Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing
Abstract
1. Introduction
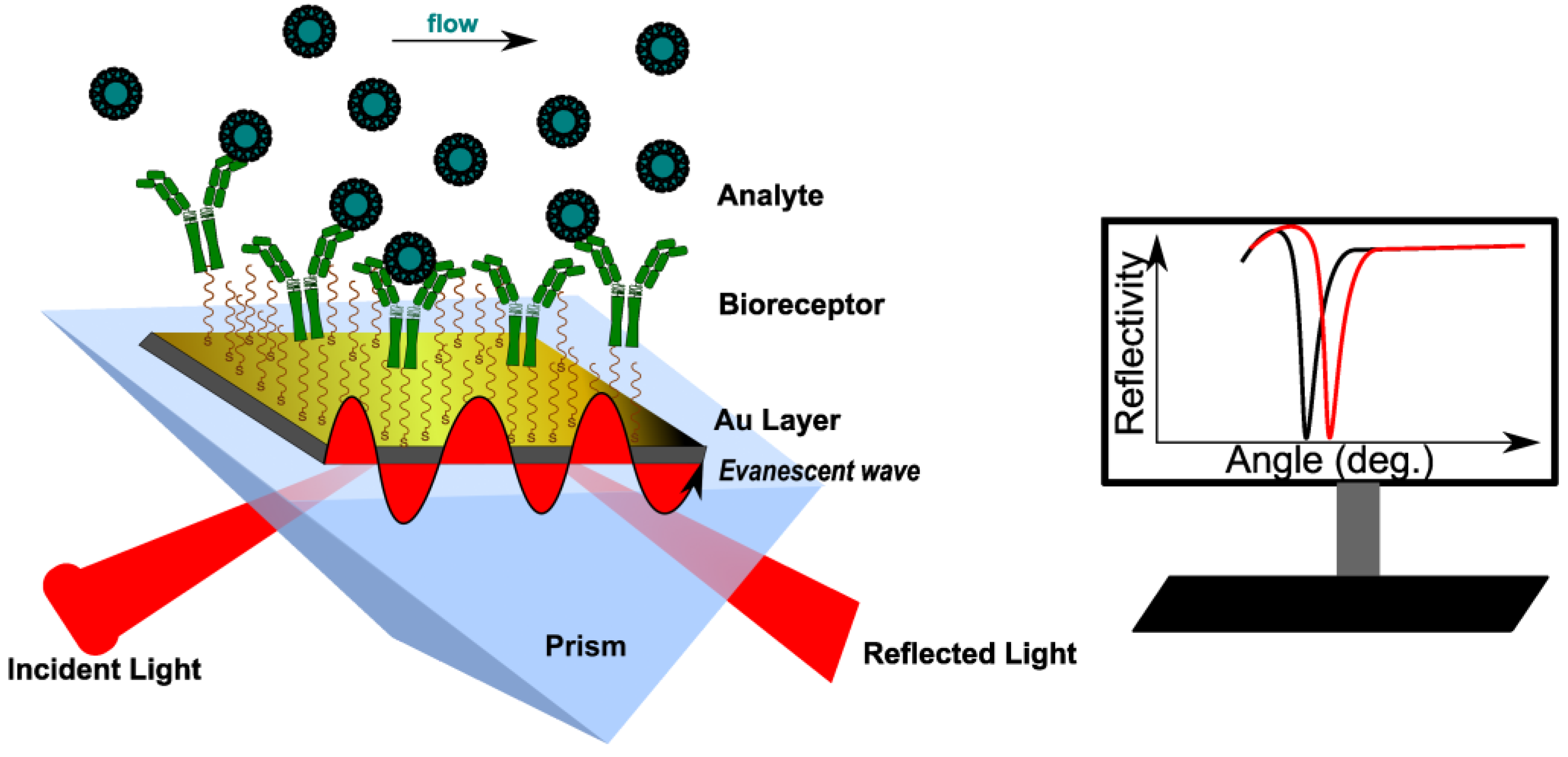
2. SPR Operating Principle
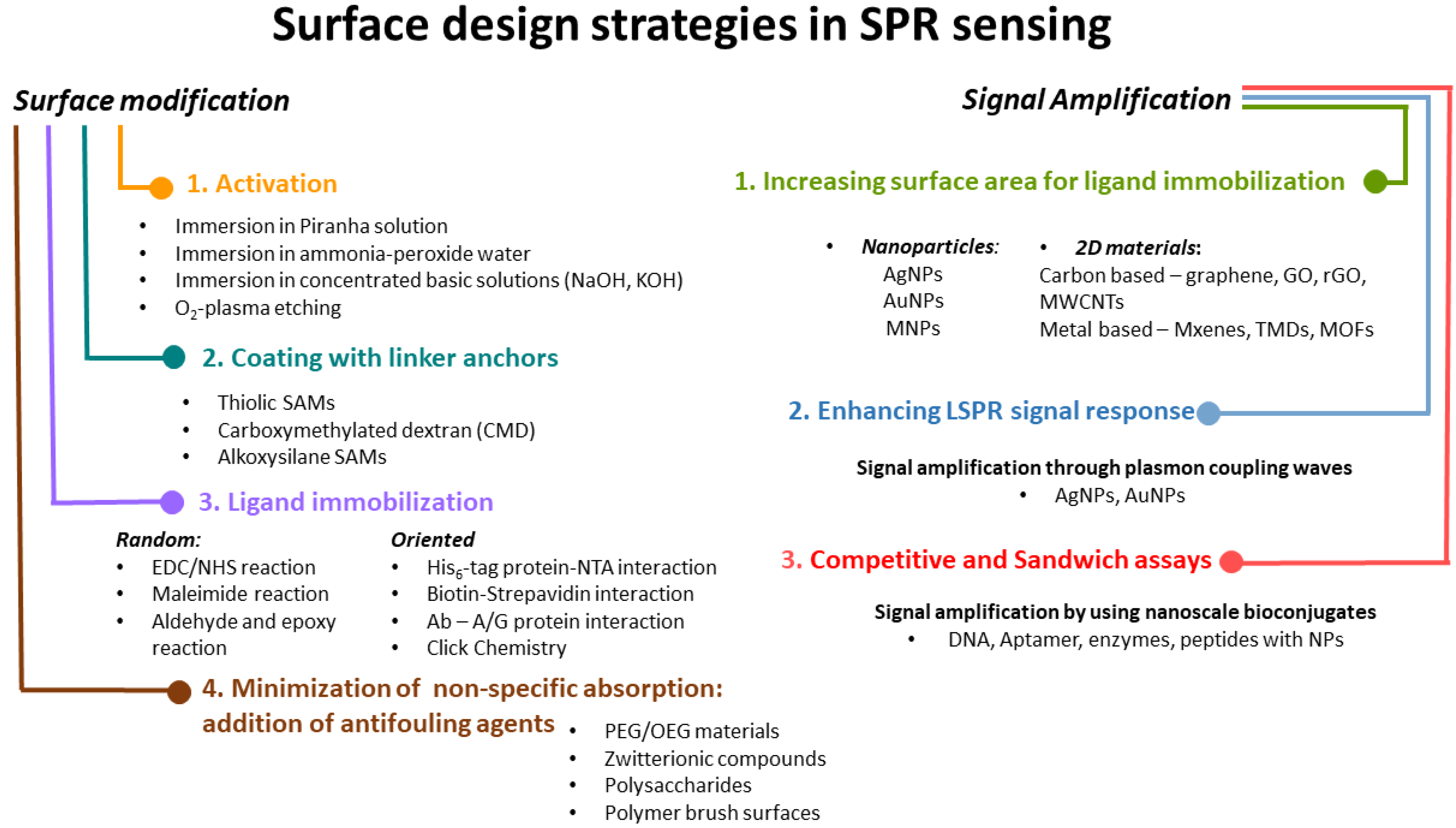
3. Surface Modification in SPR Assays: Coating Strategies
3.1. Surface Activation
3.2. Thiol Anchoring
3.3. Dextran-Based Hydrogel Attachment
3.4. Silane Attachment
4. Ligand Immobilization onto Coated SPR Chip
4.1. Random Covalent Immobilization via EDC/NHS Esters
4.2. Random Covalent Binding through Maleimide Reaction
4.3. Random Covalent Reactions Involving Aldehyde and Epoxy Groups
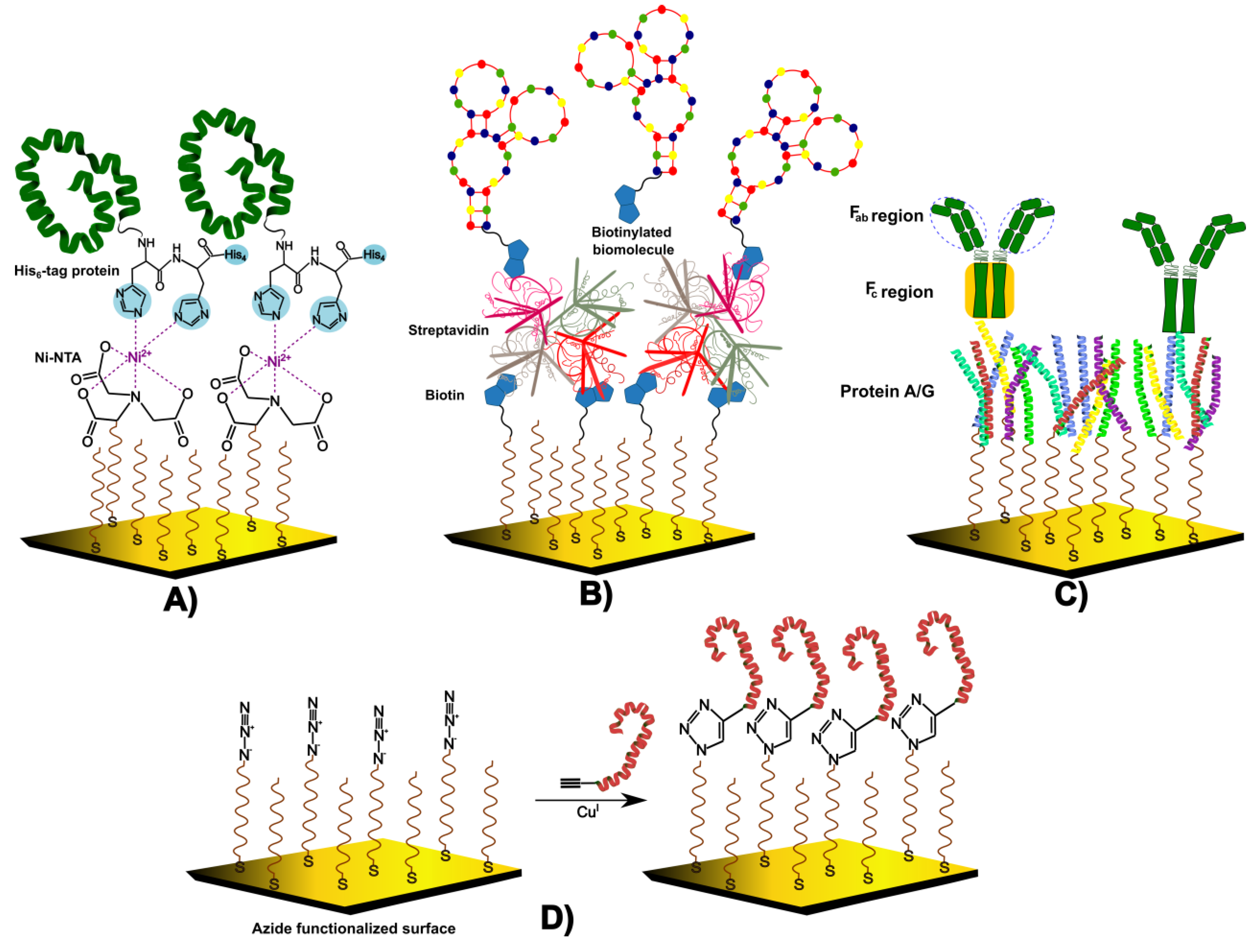
4.4. Oriented Immobilization Strategies
4.5. Minimizing Non-Specific Adsorption: Addition of Anti-Fouling Agents
5. Strategies for Signal Amplification
5.1. Gold and Silver Nanostructures for Surface Modification in Localised Surface Plasmon Resonance (LSPR) Experiments
5.2. Magnetic Nanoparticles (MNPs) Bioconjugates for Signal Enhancement in SPR Assays
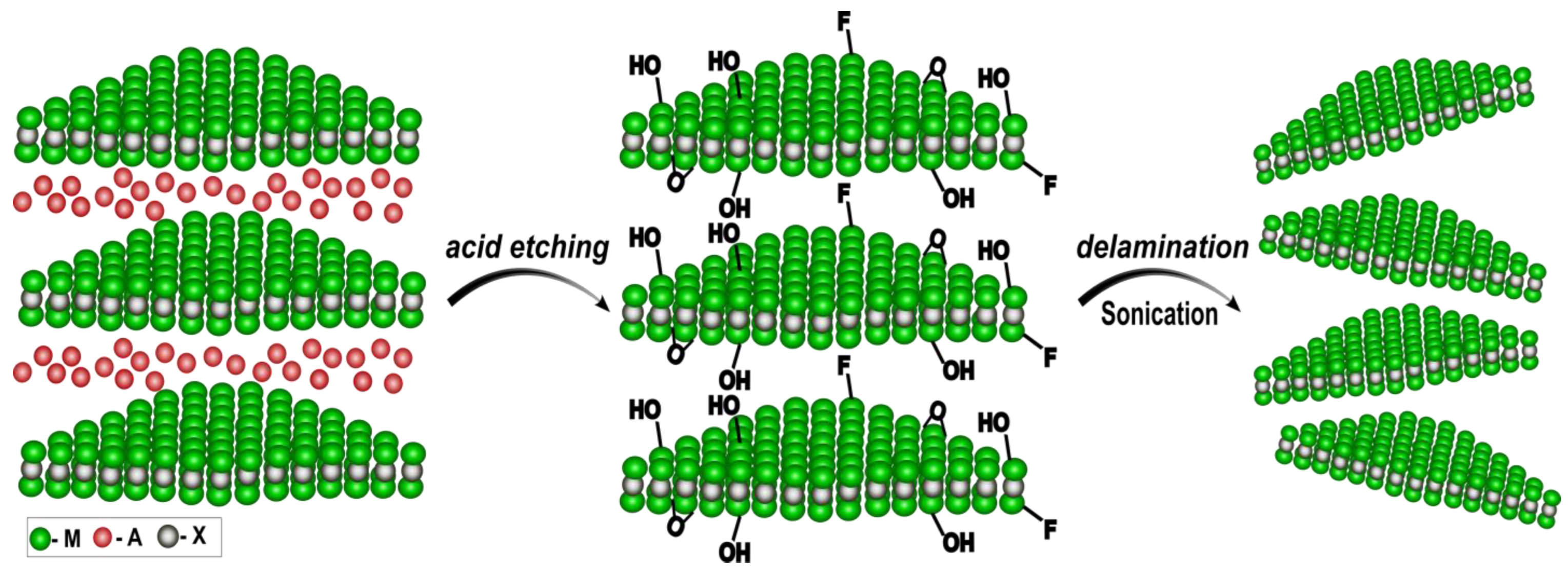
5.3. 2D Materials for Increasing Surface Loading of Biomolecules
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gacem, A.; Rajendran, S.; Hasan, M.A.; Kakodiya, S.D.; Modi, S.; Yadav, K.K.; Awwad, N.S.; Islam, S.; Park, S.; Jeon, B.-H. Plasmon Inspired 2D Carbon Nitrides: Structural, Optical and Surface Characteristics for Improved Biomedical Applications. Crystals 2022, 12, 1213. [Google Scholar] [CrossRef]
- Anand, U.; Chandel, A.K.S.; Oleksak, P.; Mishra, A.; Krejcar, O.; Raval, I.H.; Dey, A.; Kuca, K. Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors. Appl. Microbiol. Biotechnol. 2022, 106, 2827–2853. [Google Scholar] [CrossRef] [PubMed]
- Dursun, A.D.; Borsa, B.A.; Bayramoglu, G.; Arica, M.Y.; Ozalp, V.C. Surface plasmon resonance aptasensor for Brucella detection in milk. Talanta 2022, 239, 123074. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Cho, Y.-W.; Kim, T.-H. Recent Advances in Surface Plasmon Resonance Sensors for Sensitive Optical Detection of Pathogens. Biosensors 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Sharma, A.; Srivastava, N.; Flora, S.J.S. Surface plasmon resonance: A promising approach for label-free early cancer diagnosis. Clin. Chim. Acta 2022, 527, 79–88. [Google Scholar] [CrossRef]
- Patching, S.G. Surface plasmon resonance spectroscopy for characterisation of membrane protein–ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 43–55. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. The performance enhancement of surface plasmon resonance optical sensors using nanomaterials: A review. Coord. Chem. Rev. 2022, 458, 214424. [Google Scholar] [CrossRef]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Murillo Almuzara, C.; D’Auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. SPR and SPR Imaging: Recent Trends in Developing Nanodevices for Detection and Real-Time Monitoring of Biomolecular Events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. Plasmonic Biosensors for Medical Applications. In Encyclopedia of Sensors and Biosensors, 1st ed.; Narayan, R., Ed.; Elsevier: Oxford, UK, 2023; pp. 402–420. [Google Scholar]
