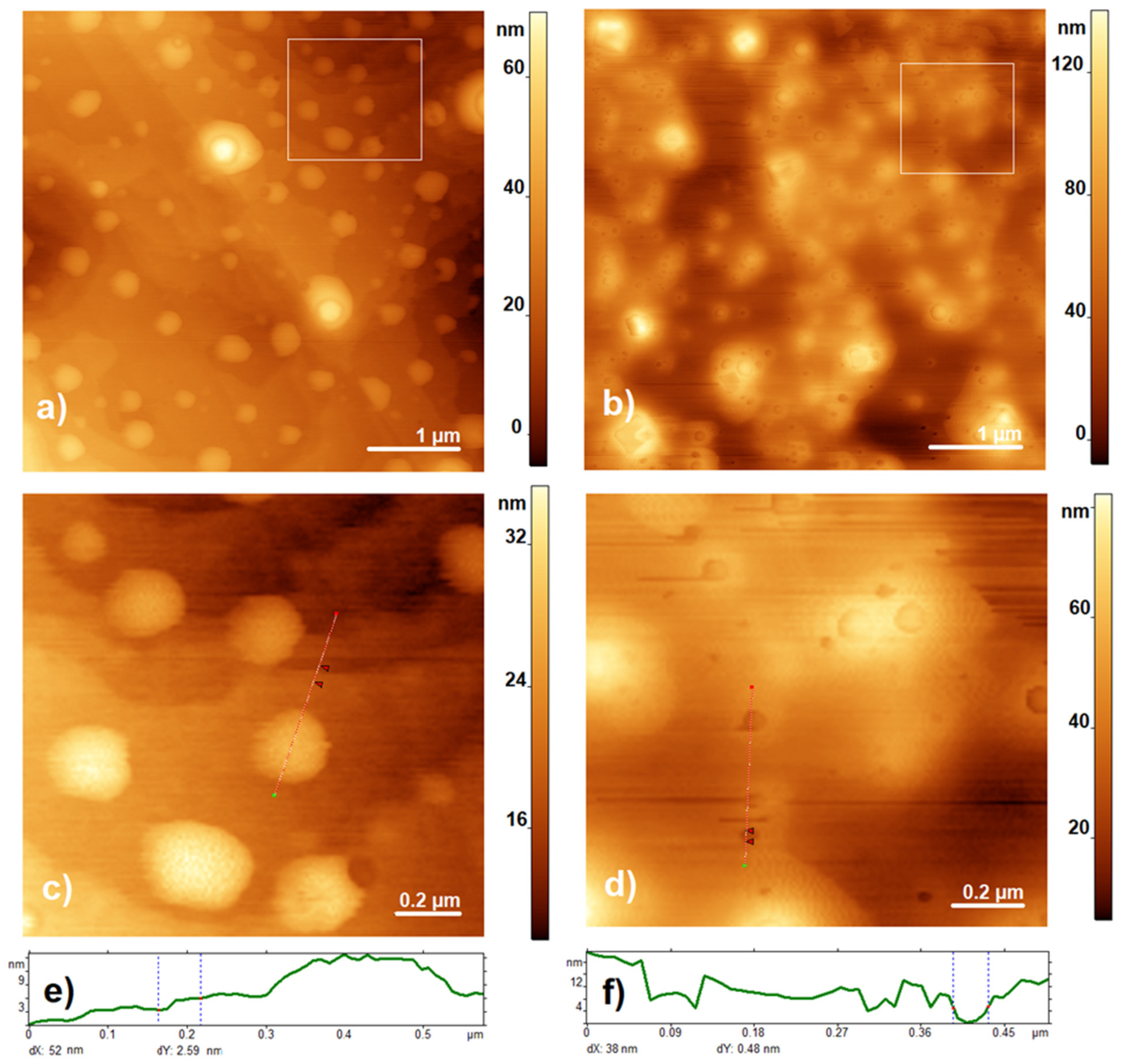
2.4. AFM Measurements
3. Results and Discussion
3.1. Characterization of the Composite by AFM
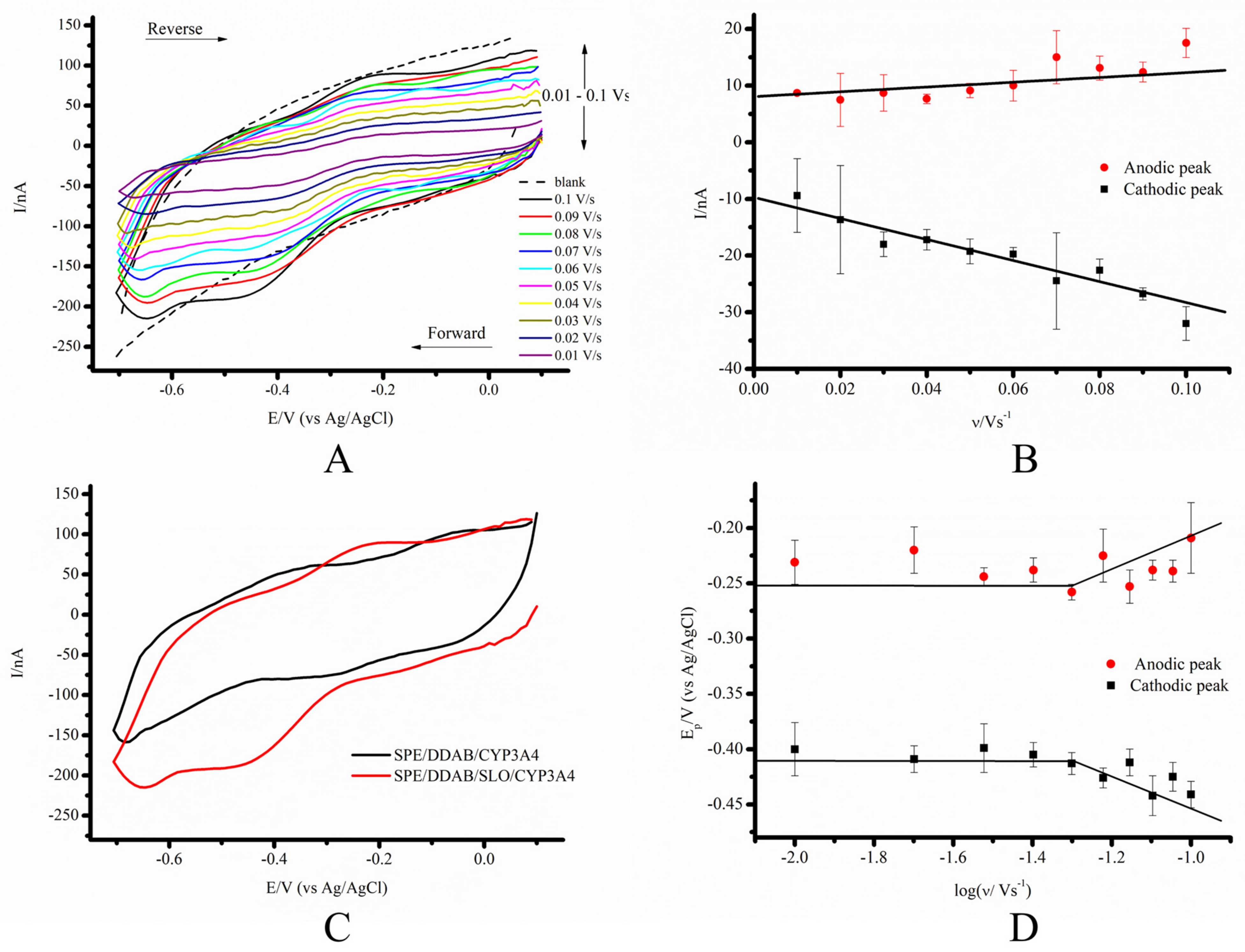
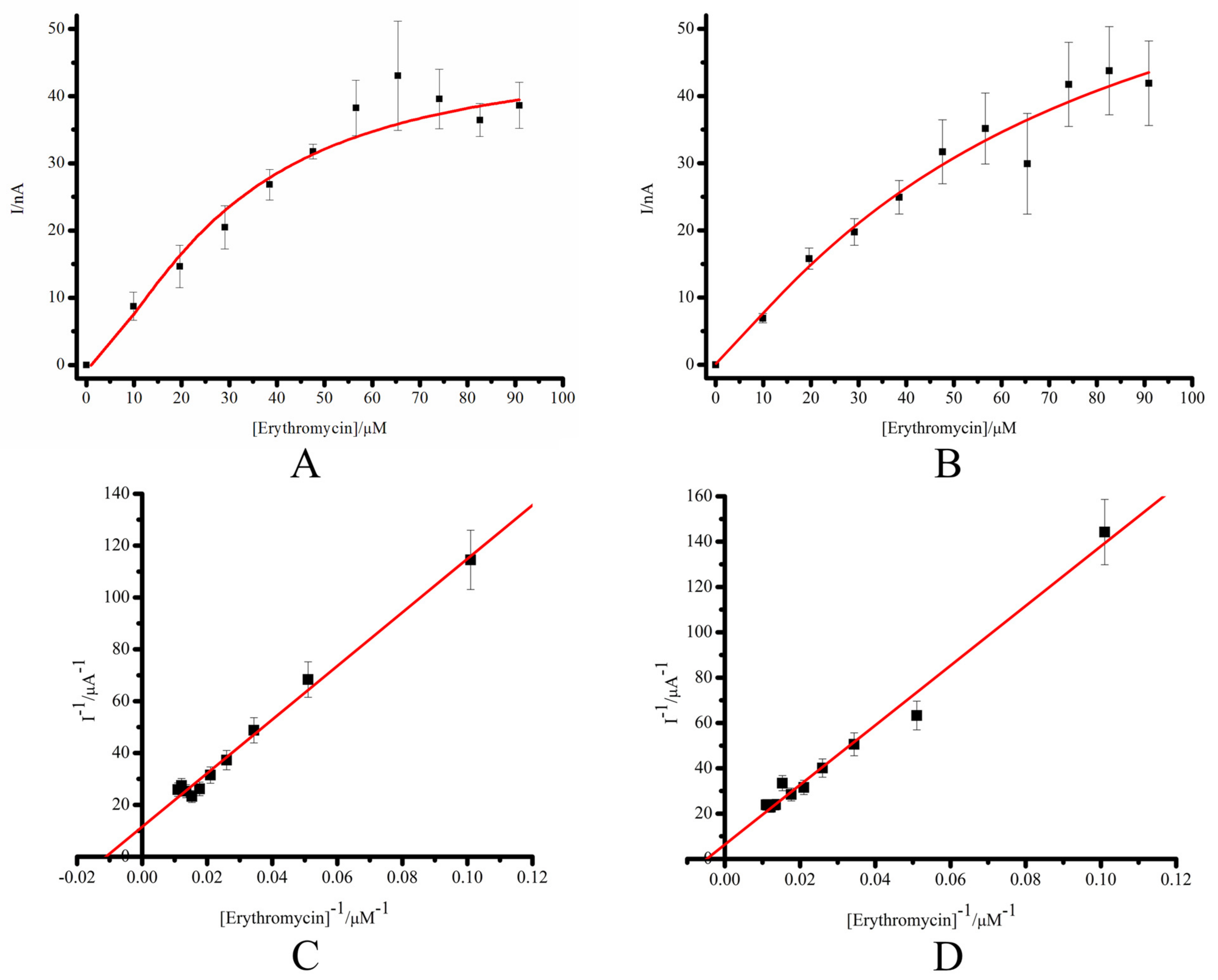
3.2. Comparative Characterization of the Electrochemical System SPE/DDAB/CYP3A4 and SPE/DDAB/SLO/CYP3A4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry; de Montellano, P.R.O., Ed.; Springer: New York, NY, USA, 2015; pp. 523–785. [Google Scholar] [CrossRef]
- Bernhardt, R.; Urlacher, V.B. Cytochromes P450 as promising catalysts for biotechnological application: Chances and limitations. Appl. Microbiol. Biotechnol. 2014, 98, 6185–6203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug. Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Bulko, T.V.; Archakov, A.I. Electrochemical reduction of cytochrome P450 as an approach to the construction of biosensors and bioreactors. J. Inorg. Biochem. 2005, 99, 1051–1063. [Google Scholar] [CrossRef]
- Mi, L.; Wang, Z.; Yang, W.; Huang, C.; Zhou, B.; Hu, Y.; Liu, S. Cytochromes P450 in biosensing and biosynthesis applications: Recent progress and future perspectives. Trends Anal. Chem. 2023, 158, 116791. [Google Scholar] [CrossRef]
- Morant, M.; Bak, S.; Møller, B.L.; Werck-Reichhart, D. Plant cytochromes P450: Tools for pharmacology, plant protection and phytoremediation. Curr. Opin. Biotechnol. 2003, 14, 151–162. [Google Scholar] [CrossRef]
- Jennewein, S.; Croteau, R. Taxol: Biosynthesis, molecular genetics, and biotechnological applications. Appl. Microbiol. Biotechnol. 2001, 57, 13–19. [Google Scholar] [CrossRef]
- Falck, J.R.; Reddy, Y.K.; Haines, D.C.; Reddy, K.M.; Krishna, U.M.; Graham, S.; Murry, B.; Peterson, J.A. Practical, enantiospecific syntheses of 14,15-EET and leukotoxin B (vernolic acid). Tetrahedron Lett. 2001, 42, 4131–4133. [Google Scholar] [CrossRef]
- Kumar, S. Engineering cytochrome P450 biocatalysts for biotechnology, medicine and bioremediation. Expert Opin. Drug. Metab. Toxicol. 2010, 6, 115–131. [Google Scholar] [CrossRef]
