Behind the Optimization of the Sensor Film: Bioconjugation of Triangular Gold Nanoparticles with Hemoproteins for Sensitivity Enhancement of Enzymatic Biosensors
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Characterization Techniques
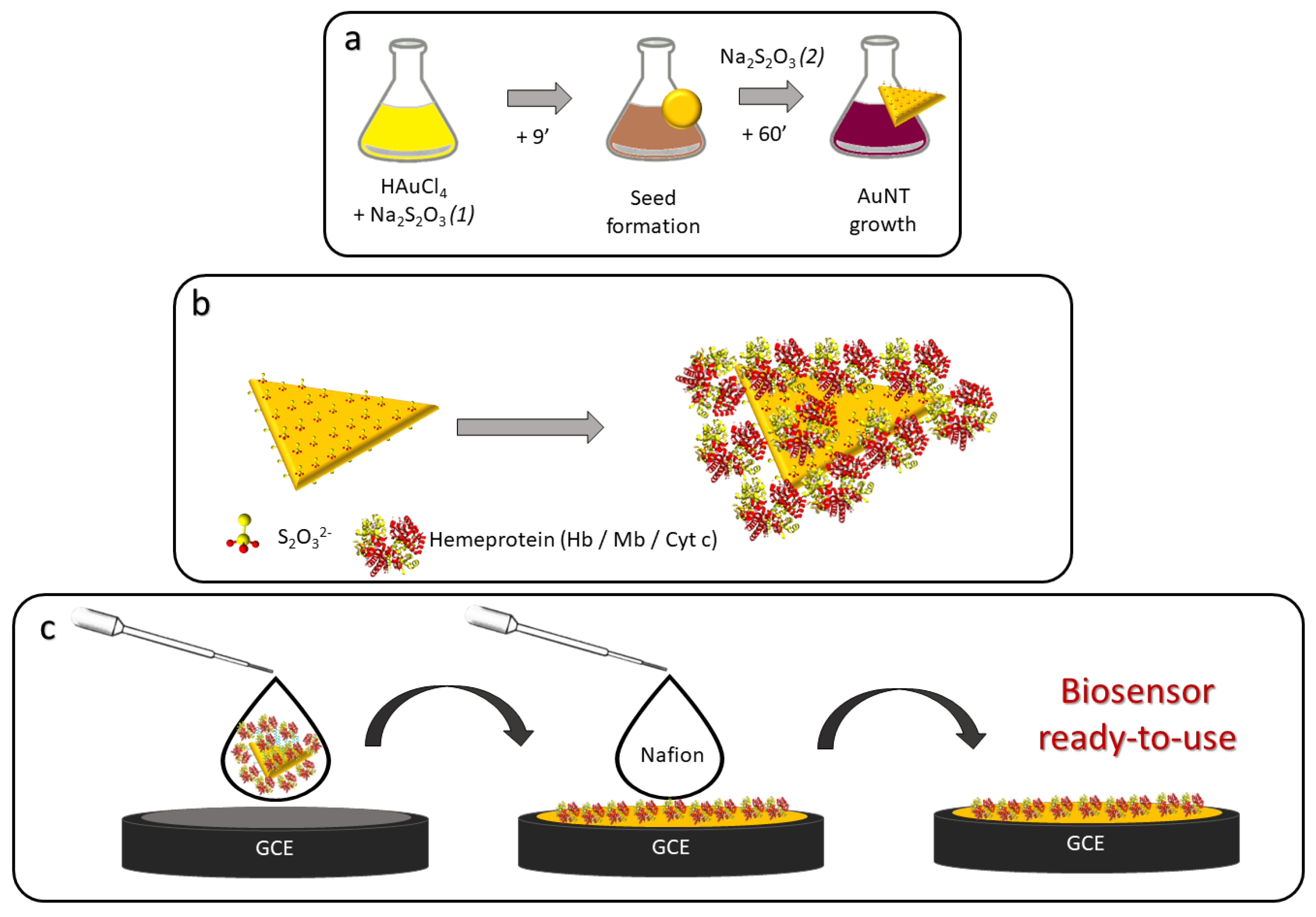
2.3. Synthesis of AuNTs
2.4. Formation of the Colloidal Suspension of Bioconjugates
2.5. Preparation of the Biosensor Platforms
3. Results and Discussion
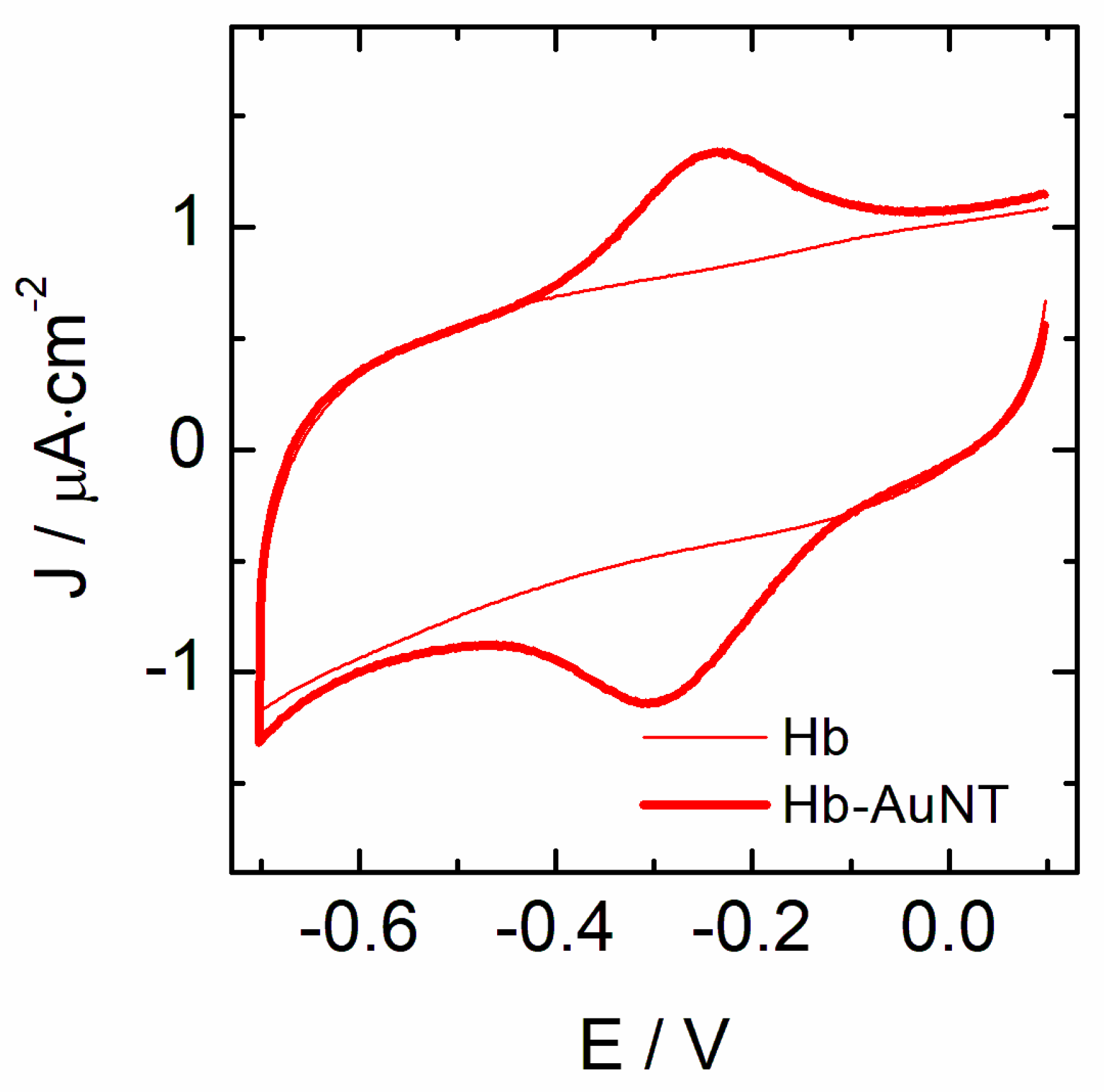
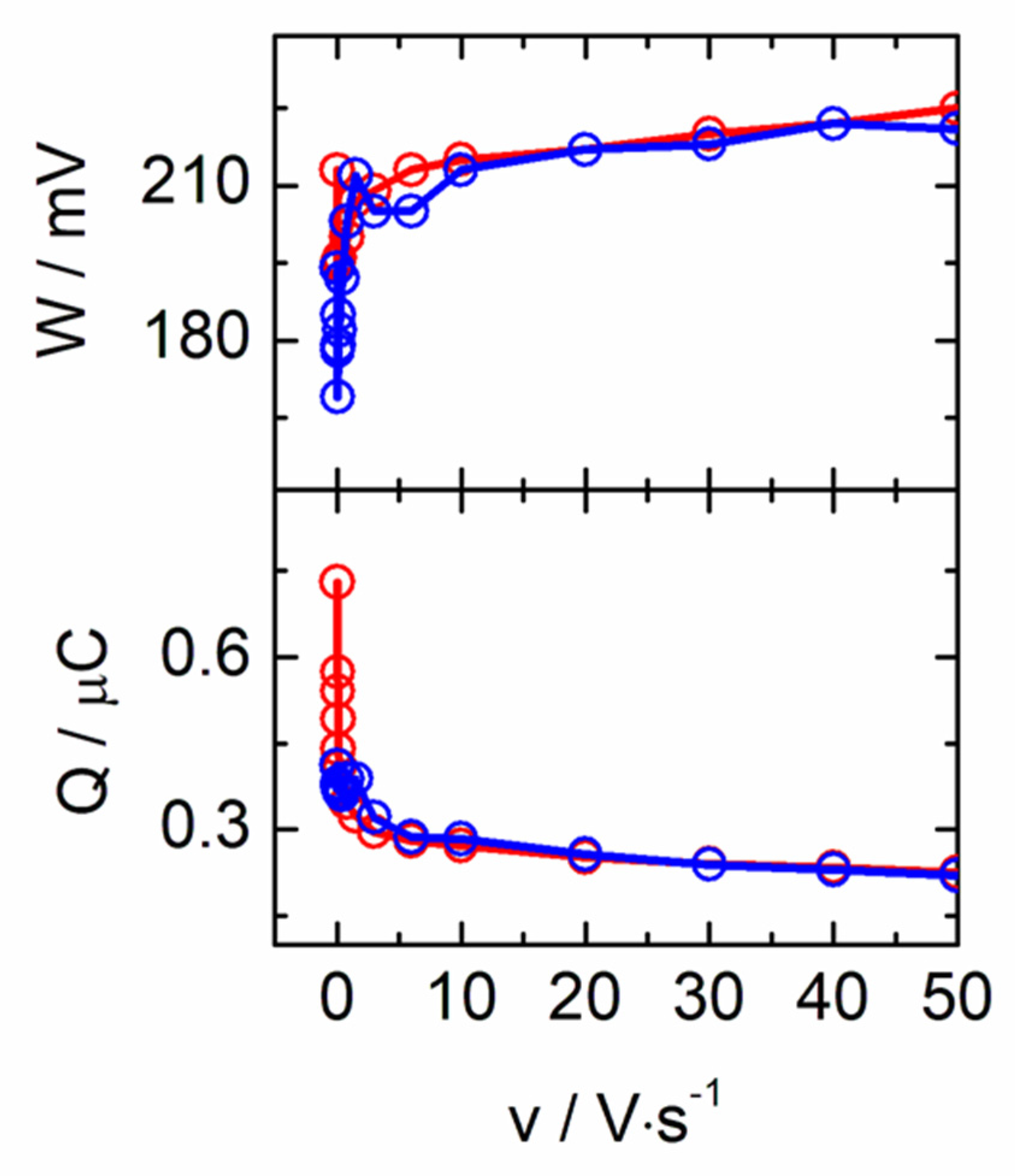
3.1. Electrochemical Behavior of Hemeproteins Immobilized on AuNT
3.2. Electrocatalysis of the Nanobioconjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagati, A.K.; Choi, J.-W. Protein Based Electrochemical Biosensors for H2O2 Detection towards Clinical Diagnostics. Electroanalysis 2014, 26, 1259–1276. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, J.R. New developments in crystallography: Exploring its technology, methods and scope in the molecular biosciences. Biosci. Rep. 2017, 37, BSR20170204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.C.; Shen, Y.; Song, Y.H.; Wang, L. On-Off ratiometric electrochemical biosensor for accurate detection of glucose. Electrochim. Acta 2017, 235, 488–494. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Atar, N.; Yola, M.L. A molecularly imprinted electrochemical biosensor based on hierarchical Ti2Nb10O29 (TNO) for glucose detection. Microchim. Acta 2022, 189, 24. [Google Scholar] [CrossRef]
- Sha, T.Z.; Liu, J.J.; Sun, M.M.; Li, L.; Bai, J.; Hu, Z.Q.; Zhou, M. Green and low-cost synthesis of nitrogen-doped graphene-like mesoporous nanosheets from the biomass waste of okara for the amperometric detection of vitamin C in real samples. Talanta 2019, 200, 300–306. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; Silva, A.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. Acs Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim. Acta 2017, 231, 209–215. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Jiang, D.F.; Xu, C.S.; Ge, Y.C.; Liu, X.H.; Wei, Q.Q.; Huang, L.P.; Ren, X.Q.; Wang, C.D.