- Dastmalchi, B.; Tassin, P.; Koschny, T.; Soukoulis, C.M. A New Perspective on Plasmonics: Confinement and Propagation Length of Surface Plasmons for Different Materials and Geometries. Adv. Opt. Mater. 2016, 4, 177–184. [Google Scholar] [CrossRef]
- Nangare, S.N.; Patil, P.O. Affinity-Based nanoarchitectured Biotransducer for Sensitivity Enhancement of Surface Plasmon Resonance Sensors for In Vitro Diagnosis: A Review. ACS Biomater. Sci. Eng. 2021, 7, 2–30. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. 6—Surface Plasmon Resonance (SPR) for Sensors and Biosensors. In Nanocharacterization Techniques; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 183–200. [Google Scholar]
- Prabowo, B.A.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 080. [Google Scholar] [CrossRef]
- Belkilani, M.; Shokouhi, M.; Farre, C.; Chevalier, Y.; Minot, S.; Bessueille, F.; Abdelghani, A.; Jaffrezic-Renault, N.; Chaix, C. Surface Plasmon Resonance Monitoring of Mono-Rhamnolipid Interaction with Phospholipid-Based Liposomes. Langmuir 2021, 37, 7975–7985. [Google Scholar] [CrossRef]
- Korhonen, K.; Granqvist, N.; Ketolainen, J.; Laitinen, R. Monitoring of drug release kinetics from thin polymer films by multi-parametric surface plasmon resonance. Int. J. Pharm. 2015, 494, 531–536. [Google Scholar] [CrossRef]
- Padra, J.T.; Pagneux, Q.; Bouckaert, J.; Jijie, R.; Sundh, H.; Boukherroub, R.; Szunerits, S.; Lindén, S.K. Mucin modified SPR interfaces for studying the effect of flow on pathogen binding to Atlantic salmon mucins. Biosens. Bioelectron. 2019, 146, 111736. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, S.; Verma, A.; Prajapati, Y.K.; Saini, J.P. Effect of silicon on the sensitivity of SPR biosensor using hybrid nanostructure of black phosphorus and MXene. Superlattices Microstruct. 2020, 145, 106591. [Google Scholar] [CrossRef]
- Niegelhell, K.; Leimgruber, S.; Grießer, T.; Brandl, C.; Chernev, B.; Schennach, R.; Trimmel, G.; Spirk, S. Adsorption Studies of Organophosphonic Acids on Differently Activated Gold Surfaces. Langmuir 2016, 32, 1550–1559. [Google Scholar] [CrossRef]
- Rodríguez-Franco, P.; Abad, L.; Muñoz-Pascual, F.X.; Moreno, M.; Baldrich, E. Effect of the transducer's surface pre-treatment on SPR aptasensor development. Sens. Actuators B Chem. 2014, 191, 634–642. [Google Scholar] [CrossRef]
- Murugan, P.; Krishnamurthy, M.; Jaisankar, S.N.; Samanta, D.; Mandal, A.B. Controlled decoration of the surface with macromolecules: Polymerization on a self-assembled monolayer (SAM). Chem. Soc. Rev. 2015, 44, 3212–3243. [Google Scholar] [CrossRef]
- Sinha, R.K. Wavelength modulation based surface plasmon resonance sensor for detection of cardiac marker proteins troponin I and troponin T. Sens. Actuators A Phys. 2021, 332, 113104. [Google Scholar] [CrossRef]