- Schneider, E.; Clark, D.S. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Mi, L.; He, F.; Jiang, L.; Shangguan, L.; Zhang, X.; Ding, T.; Liu, A.; Zhang, Y.; Liu, S. Electrochemically-driven benzo [a] pyrene metabolism via human cytochrome P450 1A1 with reductase coated nitrogen-doped graphene nano- composites. J. Electroanal. Chem. 2017, 804, 23–28. [Google Scholar] [CrossRef]
- Estabrook, R.W.; Faulkner, K.M.; Seth, M.S.; Fisher, C.W. Application of electrochemistry for P450-catalyzed reactions. Methods Enzymol. 1996, 272, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Bulko, T.V.; Koroleva, P.I.; Shikh, E.V.; Makhova, A.A.; Kisel, M.S.; Haidukevich, I.V.; Gilep, A.A. Human Cytochrome P450 2C9 and its polymorphic modifications: Electroanalysis, catalytic properties, and approaches to the regulation of enzymatic activity. Processes 2022, 10, 383. [Google Scholar] [CrossRef]
- Wu, J.; Guan, X.; Dai, Z.; He, R.; Ding, X.; Yang, L.; Ge, G. Molecular probes for human cytochrome P450 enzymes: Recent progress and future perspectives. Coord. Chem. Rev. 2021, 421, 213600. [Google Scholar] [CrossRef]
- Kuzikov, A.V.; Bulko, T.V.; Koroleva, P.I.; Masamrekh, R.A.; Babkina, S.S.; Gilep, A.A.; Shumyantseva, V.V. Cytochrome P450 3A4 as a drug metabolizing enzyme: The role of sensor system modifications in electocatalysis and electroanalysis. Biochem. Mosc. Suppl. Ser. B 2020, 66, 64–70. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Kuzikov, A.V.; Masamrekh, R.A.; Bulko, T.V.; Archakov, A.I. From electrochemistry to enzyme kinetics of cytochrome P450. Biosens. Bioelectron. 2018, 121, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Koroleva, P.I.; Bulko, T.V.; Shkel, T.V.; Gilep, A.A.; Veselovsky, A.V. Approaches for increasing the electrocatalitic efficiency of cytochrome P450 3A4. Bioelectrochemistry 2023, 149, 108277. [Google Scholar] [CrossRef]