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B-Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Dimcheva, N. Nanostructures of noble metals as functional materials in biosensors. Curr. Opin. Electrochem. 2020, 19, 35–41. [Google Scholar] [CrossRef]
- Zribi, R.; Ferlazzo, A.; Fazio, E.; Condorelli, M.; D’Urso, L.; Neri, G.; Corsaro, C.; Neri, F.; Compagnini, G. Ag Nanoplates Modified-Screen Printed Carbon Electrode to Improve Electrochemical Performances toward a Selective H2O2 Detection. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Ye, M.; Yang, C.; Sun, Y.; Wang, J.; Wang, D.; Zhao, Y.; Zhu, Z.; Liu, P.; Zhu, J.; Li, C.; et al. ZnFe2O4/Graphitic Carbon Nitride Nano/Microcomposites for the Enhanced Electrochemical Sensing of H2O2. ACS Appl. Nano Mater. 2022, 5, 10922–10932. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
- Zhe, T.; Li, M.; Li, F.; Li, R.; Bai, F.; Bu, T.; Jia, P.; Wang, L. Integrating electrochemical sensor based on MoO3/Co3O4 heterostructure for highly sensitive sensing of nitrite in sausages and water. Food Chem. 2022, 367, 130666. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, M.; Sahin, E.M.; Ayranci, E. Sensor and biosensor application of a new redox mediator: Rosmarinic acid modified screen-printed carbon electrode for electrochemical determination of NADH and ethanol. J. Electroanal. Chem. 2018, 813, 67–74. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.H.; Duan, M.; Tang, Y.; Zhu, J.F. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.M.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Chen, W.; Yin, C.; Weng, W.; Li, X.; Wang, X.; Li, G.; Sun, W. A direct electron transfer biosensor based on a horseradish peroxidase and gold nanotriangle modified electrode and electrocatalysis. Anal. Methods 2018, 10, 5297–5304. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Chavez, M.; Fernandez-Merino, A.; Sanchez-Obrero, G.; Madueno, R.; Sevilla, J.M.; Blazquez, M.; Pineda, T. Distinct thermoresponsive behaviour of oligo- and poly-ethylene glycol protected gold nanoparticles in concentrated salt solutions. Nanoscale Adv. 2021, 3, 4767–4779. [Google Scholar] [CrossRef] [PubMed]