- Jia, W.; Li, H.; Wilkop, T.; Liu, X.; Yu, X.; Cheng, Q.; Xu, D.; Chen, H.-Y. Silver decahedral nanoparticles empowered SPR imaging-SELEX for high throughput screening of aptamers with real-time assessment. Biosens. Bioelectron. 2018, 109, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Blasi, D.; Sarcina, L.; Tricase, A.; Stefanachi, A.; Leonetti, F.; Alberga, D.; Mangiatordi, G.F.; Manoli, K.; Scamarcio, G.; Picca, R.A.; et al. Enhancing the Sensitivity of Biotinylated Surfaces by Tailoring the Design of the Mixed Self-Assembled Monolayer Synthesis. ACS Omega 2020, 5, 16762–16771. [Google Scholar] [CrossRef]
- Ataman Sadık, D.; Eksi-Kocak, H.; Ertaş, G.; Boyacı, İ.H.; Mutlu, M. Mixed-monolayer of N-hydroxysuccinimide-terminated cross-linker and short alkanethiol to improve the efficiency of biomolecule binding for biosensing. Surf. Interface Anal. 2018, 50, 866–878. [Google Scholar] [CrossRef]
- Ataman Sadık, D.; Boyacı, İ.H.; Mutlu, M. Mixed monolayer decorated SPR sensing surface for thrombin detection. J. Pharm. Biomed. Anal. 2019, 176, 112822. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-W.; Han, X.; Chu, L.-Q. Polyadenine-mediated Immobilization of Aptamers on a Gold Substrate for the Direct Detection of Bacterial Pathogens. Anal. Sci. 2019, 35, 967–972. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.D.; Malinowska, E. SPRi-Based Biosensing Platforms for Detection of Specific DNA Sequences Using Thiolate and Dithiocarbamate Assemblies. Front. Chem. 2018, 6, 173. [Google Scholar] [CrossRef]
- Simon, L.; Lautner, G.; Gyurcsányi, R.E. Reliable micro spotting methodology for peptide-nucleic acid layers with high hybridization efficiency on gold SPR imaging chips. Anal. Methods 2015, 7, 6077–6082. [Google Scholar] [CrossRef]
- Ambrosetti, E.; Conti, M.; Teixeira, A.I.; Zilio, S.D. Patterned Carboxymethyl-Dextran Functionalized Surfaces Using Organic Mixed Monolayers for Biosensing Applications. ACS Appl. Bio Mater. 2022, 5, 3310–3319. [Google Scholar] [CrossRef]
- Wang, Y.; Partridge, A.; Wu, Y. Comparison of a carboxylated terthiophene surface with carboxymethylated dextran layer for surface plasmon resonance detection of progesterone. Anal. Biochem. 2016, 508, 46–49. [Google Scholar] [CrossRef]
- Bertokova, A.; Svecova, N.; Kozics, K.; Gabelova, A.; Vikartovska, A.; Jane, E.; Hires, M.; Bertok, T.; Tkac, J. Exosomes from prostate cancer cell lines: Isolation optimisation and characterisation. Biomed. Pharmacother. 2022, 151, 113093. [Google Scholar] [CrossRef]
- Xiao, Y.; Tai, Y.; Quan, X.; Zhao, C.; Liu, R.; Tong, H.; Huang, Z.; Tang, C.; Gao, J. Quantification of chromogranin A using a surface plasmon resonance-based biosensor. Anal. Methods 2021, 13, 3772–3778. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef]
- Jain, S.; Paliwal, A.; Gupta, V.; Tomar, M. SPR based refractive index modulation of nanostructured SiO2 films grown using GLAD assisted RF sputtering technique. Surf. Interfaces 2022, 34, 102355. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Soler, M.; Lechuga, L.M. Biochemistry strategies for label-free optical sensor biofunctionalization: Advances towards real applicability. Anal. Bioanal. Chem. 2022, 414, 5071–5085. [Google Scholar] [CrossRef]
- Bañuls, M.-J.; Puchades, R.; Maquieira, Á. Chemical surface modifications for the development of silicon-based label-free integrated optical (IO) biosensors: A review. Anal. Chim. Acta 2013, 777, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Schubert, U.S.; Hoeppener, S. Surface chemical reactions on self-assembled silane based monolayers. Chem. Soc. Rev. 2021, 50, 6507–6540. [Google Scholar] [CrossRef]
- Écija-Arenas, Á.; Kirchner, E.-M.; Hirsch, T.; Fernández-Romero, J.M. Development of an aptamer-based SPR-biosensor for the determination of kanamycin residues in foods. Anal. Chim. Acta 2021, 1169, 338631. [Google Scholar] [CrossRef]
- Tabasi, O.; Falamaki, C. Controlled immobilization of IgG1 on carboxymethyl and amino-dextran SPR chips under external vertical electric fields. Appl. Surf. Sci. 2019, 490, 251–259. [Google Scholar] [CrossRef]
- Yuan, P.-X.; Deng, S.-Y.; Zheng, C.-Y.; Cosnier, S.; Shan, D. In situ formed copper nanoparticles templated by TdT-mediated DNA for enhanced SPR sensor-based DNA assay. Biosens. Bioelectron. 2017, 97, 1–7. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Z.; Xia, L.; Zhang, H. Construction of an enzyme-based all-fiber SPR biosensor for detection of enantiomers. Biosens. Bioelectron. 2022, 198, 113836. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; Guardia, M.D.L. Peptide based biosensors. TrAC Trends Anal. Chem. 2018, 107, 1–20. [Google Scholar] [CrossRef]
- Liu, E.Y.; Jung, S.; Yi, H. Improved Protein Conjugation with Uniform, Macroporous Poly(acrylamide-co-acrylic acid) Hydrogel Microspheres via EDC/NHS Chemistry. Langmuir 2016, 32, 11043–11054. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Chapter 4—Zero-Length Crosslinkers. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 259–273. [Google Scholar]
- Delgado, J.D.; Surmaitis, R.L.; Abou Shaheen, S.; Schlenoff, J.B. Engineering Thiolated Surfaces with Polyelectrolyte Multilayers. ACS Appl. Mater. Interfaces 2019, 11, 3524–3535. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, H.J.; Jamison, A.C.; Lee, T.R. Robust Maleimide-Functionalized Gold Surfaces and Nanoparticles Generated Using Custom-Designed Bidentate Adsorbates. Langmuir 2016, 32, 7306–7315. [Google Scholar] [CrossRef]
- Mulligan, C.; Mindell, J.A. Chapter Seven—Pinning Down the Mechanism of Transport: Probing the Structure and Function of Transporters Using Cysteine Cross-Linking and Site-Specific Labeling. In Methods in Enzymology; Ziegler, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 594, pp. 165–202. [Google Scholar]
- Qu, J.-H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR biosensing technology: An overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta 2020, 1104, 10–27. [Google Scholar] [CrossRef]
- Huang, C.-J. 5—Advanced surface modification technologies for biosensors. In Chemical, Gas, and Biosensors for Internet of Things and Related Applications; Mitsubayashi, K., Niwa, O., Ueno, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–86. [Google Scholar]
- Farkaš, P.; Bystrický, S. Chemical conjugation of biomacromolecules: A mini-review. Chem. Pap. 2010, 64, 683–695. [Google Scholar] [CrossRef]
- Meldal, M.; Schoffelen, S. Recent advances in covalent, site-specific protein immobilization. F1000Research 2016, 5, 2303. [Google Scholar] [CrossRef]
- Spriestersbach, A.; Kubicek, J.; Schäfer, F.; Block, H.; Maertens, B. Purification of His-Tagged Proteins. Methods Enzymol. 2015, 559, 1–15. [Google Scholar] [CrossRef]
- Katrlík, J.; Holazová, A.; Medovarská, I.; Seilerová, I.; Gemeiner, P.; Bystrický, S. SPR biosensor chip based on mannan isolated from Candida dubliniensis yeasts applied in immunization effectiveness testing. Sens. Actuators B Chem. 2022, 350, 130883. [Google Scholar] [CrossRef]
- Rispens, T.; Vidarsson, G. Chapter 9—Human IgG Subclasses. In Antibody Fc; Ackerman, M.E., Nimmerjahn, F., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 159–177. [Google Scholar]
- Parkkila, P.; Härkönen, K.; Ilvonen, P.; Laitinen, S.; Viitala, T. Protein A/G-based surface plasmon resonance biosensor for regenerable antibody-mediated capture and analysis of nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130015. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Li, Z.; Xu, H.; Guo, L.; Wang, L.; Wang, Y.; Luo, L.; Wang, J.; Zhang, P.; et al. Label-free differentiation and quantification of ricin, abrin from their agglutinin biotoxins by surface plasmon resonance. Talanta 2022, 238, 122860. [Google Scholar] [CrossRef] [PubMed]
- Paiva, T.O.; Almeida, I.; Marquês, J.T.; Liu, W.; Niu, Y.; Jin, G.; Viana, A.S. Nanostructured interfaces with site-specific bioreceptors for immunosensing. Appl. Surf. Sci. 2017, 412, 455–463. [Google Scholar] [CrossRef]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef]
- Maalouli, N.; Barras, A.; Siriwardena, A.; Bouazaoui, M.; Boukherroub, R.; Szunerits, S. Comparison of photo- and Cu(i)-catalyzed “click” chemistries for the formation of carbohydrate SPR interfaces. Analyst 2013, 138, 805–812. [Google Scholar] [CrossRef]
- Trilling, A.K.; Hesselink, T.; van Houwelingen, A.V.; Cordewener, J.H.G.; Jongsma, M.A.; Schoffelen, S.; van Hest, J.C.M.; Zuilhof, H.; Beekwilder, J. Orientation of llama antibodies strongly increases sensitivity of biosensors. Biosens. Bioelectron. 2014, 60, 130–136. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- Emilsson, G.; Schoch, R.L.; Feuz, L.; Höök, F.; Lim, R.Y.H.; Dahlin, A.B. Strongly Stretched Protein Resistant Poly(ethylene glycol) Brushes Prepared by Grafting-To. ACS Appl. Mater. Interfaces 2015, 7, 7505–7515. [Google Scholar] [CrossRef]
- Su, H.; Li, S.; Kerman, K. Novel thiolated-PEG linker molecule for biosensor development on gold surfaces. Biosens. Bioelectron. 2019, 141, 111477. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, B.; Zhang, Y.; Liu, Y.; Chen, H.; Xiao, S.; Chang, Y.; Yang, J.; Zheng, J. Micro- and macroscopically structured zwitterionic polymers with ultralow fouling property. J. Colloid Interface Sci. 2020, 578, 242–253. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, C.Y.; Lei, Y.; Song, G.; Yao, Y. Plasmonic nanomaterials: A versatile phototheranostic platform of cancers. Mater. Today 2022, 62, 168–189. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, J.; Jiang, Y.; Jiang, L.; Chen, X. Plasmonic Enhanced Optoelectronic Devices. Plasmonics 2014, 9, 859–866. [Google Scholar] [CrossRef]
- Bhatia, P.; Verma, S.S. Enhancement of LSPR properties of temperature-dependent gold nanoparticles. Mater. Today Proc. 2022, 78, 871–876. [Google Scholar] [CrossRef]
- Xu, T.; Geng, Z. Strategies to improve performances of LSPR biosensing: Structure, materials, and interface modification. Biosens. Bioelectron. 2021, 174, 112850. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.K.; Chun, H.S.; Jo, C.-H.; Choi, B.G.; Huh, Y.S.; et al. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122. [Google Scholar] [CrossRef]
- Hosseinniya, S.; Rezayan, A.H.; Ghasemi, F.; Malekmohamadi, M.; Taheri, R.A.; Hosseini, M.; Alvandi, H. Fabrication and evaluation of optical nanobiosensor based on localized surface plasmon resonance (LSPR) of gold nanorod for detection of CRP. Anal. Chim. Acta 2023, 1237, 340580. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef]
- Park, Y.I.; Im, H.; Weissleder, R.; Lee, H. Nanostar Clustering Improves the Sensitivity of Plasmonic Assays. Bioconj. Chem. 2015, 26, 1470–1474. [Google Scholar] [CrossRef]
- Abass Sofi, M.; Sunitha, S.; Ashaq Sofi, M.; Khadheer Pasha, S.K.; Choi, D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 101791. [Google Scholar] [CrossRef]
- Parit, S.B.; Karade, V.C.; Patil, R.B.; Pawar, N.V.; Dhavale, R.P.; Tawre, M.; Pardesi, K.; Jadhav, U.U.; Dawkar, V.V.; Tanpure, R.S.; et al. Bioinspired synthesis of multifunctional silver nanoparticles for enhanced antimicrobial and catalytic applications with tailored SPR properties. Mater. Today Chem. 2020, 17, 100285. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, S.; Liu, C.; Wang, T.; Qiu, Z.; Wang, X.; Waterhouse, G.I.N.; Xu, C.; Yin, H. Performance comparison of surface plasmon resonance biosensors based on ultrasmall noble metal nanoparticles templated using bovine serum albumin. Microchem. J. 2020, 155, 104737. [Google Scholar] [CrossRef]
- Wang, X.; Hou, T.; Lin, H.; Lv, W.; Li, H.; Li, F. In situ template generation of silver nanoparticles as amplification tags for ultrasensitive surface plasmon resonance biosensing of microRNA. Biosens. Bioelectron. 2019, 137, 82–87. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Dolci, M.; Bryche, J.-F.; Leuvrey, C.; Zafeiratos, S.; Gree, S.; Begin-Colin, S.; Barbillon, G.; Pichon, B.P. Robust clicked assembly based on iron oxide nanoparticles for a new type of SPR biosensor. J. Mater. Chem. C 2018, 6, 9102–9110. [Google Scholar] [CrossRef]
- Zamfir, L.-G.; Geana, I.; Bourigua, S.; Rotariu, L.; Bala, C.; Errachid, A.; Jaffrezic-Renault, N. Highly sensitive label-free immunosensor for ochratoxin A based on functionalized magnetic nanoparticles and EIS/SPR detection. Sens. Actuators B Chem. 2011, 159, 178–184. [Google Scholar] [CrossRef]
- Jia, Y.; Peng, Y.; Bai, J.; Zhang, X.; Cui, Y.; Ning, B.; Cui, J.; Gao, Z. Magnetic nanoparticle enhanced surface plasmon resonance sensor for estradiol analysis. Sens. Actuators B Chem. 2018, 254, 629–635. [Google Scholar] [CrossRef]
- Li, Q.; Dou, X.; Zhao, X.; Zhang, L.; Luo, J.; Xing, X.; Yang, M. A gold/Fe3O4 nanocomposite for use in a surface plasmon resonance immunosensor for carbendazim. Microchim. Acta 2019, 186, 313. [Google Scholar] [CrossRef]
- Yoo, H.; Shin, J.; Sim, J.; Cho, H.; Hong, S. Reusable surface plasmon resonance biosensor chip for the detection of H1N1 influenza virus. Biosens. Bioelectron. 2020, 168, 112561. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-dimensional nanomaterials: Fascinating materials in biomedical field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, R.A.; Sagor, R.H.; Khan, M.Z.M. Surface plasmon refractive index biosensors: A review of optical fiber, multilayer 2D material and gratings, and MIM configurations. Opt. Laser Technol. 2023, 159, 108939. [Google Scholar] [CrossRef]
- Ullah, S.; Yang, X.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene transfer methods: A review. Nano Res. 2021, 14, 3756–3772. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yuan, R.-s.; Wang, X. Selective reduction of graphene oxide. New Carbon Mater. 2014, 29, 61–66. [Google Scholar] [CrossRef]
- Chiu, N.-F.; Fan, S.-Y.; Yang, C.-D.; Huang, T.-Y. Carboxyl-functionalized graphene oxide composites as SPR biosensors with enhanced sensitivity for immunoaffinity detection. Biosens. Bioelectron. 2017, 89, 370–376. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Ma, P.; Zhang, D.; Li, S.; Wang, X.; Song, D. Gold nanostar-enhanced surface plasmon resonance biosensor based on carboxyl-functionalized graphene oxide. Anal. Chim. Acta 2016, 913, 137–144. [Google Scholar] [CrossRef]
- Nangare, S.; Patil, P. Chitosan mediated layer-by-layer assembly based graphene oxide decorated surface plasmon resonance biosensor for highly sensitive detection of β-amyloid. Int. J. Biol. Macromol. 2022, 214, 568–582. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer's biomarkers; an effective step in early and accurate diagnosis. Biosens. Bioelectron. 2020, 167, 112511. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Chiu, N.-F.; Yang, H.-T. High-Sensitivity Detection of the Lung Cancer Biomarker CYFRA21-1 in Serum Samples Using a Carboxyl-MoS2 Functional Film for SPR-Based Immunosensors. Front. Bioeng. Biotechnol. 2020, 8, 234. [Google Scholar] [CrossRef]
- Nie, W.; Wang, Q.; Yang, X.; Zhang, H.; Li, Z.; Gao, L.; Zheng, Y.; Liu, X.; Wang, K. High sensitivity surface plasmon resonance biosensor for detection of microRNA based on gold nanoparticles-decorated molybdenum sulfide. Anal. Chim. Acta 2017, 993, 55–62. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Xu, Y.; Wu, J.; Jia, G.; Ji, F.; Fang, X.; Chen, F.; Cui, X. Ultrasensitive and Selective Determination of Carcinoembryonic Antigen Using Multifunctional Ultrathin Amino-Functionalized Ti3C2-MXene Nanosheets. Anal. Chem. 2020, 92, 3354–3360. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Liu, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; et al. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef]
- Chen, R.; Kan, L.; Duan, F.; He, L.; Wang, M.; Cui, J.; Zhang, Z.; Zhang, Z. Surface plasmon resonance aptasensor based on niobium carbide MXene quantum dots for nucleocapsid of SARS-CoV-2 detection. Microchim. Acta 2021, 188, 316. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Y.; Zhou, J.; Wang, X.; Jia, B.; Miyan, R.; Zhang, T.; Sang, W.; Wang, Y.; Qiu, H.; et al. Optothermophoretic flipping method for biomolecule interaction enhancement. Biosens. Bioelectron. 2022, 204, 114084. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M. Handbook of Surface Plasmon Resonance, 2nd ed.; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]







| Coating Biomaterial | Target | Sensitivity (Deg or RIU) | LOD | Dynamic Range | Ref. |
|---|---|---|---|---|---|
| Aptamer/CVD graphene | Kanamycin | 2.1·10−3 RIU | 0.28 µM | 1–100 µM | [40] |
| Aptamer/rGO | 1.9·10−3 RIU | 1.79 µM | 5.88–100 µM | ||
| BSA/COOH-GO | Ab-BSA | 35.5 m° | 0.01 pg/ml | 0.01–100 pg/mL | [92] |
| BSA/GO | 9.13 m° | - | - | ||
| AuNSt-antigen/COOH-GO | pig IgG | - | 0.0375 μg/mL | 0.0375–40 μg/mL | [93] |
| Ab-β-amyloid/AgNPs-Cs-PSS/GO | β-amyloid protein | 280 RIU | 1.21 fg/mL | 2 fg/mL–400 ng/mL | [94] |
| Ab-CYFRA 21-1/COOH-MoS2 | CYFRA 21-1 | 355.69 m° | 0.05 pg/mL | 0.05 pg/mL–100 ng/mL | [97] |
| DNA/MoS2-AuNPs | microRNA-141 | 193 m° | 0.05 fM | 0–50 pM | [98] |
| Ab-conjugated SPA/HAuNPs/N–Ti3C2-MXene | Carcinoembryonic antigen | - | 0.15 fM | 0.001−1000 pM | [100] |
| MWCNTs-(PDA)-AuNPs-modified with Ab2/Ab1-conjugated SPA/AuNPs/Ti3C2-MXene | Carcinoembryonic antigen | 369 m° | 0.07 fM | 2 × 10−16–2 × 10−8 M | [101] |
| Aptamer/Nb2C-MXene-QDs | N-gene of SARS-CoV-2 | 270 RIU | 4.9 pg/mL | 0.05–100 ng/mL | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topor, C.-V.; Puiu, M.; Bala, C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors 2023, 13, 465. https://doi.org/10.3390/bios13040465
Topor C-V, Puiu M, Bala C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors. 2023; 13(4):465. https://doi.org/10.3390/bios13040465
Chicago/Turabian StyleTopor, Cristina-Virginia, Mihaela Puiu, and Camelia Bala. 2023. "Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing" Biosensors 13, no. 4: 465. https://doi.org/10.3390/bios13040465
APA StyleTopor, C.-V., Puiu, M., & Bala, C. (2023). Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors, 13(4), 465. https://doi.org/10.3390/bios13040465