- Shangguan, L.; Wei, Y.; Liu, X.; Yu, J.; Liu, S. Confining a bi-enzyme inside the nanochannels of a porous aluminum oxide membrane for accelerating the enzymatic reactions. Chem. Comm. 2017, 53, 2673–2676. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Kuzikov, A.V.; Masamrekh, R.A.; Filippova, T.A.; Koroleva, P.I.; Agafonova, L.E.; Bulko, T.V.; Archakov, A.I. Enzymology on an electrode and in a nanopore: Analysis algorithms, enzyme kinetics, and perspectives. BioNanoScience 2022, 12, 1341–1355. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Koroleva, P.I.; Gilep, A.A.; Napolskii, K.S.; Ivanov, Y.D.; Kanashenko, S.L.; Archakov, A.I. Increasing the Efficiency of Cytochrome P450 3A4 Electrocatalysis Using Electrode Modification with Spatially Ordered Anodic Aluminum Oxide-Based Nanostructures for Investigation of Metabolic Transformations of Drugs. Dokl. Biochem. Biophys. 2022, 506, 215–219. [Google Scholar] [CrossRef]
- Walkera, A.; Walgamaa, C.; Nerimetlaa, R.; Alavib, S.H.; Echeverriac, E.; Harimkarb, S.P.; McIlroyc, D.N.; Krishnan, S. Roughened graphite biointerfaced with P450 liver microsomes: Surface and electrochemical characterizations. Colloids Surf. B 2020, 189, 110790. [Google Scholar] [CrossRef] [PubMed]
- Nerimetla, R.; Walgama, C.; Singh, V.; Hartson, S.D.; Krishnan, S. Mechanistic insights into Voltage-Driven Biocatalysis of a cytochrome P450 bactosomal film on a self- assembled monolayer. ACS Catal. 2017, 7, 3446–3453. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Guengerich, F.P.; Ma, L.; Li, S.; Zhang, W. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 2020, 295, 833–849. [Google Scholar] [CrossRef]
- Palmer, M.; Harris, R.; Freytag, C.; Kehoe, M.; Tranum-Jensen, J.; Bhakdi, S. Assembly mechanism of the oligomeric streptolysin O pore: The early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 1998, 17, 1598–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkop, T.; Xu, D.; Cheng, Q. Electrochemical Characterization of Pore Formation by Bacterial Protein Toxins on Hybrid Supported Membranes. Langmuir 2008, 24, 5615–5621. [Google Scholar] [CrossRef]
- Teng, K.W.; Ishitsuka, Y.; Ren, P.; Youn, Y.; Deng, X.; Ge, P.; Lee, S.H.; Belmont, A.S.; Selvin, P.R. Labeling proteins inside living cells using external fluorophores for microscopy. eLife 2016, 5, e20378. [Google Scholar] [CrossRef]
- Sadeghi, S.J.; Fantuzzi, A.; Gilardi, G. Breakthrough in P450 bioelectrochemistry and future perspectives. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 237–248. [Google Scholar] [CrossRef]
- Cheropkina, H.; Catucci, G.; Cesano, F.; Marucco, A.; Gilardi, G.; Sadeghi, S.J. Bioelectrochemical platform with human monooxygenases: FMO1 and CYP3A4 tandem reactions with phorate. Bioelectrochemistry 2023, 150, 108327. [Google Scholar] [CrossRef]
- Gilep, A.A.; Guryev, O.V.; Usanov, S.A.; Estabrook, R.W. Reconstitution of the enzymatic activities of cytochrome P450s using recombinant flavocytochromes containing rat cytochrome b(5) fused to NADPH–cytochrome P450 reductase with various membrane-binding segments. Arch. Biochem. Biophys. 2001, 390, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Sato, R. The Carbon Monoxide-binding Pigment of Liver Microsomes: II. Solubilization, purification, and properties. J. Biol. Chem. 1964, 239, 2379–2385. [Google Scholar] [CrossRef]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masamrekh, R.A.; Kuzikov, A.V.; Haurychenka, Y.I.; Shcherbakov, K.A.; Veselovsky, A.V.; Filimonov, D.A.; Dmitriev, A.V.; Zavialova, M.G.; Gilep, A.A.; Shkel, T.V.; et al. In Vitro interactions of abiraterone, erythromycin, and CYP3A4: Implications for drug–drug interactions. Fundam. Clin. Pharmacol. 2020, 34, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.J.; Ferrero, S.; Di Nardo, G.; Gilardi, G. Drug–drug interactions and cooperative effects detected in electrochemically driven human cytochrome P450 3A4. Bioelectrochemistry 2012, 86, 87–91. [Google Scholar] [CrossRef]
- Filonov, A.; Yaminsky, I. Scanning Probe Microscopy Image Processing Software User’s Manual “FemtoScan”; Version 2.2.90; Advanced Technologies Center: Moscow, Russia, 2012; p. 82. [Google Scholar]
- Li, X.-F.; Zhang, G.-Y.; Dong, J.-F.; Zhou, X.-H.; Hong, X.-L. An Atomic Force Microscopy Study on Small Unilamellar Vesicle Structures on Mica. Chin. J. Chem. 2006, 24, 311–315. [Google Scholar] [CrossRef]
- Boussaad, S.; Tao, N.J. Electron Transfer and Adsorption of Myoglobin on Self-Assembled Surfactant Films: An Electrochemical Tapping-Mode AFM Study. J. Am. Chem. Soc. 1999, 121, 4510–4515. [Google Scholar] [CrossRef]
- Randles, J.E.B. A cathode-ray polarograph. Part II—The current-voltage curves. Trans. Faraday Soc. 1948, 44, 327–338. [Google Scholar] [CrossRef]
- Sevcik, A. Oscillographic polarography with periodical triangular voltage. Collect. Czech ChemCommun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 32. [Google Scholar]
- Chen, H.C.; Chang, C.C.; Yang, K.H.; Mai, F.D.; Tseng, C.L.; Chen, L.Y.; Hwang, B.J.; Liu, Y.C. Polypyrrole electrode with a greater electroactive surface electrochemically polymerized in plasmon-activated water. J. Taiwan Inst. Chem. Eng. 2018, 82, 252–260. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Bulko, T.V.; Kozin, M.S.; Zhang, W.; Köhler, M.; Romanenko, I.; Yuan, J.; Schacher, F.H.; Pergushov, D.V.; Shumyantseva, V.V. Long-term stable poly(ionic liquid)/MWCNTs inks enable enhanced surface modification for electrooxidative detection and quantification of dsDNA. Polymer 2019, 168, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.J. The interaction of proteins with solid surfaces. Curr. Opin. Structur. Biol. 2004, 14, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F. Enzyme Bioelectrochemistry in Cast Biomembrane-Like Films. Acc. Chem. Res. 1998, 31, 363–369. [Google Scholar] [CrossRef]
- Baas, B.J.; Denisov, I.G.; Sligar, S.G. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch. Biochem. Biophys. 2004, 430, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Panicco, P.; Castrignanò, S.; Sadeghi, S.J.; Di Nardo, G.; Gilardi, G. Engineered human CYP2C9 and its main polymorphic variants for bioelectrochemical measurements of catalytic response. Bioelectrochemistry 2021, 138, 107729. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.W.; Bard, A.J. Electroanalytical Chemistry; Marcel Dekker: New York, NY, USA, 2001; p. 191. [Google Scholar]
- Rusling, J.F.; Wang, B.; Yun, S. Electrochemistry of redox enzymes. In Bioelectrochemistry: Fundametals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; JohnWiley & Sons, Ltd.: New Jersey, NJ, USA, 2008; pp. 39–85. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Dodhia, V.R.; Sassone, C.; Fantuzzi, A.; Di Nardo, G.; Sadeghi, S.J.; Gilardi, G. Modulating the coupling efficiency of human cytochrome P450 CYP3A4 at electrode surfaces through protein engineering. Electrochem. Commun. 2008, 10, 1744–1747. [Google Scholar] [CrossRef]
- Zanger, U.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Marchant, J.M.; Petsky, H.L.; Morris, P.S.; Chang, A.B. Antibiotics for prolonged wet cough in children. Cochrane Database Syst. Rev. 2018, 7, CD004822. [Google Scholar] [CrossRef] [Green Version]
- Amsden, G.W. Erythromycin, clarithromycin, and azithromycin: Are the differences real? Clin. Ther. 1996, 18, 56–72. [Google Scholar] [CrossRef]
- Riley, R.J.; Howbrook, D. In Vitro analysis of the activity of the major human hepatic CYP enzyme (CYP3A4) using [N-methyl-14C]-erythromycin. J. Pharmacol. Toxicol. Methods 1997, 38, 189–193. [Google Scholar] [CrossRef]


| Electrode | Ec, V | Ea, V | E0′, V | ΔE, V | ks, s−1 | Γ0, mol/cm2 |
|---|---|---|---|---|---|---|
| SPE/DDAB/ CYP3A4 [14] | −0.377 ± 0.008 | −0.227 ± 0.010 | −0.302 ± 0.010 | −0.150 ± 0.009 | 0.51 ± 0.030 | 2.7 ± 0.2 ×10−11 |
| SPE/DDAB/SLO/ CYP3A4 | −0.441 ± 0.016 | −0.209 ± 0.032 | −0.325 ± 0.024 | −0.230 ± 0.044 | 0.203 ± 0.038 | 1.75 ± 0.26 × 10−11 |
| Electrode | Ered, V | Ecat, V | Eonset, V | IO2, A × 10−7 | IEr, A × 10−7 | IEr/IO2 | Sensitivity, nA µM−1 cm−1 |
|---|---|---|---|---|---|---|---|
| SPE/DDAB/ CYP3A4 | −0.438 ± 0.006 | −0.438 ± 0.006 | −0.228 ± 0.003 | −3.71 ± 0.51 | −6.05 ± 0.63 | 1.63 ± 0.14 | 193 ± 21 |
| SPE/DDAB/ SLO/CYP3A4 | −0.462 ± 0.001 | −0.445 ± 0.006 | −0.264 ± 0.020 | −9.01 ± 1.59 | −12.22 ± 2.49 | 1.57 ± 0.17 | 389 ± 45 |
| Electrode | Icat max, A | KM, M | Vmax, M·min−1 |
|---|---|---|---|
| SPE/DDAB/CYP3A4 | 8.69 ± 0.94 × 10−8 | 8.98 ± 1.2 × 10−5 | 1.52 ± 0.34 × 10−10 |
| SPE/DDAB/SLO/CYP3A4 | 15.8 ± 1.7 × 10−8 | 20.7 ± 2.5 × 10−5 | 4.52 ± 0.33 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroleva, P.I.; Gilep, A.A.; Kraevsky, S.V.; Tsybruk, T.V.; Shumyantseva, V.V. Improving the Efficiency of Electrocatalysis of Cytochrome P450 3A4 by Modifying the Electrode with Membrane Protein Streptolysin O for Studying the Metabolic Transformations of Drugs. Biosensors 2023, 13, 457. https://doi.org/10.3390/bios13040457
Koroleva PI, Gilep AA, Kraevsky SV, Tsybruk TV, Shumyantseva VV. Improving the Efficiency of Electrocatalysis of Cytochrome P450 3A4 by Modifying the Electrode with Membrane Protein Streptolysin O for Studying the Metabolic Transformations of Drugs. Biosensors. 2023; 13(4):457. https://doi.org/10.3390/bios13040457
Chicago/Turabian StyleKoroleva, Polina I., Andrei A. Gilep, Sergey V. Kraevsky, Tatiana V. Tsybruk, and Victoria V. Shumyantseva. 2023. "Improving the Efficiency of Electrocatalysis of Cytochrome P450 3A4 by Modifying the Electrode with Membrane Protein Streptolysin O for Studying the Metabolic Transformations of Drugs" Biosensors 13, no. 4: 457. https://doi.org/10.3390/bios13040457
APA StyleKoroleva, P. I., Gilep, A. A., Kraevsky, S. V., Tsybruk, T. V., & Shumyantseva, V. V. (2023). Improving the Efficiency of Electrocatalysis of Cytochrome P450 3A4 by Modifying the Electrode with Membrane Protein Streptolysin O for Studying the Metabolic Transformations of Drugs. Biosensors, 13(4), 457. https://doi.org/10.3390/bios13040457