- Viudez, A.J.; Madueno, R.; Pineda, T.; Blazquez, M. Stabilization of gold nanoparticles by 6-mercaptopurine monolayers. Effects of the solvent properties. J. Phys. Chem. B 2006, 110, 17840–17847. [Google Scholar] [CrossRef] [PubMed]
- Viudez, A.J.; Madueno, R.; Blazquez, M.; Pineda, T. Synthesis, Characterization, and Double Layer Capacitance Charging of Nanoclusters Protected by 6-Mercaptopurine. J. Phys. Chem. C 2009, 113, 5186–5192. [Google Scholar] [CrossRef]
- Zuccarello, L.; Barbosa, C.; Todorovic, S.; Silveira, C.M. Electrocatalysis by heme enzymes-applications in biosensing. Catalysts 2021, 11, 218. [Google Scholar] [CrossRef]
- del Cano, R.; Mateus, L.; Sanchez-Obrero, G.; Sevilla, J.M.; Madueno, R.; Blazquez, M.; Pineda, T. Hemoglobin becomes electroactive upon interaction with surface-protected Au nanoparticles. Talanta 2018, 176, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gálvez, L.; del Caño, R.; Menéndez-Luque, I.; García-Nieto, D.; Rodríguez-Peña, M.; Luna, M.; Pineda, T.; Pariente, F.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescent nanostructured DNA biosensor for SARS-CoV-2 detection. Talanta 2022, 240, 123203. [Google Scholar] [CrossRef]
- del Cano, R.; Garcia-Mendiola, T.; Garcia-Nieto, D.; Alvaro, R.; Luna, M.; Iniesta, H.A.; Coloma, R.; Diaz, C.R.; Milan-Rois, P.; Castellanos, M.; et al. Amplification-free detection of SARS-CoV-2 using gold nanotriangles functionalized with oligonucleotides. Microchim. Acta 2022, 189, 171. [Google Scholar] [CrossRef]
- Pina-Coronado, C.; Martínez-Sobrino, Á.; Gutiérrez-Gálvez, L.; Del Caño, R.; Martínez-Periñán, E.; García-Nieto, D.; Rodríguez-Peña, M.; Luna, M.; Milán-Rois, P.; Castellanos, M.; et al. Methylene Blue functionalized carbon nanodots combined with different shape gold nanostructures for sensitive and selective SARS-CoV-2 sensing. Sens. Actuators B Chem. 2022, 369, 132217. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Conejo-Valverde, P.; Soto-Cruz, J.; Bergueiro, J.; Calderón, M.; Rojas-Carrillo, O.; Kano, K.; Gorton, L. The influence of the shape of Au nanoparticles on the catalytic current of fructose dehydrogenase. Anal. Bioanal. Chem. 2019, 411, 7645–7657. [Google Scholar] [CrossRef] [Green Version]
- Terwilliger, N.B. Functional adaptations of oxygen-transport proteins. J. Exp. Biol. 1998, 201, 1085–1098. [Google Scholar] [CrossRef]
- Michel, H.; Behr, J.; Harrenga, A.; Kannt, A. Cytochrome C oxidase: Structure and spectroscopy. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 329–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Zhang, J.; Li, Z.P.; Zhang, P.X.; Li, Y.X.; Liu, G.H.; Wang, Y.; Yue, Z. Photoelectrochemical determination of hydrogen peroxide using a gold electrode modified with fluorescent gold nanoclusters and graphene oxide. Microchim. Acta 2017, 184, 677–686. [Google Scholar] [CrossRef]
- Shleev, S.; Andoralov, V.; Pankratov, D.; Falk, M.; Aleksejeva, O.; Blum, Z. Oxygen Electroreduction versus Bioelectroreduction: Direct Electron Transfer Approach. Electroanalysis 2016, 28, 2270–2287. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Nielsen, M.; Fapyane, D. Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate. Sensors 2020, 20, 4326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jasinski, J.B.; Howell, J.L.; Patel, D.; Stephens, D.P.; Gobin, A.M. Tunability and stability of gold nanoparticles obtained from chloroauric acid and sodium thiosulfate reaction. Nanoscale Res. Lett. 2012, 7, 337. [Google Scholar] [CrossRef] [Green Version]
- Pelaz, B.; Grazu, V.; Ibarra, A.; Magen, C.; del Pino, P.; de la Fuente, J.M. Tailoring the Synthesis and Heating Ability of Gold Nanoprisms for Bioapplications. Langmuir 2012, 28, 8965–8970. [Google Scholar] [CrossRef]
- Pineda, T.; Sevilla, J.M.; Roman, A.J.; Blazquez, M. Electrochemical evidence on the molten globule conformation of cytochrome c. Biochim. Et Biophys. Acta-Protein Struct. Mol. Enzymol. 1997, 1343, 227–234. [Google Scholar] [CrossRef]
- Sevilla, J.M.; Pineda, T.; Roman, A.J.; Madueno, R.; Blazquez, M. The direct electrochemistry of cytochrome c at a hanging mercury drop electrode modified with 6-mercaptopurine. J. Electroanal. Chem. 1998, 451, 89–93. [Google Scholar] [CrossRef]
- Laviron, E.; Roullier, L.; Degrand, C. A multilayer model for the study of space distributed redox modified electrodes: Part II. Theory and application of linear potential sweep voltammetry for a simple reaction. J. Electroanal. Chem. 1980, 112, 11–23. [Google Scholar] [CrossRef]
- Clark, R.A.; Bowden, E.F. Voltammetric peak broadening for cytochrome c/alkanethiolate monolayer structures: Dispersion of formal potentials. Langmuir 1997, 13, 559–565. [Google Scholar] [CrossRef]
- Doan, T.T.; Vargo, M.L.; Gerig, J.K.; Gulka, C.P.; Trawick, M.L.; Dattelbaum, J.D.; Leopold, M.C. Electrochemical analysis of azurin thermodynamic and adsorption properties at monolayer-protected cluster film assemblies—Evidence for a more homogeneous adsorption interface. J. Colloid Interface Sci. 2010, 352, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Beissenhirtz, M.K.; Scheller, F.W.; Lisdat, F. A Superoxide Sensor Based on a Multilayer Cytochrome c Electrode. Anal. Chem. 2004, 76, 4665–4671. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, M.; Calvo, E.J. Charge Transport in Redox Polyelectrolyte Multilayer Films: The Dramatic Effects of Outmost Layer and Solution Ionic Strength. Chemphyschem 2010, 11, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. A multilayer model for the study of space distributed redox modified electrodes: Part I. Description and discussion of the model. J. Electroanal. Chem. 1980, 112, 1–9. [Google Scholar] [CrossRef]
- Peng, L.; Dong, S.Y.; Li, N.; Suo, G.C.; Huang, T.L. Construction of a biocompatible system of hemoglobin based on AuNPs-carbon aerogel and ionic liquid for amperometric biosensor. Sens. Actuat. B-Chem. 2015, 210, 418–424. [Google Scholar] [CrossRef]
- Liu, Y.; Han, T.; Chen, C.; Bao, N.; Yu, C.M.; Gu, H.Y. A novel platform of hemoglobin on core-shell structurally Fe3O4@Au nanoparticles and its direct electrochemistry. Electrochim. Acta 2011, 56, 3238–3247. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, Y.G.; Jiang, L.P.; Zhu, J.J. Synthesis, characterizations of silica-coated gold nanorods and its applications in electroanalysis of hemoglobin. Electrochem. Commun. 2008, 10, 355–358. [Google Scholar] [CrossRef]
- Xuan, J.; Jia, X.-d.; Jiang, L.-P.; Abdel-Halim, E.S.; Zhu, J.-J. Gold nanoparticle-assembled capsules and their application as hydrogen peroxide biosensor based on hemoglobin. Bioelectrochemistry 2012, 84, 32–37. [Google Scholar] [CrossRef]
- Palanisamy, S.; Cheemalapati, S.; Chen, S.-M. Highly sensitive and selective hydrogen peroxide biosensor based on hemoglobin immobilized at multiwalled carbon nanotubes-zinc oxide composite electrode. Anal. Biochem. 2012, 429, 108–115. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, W.; Tan, Y.; Ding, S.; Zhang, H. Direct electrochemistry and electroanalysis of hemoglobin adsorbed in self-assembled films of gold nanoshells. Talanta 2007, 72, 1134–1140. [Google Scholar] [CrossRef]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Direct voltammetry and electrocatalytic properties of hemoglobin immobilized on a glassy carbon electrode modified with nickel oxide nanoparticles. Electrochem. Commun. 2006, 8, 1499–1508. [Google Scholar] [CrossRef]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Mai, Z.B.; Kang, X.H.; Dai, Z.; Zou, X.Y. Clay-chitosan-gold nanoparticle nanohybrid: Preparation and application for assembly and direct electrochemistry of myoglobin. Electrochim. Acta 2008, 53, 4732–4739. [Google Scholar] [CrossRef]
- Reuillard, B.; Ly, K.H.; Hildebrandt, P.; Jeuken, L.J.C.; Butt, J.N.; Reisner, E. High Performance Reduction of H2O2 with an Electron Transport Decaheme Cytochrome on a Porous ITO Electrode. J. Am. Chem. Soc. 2017, 139, 3324–3327. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Gopalan, A.I.; Komathi, S. Direct electrochemistry of cytochrome c and biosensing for hydrogen peroxide on polyaniline grafted multi-walled carbon nanotube electrode. Sens. Actuators B-Chem. 2009, 141, 518–525. [Google Scholar] [CrossRef]
- Mani, V.; Dinesh, B.; Chen, S.M.; Saraswathi, R. Direct electrochemistry of myoglobin at reduced graphene oxide-multiwalled carbon nanotubes-platinum nanoparticles nanocomposite and biosensing towards hydrogen peroxide and nitrite. Biosens. Bioelectron. 2014, 53, 420–427. [Google Scholar] [CrossRef]
- Cao, D.F.; He, P.L.; Hu, N.F. Electrochemical biosensors utilising electron transfer in heme proteins immobilised on Fe3O4 nanoparticles. Analyst 2003, 128, 1268–1274. [Google Scholar] [CrossRef]
- Lu, X.B.; Hu, J.Q.; Yao, X.; Wang, Z.P.; Li, J.H. Composite system based on chitosan and room-temperature ionic liquid: Direct electrochemistry and electrocatalysis of hemoglobin. Biomacromolecules 2006, 7, 975–980. [Google Scholar] [CrossRef]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.J.; Xuan, J.; Zhu, J.J. Myoglobin/Gold Nanoparticles/Carbon Spheres 3-D Architecture for the Fabrication of a Novel Biosensor. Nano Res. 2009, 2, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.X.; Liu, S.Q.; Ge, B.X.; Lisdat, F.; Scheller, F.W. Electrochemistry of cytochrome c immobilized on colloidal gold modified carbon paste electrodes and its electrocatalytic activity. Electroanalysis 2002, 14, 141–147. [Google Scholar] [CrossRef]
- Ranieri, A.; Bortolotti, C.A.; Di Rocco, G.; Battistuzzi, G.; Sola, M.; Borsari, M. Electrocatalytic Properties of Immobilized Heme Proteins: Basic Principles and Applications. ChemElectroChem 2019, 6, 5172–5185. [Google Scholar] [CrossRef]
- Hu, X.J.; Chen, J.; Hu, R.H.; Zhu, Z.K.; Lai, Z.J.; Zhu, X.Y.; Zhu, H.; Koh, K.; Chen, H.X. Synergistically catalytic nanozymes based on heme-protein active site model for dual-signal and ultrasensitive detection of H2O2 in living cells. Sens. Actuators B-Chem. 2021, 333, 129564. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, L.; