Carbon Nanofiber-Ionic Liquid Nanocomposite Modified Aptasensors Developed for Electrochemical Investigation of Interaction of Aptamer/Aptamer–Antisense Pair with Activated Protein C
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus
2.1.1. Electrode Preparation
2.1.2. Chemicals
2.1.3. Preparation of DNA-Apt and AD22
2.1.4. Preparation of APC, PC, BSA, or THR Solution
2.1.5. The Preparation of CNF-IL Dispersion
2.2. Procedure
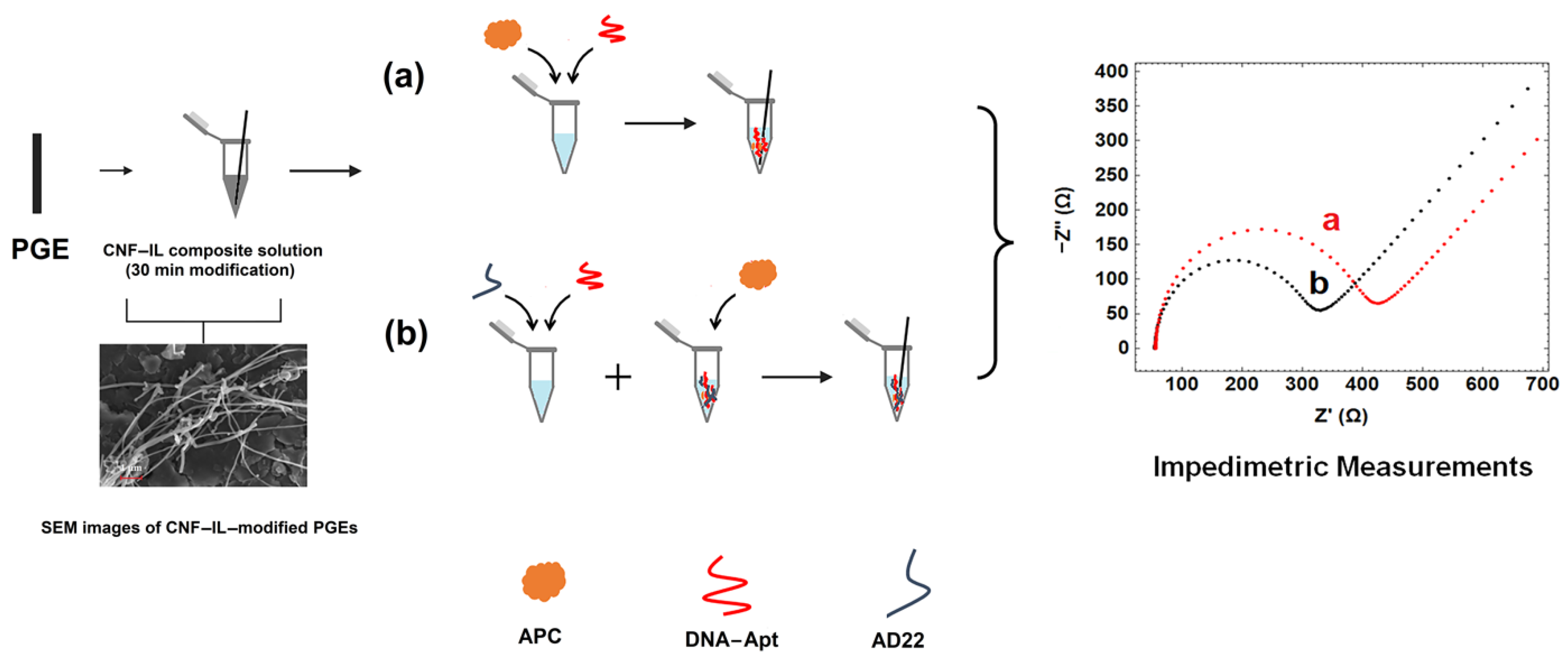
- First, the carbon nanomaterial, CNF, was dispersed in the solution of IL by sonication for 30 min.
- The obtained CNF/IL composite material was modified onto the electrode surface for 30 min.
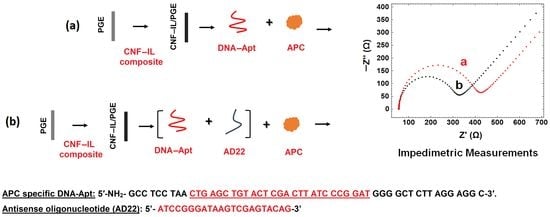
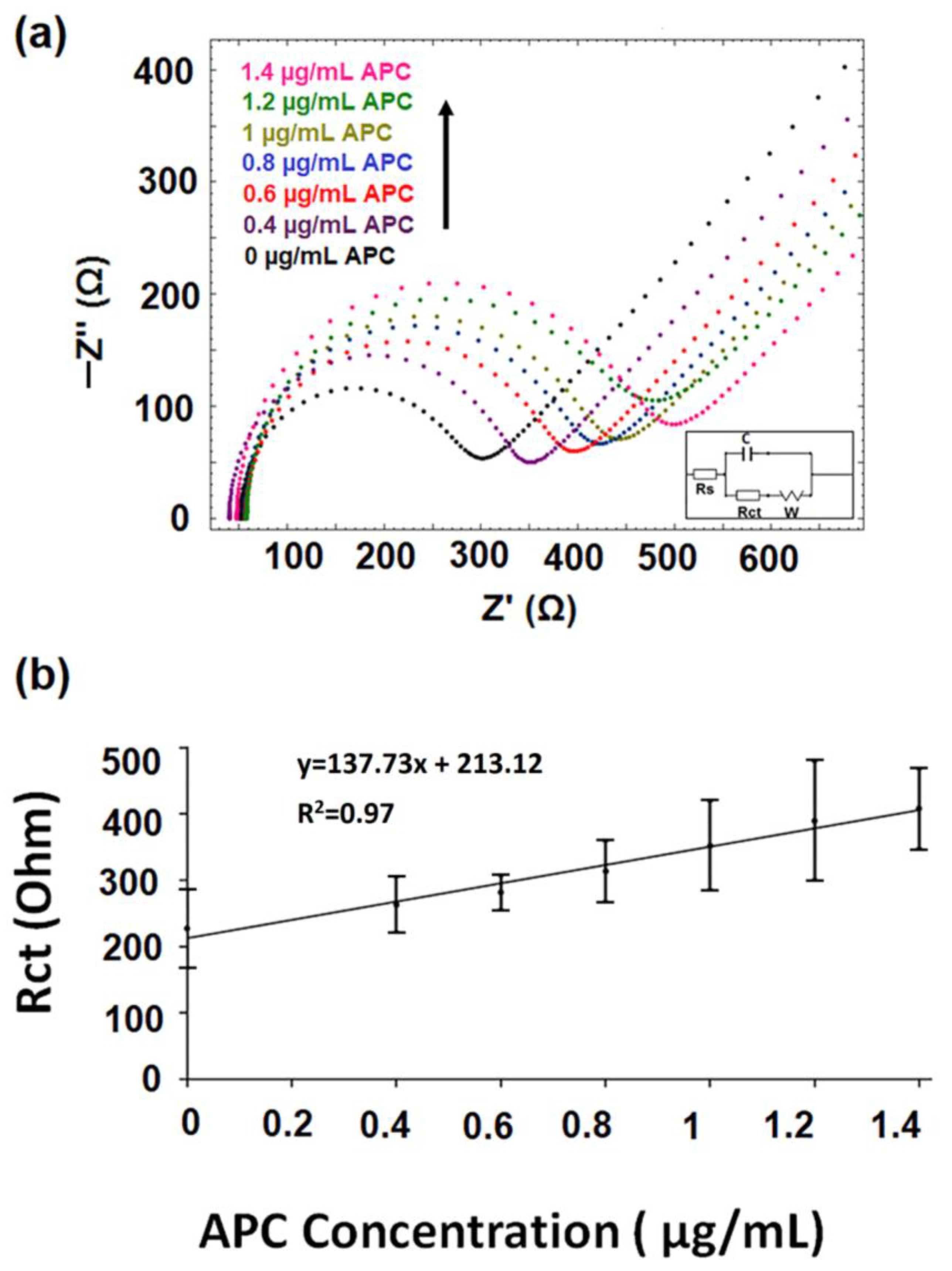
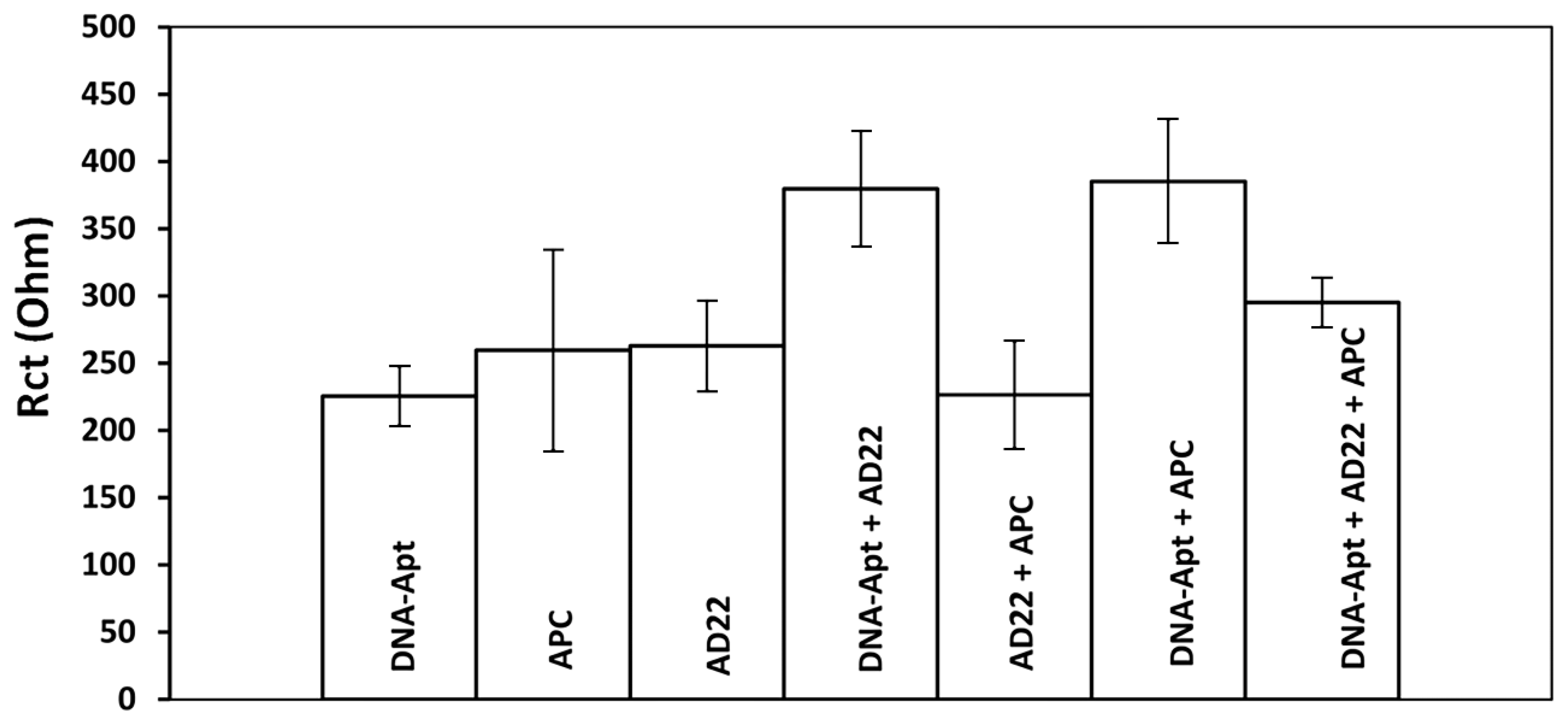
- The interaction of DNA-Apt with APC in its various concentration and the detection of APC was investigated using the EIS method and CNF/IL modified disposable electrode (a). After the aptamer binds to its target APC, an increase in Rct value was observed since the charge transfer is prevented on the electrode surface.
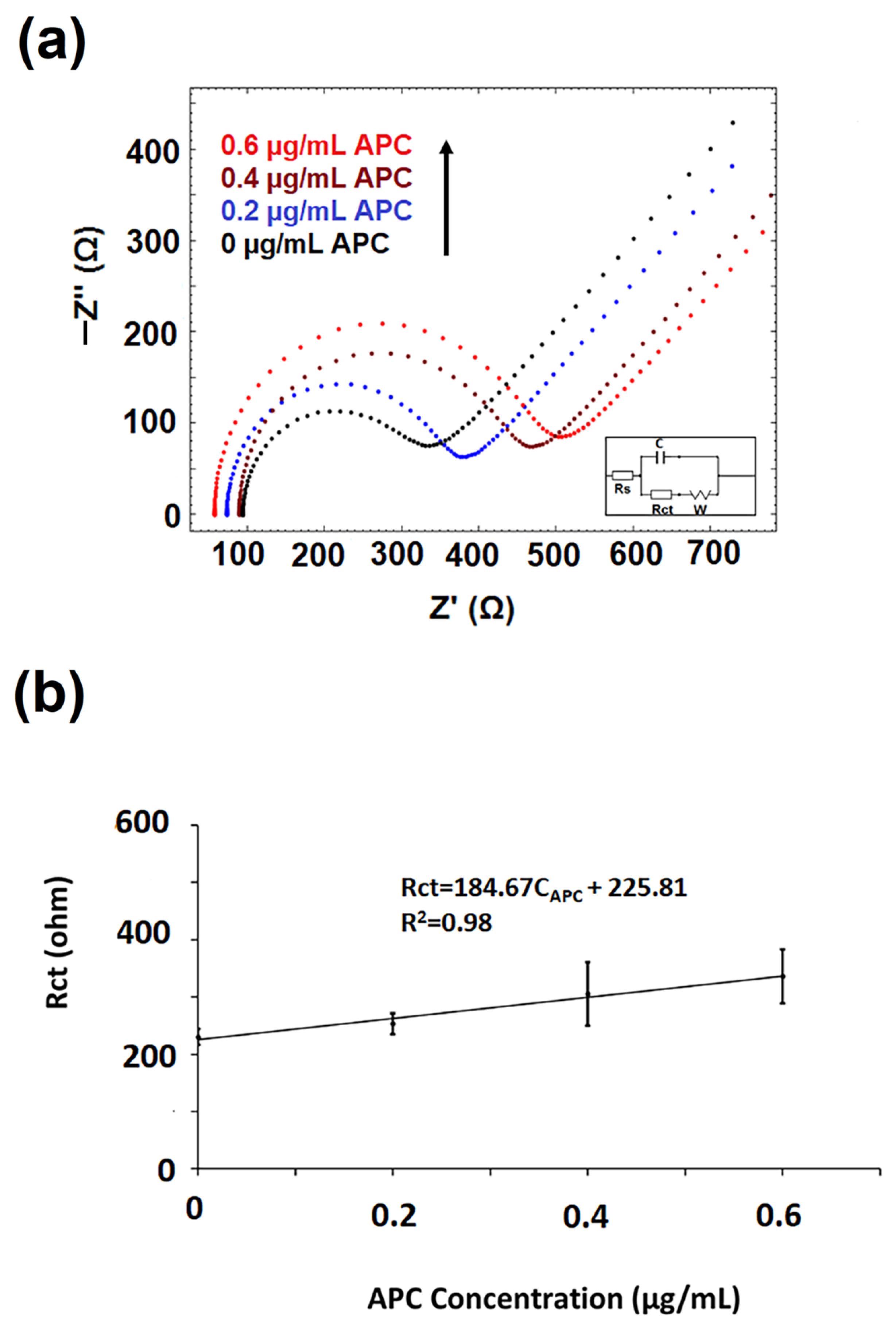
- The interaction of aptamer–antisense pair (AD22), which is effective in APC inhibition, with APC was carried out. The interaction of DNA-Apt, AD22 and APC in its various concentration and the detection of APC was investigated using the EIS method and CNF/IL modified disposable electrode First, the interaction of DNA-Apt and AD22 in the solution phase was performed for 2 min, then APC was added into the medium. After that, this sample was mixed for one minute. Accordingly, a decrease in Rct value was observed in the presence of DNA-Apt-AD22 complex containing APC (b).
2.2.1. Preparation of IL-CNF/PGEs
2.2.2. Preparation of Aptamer Immobilized IL-CNF Modified Electrodes and the Interaction of Aptamer with APC
2.2.3. Impedimetric Detection of Interaction of DNA Aptamer with APC in the Presence of Its Antidote
2.2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Microscopic Characterization of the Electrodes by Using Scanning Electron Microscopy (SEM)
- It was observed that the carbon (%) weight level at the PGE surface gradually decreased after the modification of CNF and CNF-IL. The decrease in the carbon (%) weight level at the PGE surface was observed after CNF-IL composite modification onto the electrode surface, which is a result of the modification of the graphite layer consisting of carbon layers on the PGE surface with nanocomposite material.
- After IL modification onto the PGE surface, the presence of F and P elements was observed at the electrode surface. After modification of IL and CNF-IL onto the PGE surface, it was observed that there is a gradual decrease in the weight level of the F and P elements (%), and the highest coating at the PGE surface occurred in the presence of the CNF-IL composite modification.
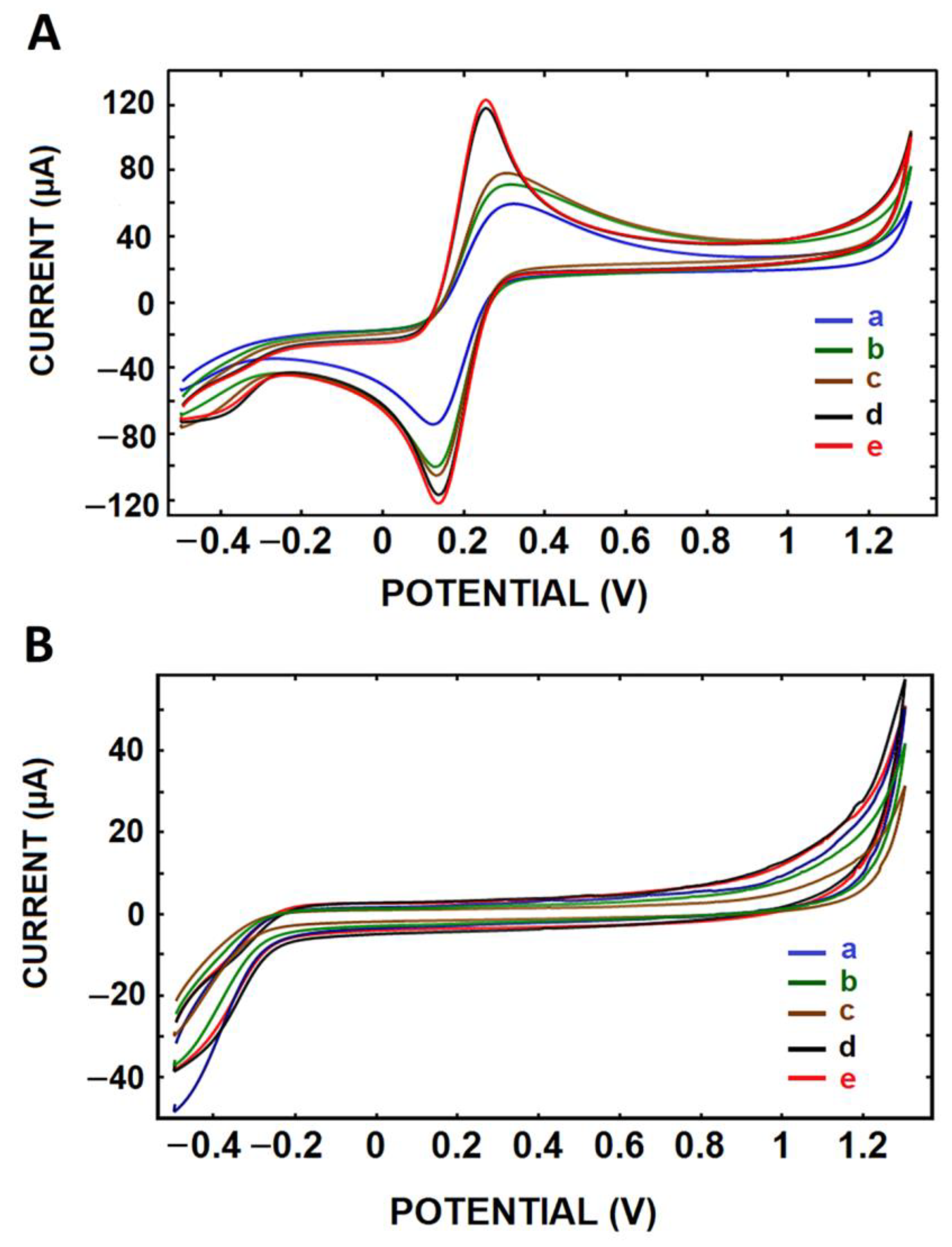
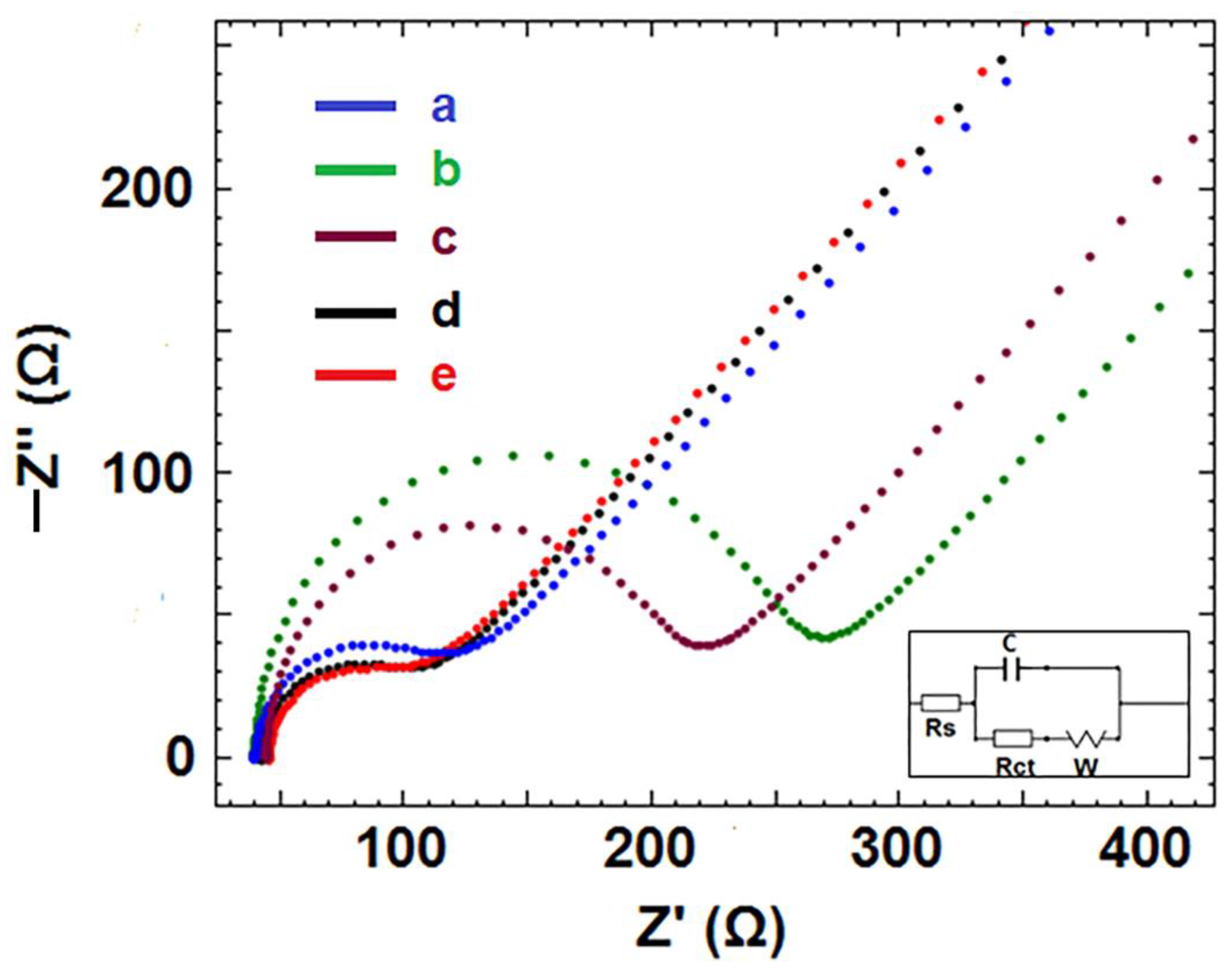
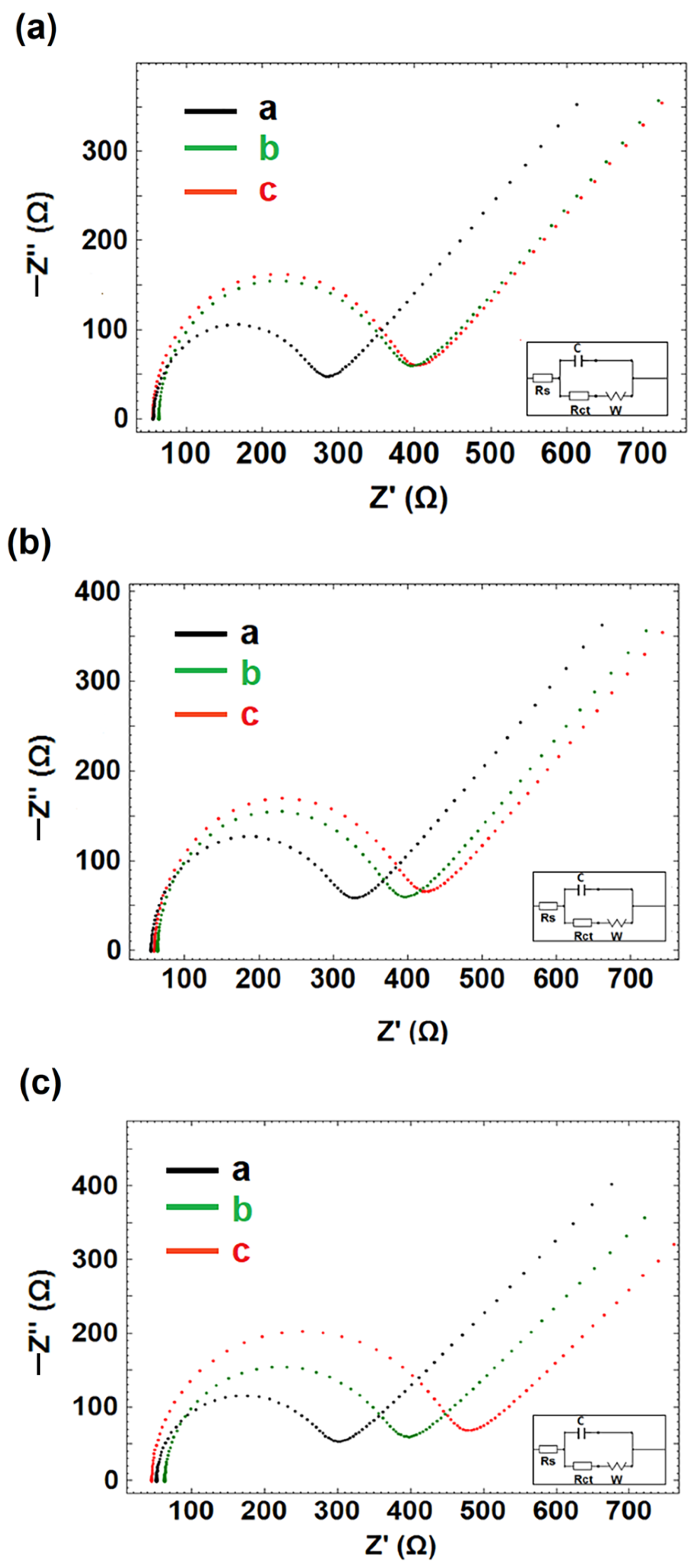
3.2. Electrochemical Characterization of CNF/IL Modified Electrodes
3.3. The Optimization of DNA-Apt Concentration
3.4. The Optimization of APC Concentration in Buffer Medium
3.5. The Optimization of AD22 Concentration
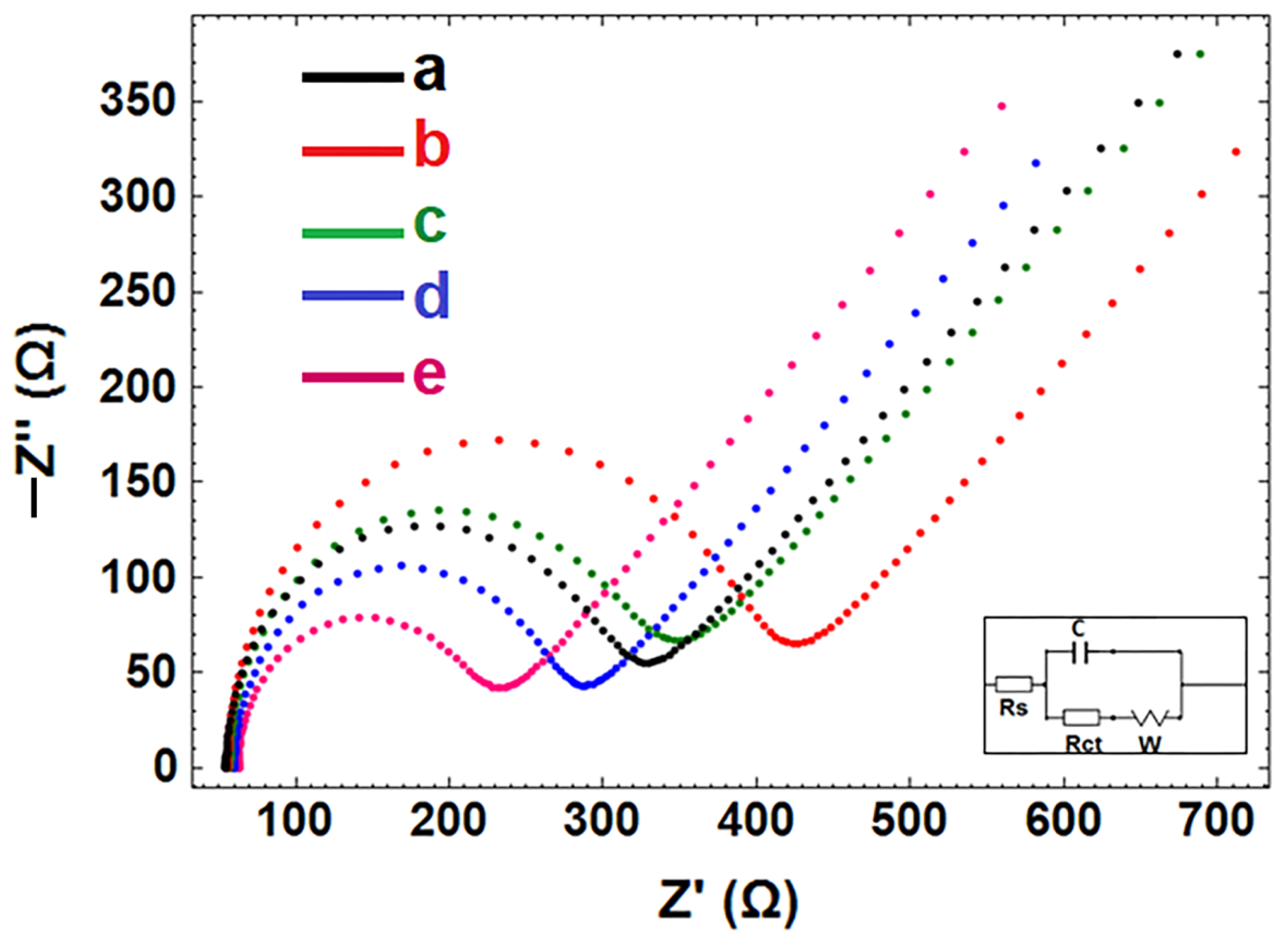
3.6. The Optimization of Interaction Time of Aptamer-AD22 with Protein, APC
- In the presence of interaction between AD22 and DNA-Apt, a decrease of 15.28% was observed in contrast to the mean Rct value (344.67 ± 39.17 Ohm and RSD% = 11.36%, n = 3). Different APC interaction times (i.e, 2 and 5 min), the change in response resulted in a decrease of 0.29% and an increase of 18.76%, respectively.
- In the presence of interaction between DNA-Apt and APC, a decrease of 6.80% was observed in contrast to the mean Rct value (313.13 ± 96.76 Ohm and RSD% = 30.87%, n = 3). Different APC interaction times (i.e., 2 and 5 min), the change in response resulted in an increase of 26.50% and an increase of 52.54%, respectively.
3.7. The Applicability of APC Aptasensor in Artificial Serum Medium
3.8. Selectivity Studies
3.9. Impedimetric Detection of Interaction of Aptamer-AD22 with APC in FBS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdem, A. Nanomaterial-based electrochemical DNA sensing strategies. Talanta 2007, 74, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef]
- Ramnani, P.; Saucedo, N.; Mulchandani, A. Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 2016, 143, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Liu, Y.; You, T. Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A. Chitosan-carbon Nanofiber Modified Single-use Graphite Electrodes Developed for Electrochemical Detection of DNA Hybridization Related to Hepatitis B Virus. Electroanalysis 2016, 28, 2514–2521. [Google Scholar] [CrossRef]
- Apetrei, I.; Apetrei, C. Highly sensitive voltamperometric determination of pyritinol using carbon nanofiber/gold nanoparticle composite screen-printed carbon electrode. Int. J. Nanomed. 2017, 12, 5177–5188. [Google Scholar] [CrossRef] [Green Version]
- Erdem, A.; Congur, G.; Mayer, G. Aptasensor platform based on carbon nanofibers enriched screen printed electrodes for impedimetric detection of thrombin. J. Electroanal. Chem. 2015, 758, 12–19. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, X.; Wang, M.; Liu, Y.; Gao, L.; Dong, S. Electrochemical and bioelectrochemistry properties of room-temperature ionic liquids and carbon composite materials. Anal. Chem. 2004, 76, 4960–4967. [Google Scholar] [CrossRef]
- Eksin, E.; Muti, M.; Erdem, A. Chitosan/Ionic Liquid Composite Electrode for Electrochemical Monitoring of the Surface-Confined Interaction Between Mitomycin C and DNA. Electroanalysis 2013, 25, 2321–2329. [Google Scholar] [CrossRef]
- Erdem, A.; Muti, M.; Mese, F.; Eksin, E. Chitosan-ionic liquid modified single-use sensor for electrochemical monitoring of sequence-selective DNA hybridization. Colloids Surf. B Biointerfaces 2014, 114, 261–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Zhan, D.; Liu, H.; Zhao, Q.; Kou, Y.; Shao, Y.; Li, M.; Zhuang, Q.; Zhu, Z. Selective detection of dopamine in the presence of ascorbic acid and uric acid by a carbon nanotubes-ionic liquid gel modified electrode. Talanta 2005, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Dong, S. Electrochemical Characteristics of Glucose Oxidase Adsorbed at Carbon Nanotubes Modified Electrode with Ionic Liquid as Binder. Electroanalysis 2007, 19, 55–59. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Liu, Y.; Zhao, J.; Ju, H. Highly sensitive amperometric biosensors for phenols based on polyaniline–ionic liquid–carbon nanofiber composite. Biosens. Bioelectron. 2009, 24, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiao, K.; Wang, X. Paste Electrode Based on Short Single-Walled Carbon Nanotubes and Room Temperature Ionic Liquid: Preparation, Characterization and Application in DNA Detection. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1361–1366. [Google Scholar] [CrossRef]
- Lu, X.; Hu, J.; Yao, X.; Wang, Z.; Li, J. Composite system based on chitosan and room-temperature ionic liquid: Direct electrochemistry and electrocatalysis of hemoglobin. Biomacromolecules 2006, 7, 975–980. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Doherty, A.P.; Murnaghan, K.D.; Brett, C.M. Application of some room temperature ionic liquids in the development of biosensors at carbon film electrodes. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, G.R.; Bansal, S.; Saharan, P.; Bansal, P.; Mehta, S.K. Applications of surface modified ionic liquid/nanomaterial composite in electrochemical sensors and biosensors. BioNanoScience 2013, 3, 241–253. [Google Scholar] [CrossRef]
- Shiddiky, M.J.; Torriero, A.A. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Rahim, A.; Muhammad, N.; Abbas, F. Ionic Liquids Modified Sensors and Biosensors for Detection of Environmental Contaminants. Nanosens. Technol. Environ. Monit. 2020, 259–273. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Işın, D.; Eksin, E.; Erdem, A. Graphene-Oxide and Ionic Liquid Modified Electrodes for Electrochemical Sensing of Breast Cancer 1 Gene. Biosensors 2022, 12, 95. [Google Scholar] [CrossRef]
- Famulok, M.; Hartig, J.; Mayer, G. Functional Aptamers and Aptazymes in Biotechnology, Diagnostics, and Therapy. Chem. Rev. 2007, 107, 3715–3743. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Muti, M. Chitosan–graphene oxide based aptasensor for the impedimetric detection of lysozyme. Colloids Surf. B Biointerfaces 2014, 115, 205–211. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef]
- Willner, I.; Zayats, M. Electronic Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2007, 46, 6408–6418. [Google Scholar] [CrossRef]
- Koyun, S.; Akgönüllü, S.; Yavuz, H.; Erdem, A.; Denizli, A. Surface plasmon resonance aptasensor for detection of human activated protein C. Talanta 2019, 194, 528–533. [Google Scholar] [CrossRef]
- Rohrbach, F.; Karadeniz, H.; Erdem, A.; Famulok, M.; Mayer, G. Label-free impedimetric aptasensor for lysozyme detection based on carbon nanotube-modified screen-printed electrodes. Anal. Biochem. 2012, 421, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Roupioz, Y.; Gasparutto, D.; Livache, T.; Buhot, A. Solution-phase vs surface-phase aptamer-protein affinity from a label-free kinetic biosensor. PLoS ONE 2013, 8, e75419. [Google Scholar] [CrossRef] [Green Version]
- Daems, E.; Moro, G.; Campos, R.; De Wael, K. Mapping the gaps in chemical analysis for the characterisation of aptamer-target interactions. Trac Trends Anal. Chem. 2021, 142, 116311. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A highly selective electrochemical impedance spectroscopy-based aptasensor for sensitive detection of acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef]
- Xie, W.; Han, C.; Qian, Y.; Ding, H.; Chen, X.; Xi, J. Determination of neonicotinoid pesticides residues in agricultural samples by solid-phase extraction combined with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 4426–4433. [Google Scholar] [CrossRef]
- Zhang, X.; Mobley, N.; Zhang, J.; Zheng, X.; Lu, L.; Ragin, O.; Smith, C.J. Analysis of agricultural residues on tea using d-SPE sample preparation with GC-NCI-MS and UHPLC-MS/MS. J. Agric. Food Chem. 2010, 58, 11553–11560. [Google Scholar] [CrossRef]
- Sugawara, Y.; Gee, S.J.; Sanborn, J.R.; Gilman, S.D.; Hammock, B.D. Development of a highly sensitive enzyme-linked immunosorbent assay based on polyclonal antibodies for the detection of polychlorinated dibenzo-p-dioxins. Anal. Chem. 1998, 70, 1092–1099. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.; Tan, Z.; Wang, H. Electrochemical impedance spectroscopy aptasensor for ultrasensitive detection of adenosine with dual backfillers. Biosens. Bioelectron. 2014, 60, 218–223. [Google Scholar] [CrossRef]
- Griffin, J.; Fernández, J.; Gale, A.; Mosnier, L. Activated protein C. J. Thromb. Haemost. 2007, 5, 73–80. [Google Scholar] [CrossRef]
- Colin, G.; Annane, D. Corticosteroids and Human Recombinant Activated Protein C for Septic Shock. Clin. Chest Med. 2008, 29, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ruf, W. New players in the sepsis-protective activated protein C pathway. J. Clin. Investig. 2010, 120, 3084–3087. [Google Scholar] [CrossRef] [PubMed]
- Solá, E.; Navarro, S.; Medina, P.; Vayá, A.; Estellés, A.; Hernández-Mijares, A.; España, F. Activated protein C levels in obesity and weight loss influence. Thromb. Res. 2009, 123, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.; van ’T Veer, C.; Poulussen, N.; Meijers, J.; Levi, M.; Esmon, C.; van der Poll, T. The cytoprotective effects of endogenous activated protein C reduce activation of coagulation during murine pneumococcal pneumonia and sepsis. Thromb. Res. 2015, 135, 537–543. [Google Scholar] [CrossRef]
- Wada, H.; Matsumoto, T.; Yamashita, Y.; Hatada, T. Disseminated intravascular coagulation: Testing and diagnosis. Clin. Chim. Acta 2014, 436, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, D.; España, F.; Panes, O.; Medina, P.; Pais, E.; Marshall, G.; Tagle, R.; Downey, P.; Caceres, S.; González, F.; et al. Increased activation of protein C, but lower plasma levels of free, activated protein C in uraemic patients: Relationship with systemic inflammation and haemostatic activation. Br. J. Haematol. 2001, 113, 905–910. [Google Scholar] [CrossRef]
- Becher, T.; Müller, J.; Akin, I.; Baumann, S.; Bosch, K.; Stach, K.; Borggrefe, M.; Pötzsch, B.; Loßnitzer, D. The evolution of activated protein C plasma levels in septic shock and its association with mortality: A prospective observational study. J. Crit. Care 2018, 47, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Ghosh, K. Novel therapeutic approaches for haemophilia. Haemophilia 2015, 21, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Polderdijk, S.; Adams, T.; Ivanciu, L.; Camire, R.; Baglin, T.; Huntington, J. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood 2017, 129, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Hamedani, N.; Rühl, H.; Zimmermann, J.; Heiseler, T.; Oldenburg, J.; Mayer, G.; Pötzsch, B.; Müller, J. In Vitro Evaluation of Aptamer-Based Reversible Inhibition of Anticoagulant Activated Protein C as a Novel Supportive Hemostatic Approach. Nucleic Acid Ther. 2016, 26, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Chabata, C.V.; Frederiksen, J.; Sullenger, B.; Gunaratne, R. Emerging applications of aptamers for anticoagulation and hemostasis. Curr. Opin. Hematol. 2018, 25, 382–388. [Google Scholar] [CrossRef]
- Balkaransingh, P.; Young, G. Novel therapies and current clinical progress in hemophilia A. Ther. Adv. Hematol. 2017, 9, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oto, J.; Fernández-Pardo, Á.; Miralles, M.; Plana, E.; España, F.; Navarro, S.; Medina, P. Activated protein C assays: A review. Clin. Chim. Acta 2020, 502, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.; Griffin, J. Direct detection of activated protein C in blood from human subjects. Blood 1992, 79, 2340–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orthner, C.; Kolen, B.; Drohan, W. A sensitive and facile assay for the measurement of activated protein C activity levels in vivo. Thromb. Haemost. 1993, 69, 441–447. [Google Scholar] [CrossRef]
- Katsuura, Y.; Mochizuki, T.; Tamura, M.; Hoshide, S.; Kiyoki, M.; Nakagaki, T.; Miyamoto, S. Species specificity of anticoagulant activity of activated human protein C. Involvement of factor V as well as protein S. Thromb. Res. 1996, 82, 147–157. [Google Scholar] [CrossRef]
- Takazoe, K.; Ogawa, H.; Yasue, H.; Sakamoto, T.; Oshima, S.; Arai, H.; Moriyama, Y.; Shimomura, H.; Hirai, N.; Kaikita, K.; et al. Association of Plasma Levels of Activated Protein C with Recanalization of the Infarct-Related Coronary Artery after Thrombolytic Therapy in Acute Myocardial Infarction. Thromb. Res. 1999, 95, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, X.; Gu, J.; Hunter, J.; Ferrell, G.; Lupu, F.; Esmon, N.; Esmon, C. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J. Thromb. Haemost. 2005, 3, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Kass, B.; Beeler, D.; Rosenberg, R. Detection of protein C activation in humans. J. Clin. Investig. 1984, 74, 2033–2041. [Google Scholar] [CrossRef]
- España, F.; Zuazu, I.; Vicente, V.; Estellés, A.; Marco, P.; Aznar, J. Quantification of circulating activated protein C in human plasma by immunoassays—Enzyme levels are proportional to total protein C levels. Thromb. Haemost. 1996, 75, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Martos, L.; Bonanad, S.; Ramón, L.; Cid, A.; Bonet, E.; Corral, J.; Miralles, M.; España, F.; Navarro, S.; Medina, P. A simplified assay for the quantification of circulating activated protein C. Clin. Chim. Acta 2016, 459, 101–104. [Google Scholar] [CrossRef]
- España, F.; Vayá, A.; Mira, Y.; Medina, P.; Estellés, A.; Villa, P.; Falcó, C.; Aznar, J. Low level of circulating activated protein C is a risk factor for venous thromboembolism. Thromb. Haemost. 2001, 86, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Congur, G. Dendrimer enriched single-use aptasensor for impedimetric detection of activated protein C. Colloids Surf. B Biointerfaces 2014, 117, 338–345. [Google Scholar] [CrossRef]
- Erdem, A.; Congur, G. Dendrimer modified 8-channel screen-printed electrochemical array system for impedimetric detection of activated protein C. Sens. Actuators B Chem. 2014, 196, 168–174. [Google Scholar] [CrossRef]
- Erdem, A.; Congur, G. Voltammetric aptasensor combined with magnetic beads assay developed for detection of human activated protein C. Talanta 2014, 128, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Congur, G.; Mese, F. Electrochemical detection of activated protein C using an aptasensor based on PAMAM dendrimer modified pencil graphite electrodes. Electroanalysis 2014, 26, 2580–2590. [Google Scholar] [CrossRef]
- Josic, D.; Hoffer, L.; Buchacher, A. Preparation of vitamin K-dependent proteins, such as clotting factors II, VII, IX and X and clotting inhibitor protein C. J. Chromatogr. B 2003, 790, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Ghalehno, M.H.; Mirzaei, M.; Torkzadeh-Mahani, M. Electrochemical aptasensor for activated protein C using a gold nanoparticle—Chitosan/graphene paste modified carbon paste electrode. Bioelectrochemistry 2019, 130, 107322. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Shamsipur, M.; Alizadeh, A.; Mousavi, M.F. Electrochemical behaviors of novel electroactive Au nanoparticles protected by self-assembled monolayers. J. Iran. Chem. Soc. 2013, 10, 333–338. [Google Scholar] [CrossRef]
- Chakraborty, A.; Tibarewala, D.N.; Barui, A. Impedance-based biosensors. In Bioelectronics and Medical Devices; Woodhead Publishing: Sawston, UKA, 2019; pp. 97–122. [Google Scholar] [CrossRef]
- Turner, M.W.; Hulme, B. The Plasma Proteins: An İntroduction; Pitman Medical: London, UK, 1971. [Google Scholar]
- Moro, G.; Bottari, F.; Liberi, S.; Covaceuszach, S.; Cassetta, A.; Angelini, A.; Moretto, L.M. Covalent immobilization of delipidated human serum albumin on poly (pyrrole-2-carboxylic) acid film for the impedimetric detection of perfluorooctanoic acid. Bioelectrochemistry 2020, 134, 107540. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.E.; Acero Sánchez, J.L.; Baldrich, E.; O’Sullivan, C.K. Reusable impedimetric aptasensor. Anal. Chem. 2005, 77, 6320–6323. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Chang, R.; Cardenas, J.; Wade, C.; Holcomb, J. Advances in the understanding of trauma-induced coagulopathy. Blood 2016, 128, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Eksin, E.; Polat, D.; Erdem, A. Voltammetric and Impedimetric Detection of Interaction Between Dacarbazine and Nucleic Acids. Electroanalysis 2019, 31, 2012–2019. [Google Scholar] [CrossRef]
- Erdem, A.; Eksin, E. Electrochemical Detection of Solution Phase Hybridization Related to Single Nucleotide Mutation by Carbon Nanofibers Enriched Electrodes. Materials 2019, 12, 3377. [Google Scholar] [CrossRef] [Green Version]
- Cummings, T.; Elving, P. Determination of the Electrochemically Effective Electrode Area. Anal. Chem. 1978, 50, 480–488. [Google Scholar] [CrossRef]
- Janek, R.; Fawcett, W.; Ulman, A. Impedance Spectroscopy of Self-Assembled Monolayers on Au(111): Sodium Ferrocyanide Charge Transfer at Modified Electrodes. Langmuir 1998, 14, 3011–3018. [Google Scholar] [CrossRef]
- Haemtech. Available online: http://www.haemtech.com/Enzymes/aPC.htm (accessed on 1 January 2022).
- Erdem, A.; Karadeniz, H.; Caliskan, A. Single-walled carbon nanotubes modified graphite electrodes for electrochemical monitoring of nucleic acids and biomolecular interactions. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 464–471. [Google Scholar] [CrossRef]
- Müller, J.; Isermann, B.; Dücker, C.; Salehi, M.; Meyer, M.; Friedrich, M.; Pötzsch, B. An exosite-specific ssDNA aptamer inhibits the anticoagulant functions of activated protein C and enhances inhibition by protein C inhibitor. Chem. Biol. 2009, 16, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Experimental Group | Average Rct Values Measured Only in the Presence of AD22 | Average Rct Value Measured with 0.1 µg/mL DNA-Apt in the Presence of AD22 | RSD% | Change in the Rct Value |
|---|---|---|---|---|
| 0.1 µg/mL DNA-Apt (control grup) | 213.33 ± 14.15 | - | 6.63 | 6.63 fold increase * |
| 0.05 µg/mL AD22 | 317.50 ± 82.73 | 206.00 ± 22.63 | 10.98 | 35.12% decrease |
| 0.1 µg/mL AD22 | 318.00 ± 30.14 | 366.00 ± 71.39 | 19.51 | 15.09% increase |
| 0.2 µg/mL AD22 | 345.00 ± 92.93 | 293.33 ± 64.94 | 22.14 | 14.98% decrease |
| Method | Detection Limit | Reference |
|---|---|---|
| SPR | 1.5 ng/mL | [28] |
| EIS | 0.74 µg/mL in buffer | [60] |
| 2.03 µg/mL in FBS | ||
| EIS | 1.81 µg/mL in buffer | [61] |
| 0.02 µg/mL in FBS | ||
| DPV | 2.35 µg/mL in buffer | [62] |
| EIS, CV | 0.94 µg/mL in buffer | [63] |
| 1.5 µg/mL in FBS | ||
| DPV | 0.73 × 10−5 µg/mL | [65] |
| EIS | 0.23 µg/mL in buffer | This work |
| 0.11 µg/mL in FBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maral, M.; Erdem, A. Carbon Nanofiber-Ionic Liquid Nanocomposite Modified Aptasensors Developed for Electrochemical Investigation of Interaction of Aptamer/Aptamer–Antisense Pair with Activated Protein C. Biosensors 2023, 13, 458. https://doi.org/10.3390/bios13040458
Maral M, Erdem A. Carbon Nanofiber-Ionic Liquid Nanocomposite Modified Aptasensors Developed for Electrochemical Investigation of Interaction of Aptamer/Aptamer–Antisense Pair with Activated Protein C. Biosensors. 2023; 13(4):458. https://doi.org/10.3390/bios13040458
Chicago/Turabian StyleMaral, Meltem, and Arzum Erdem. 2023. "Carbon Nanofiber-Ionic Liquid Nanocomposite Modified Aptasensors Developed for Electrochemical Investigation of Interaction of Aptamer/Aptamer–Antisense Pair with Activated Protein C" Biosensors 13, no. 4: 458. https://doi.org/10.3390/bios13040458
APA StyleMaral, M., & Erdem, A. (2023). Carbon Nanofiber-Ionic Liquid Nanocomposite Modified Aptasensors Developed for Electrochemical Investigation of Interaction of Aptamer/Aptamer–Antisense Pair with Activated Protein C. Biosensors, 13(4), 458. https://doi.org/10.3390/bios13040458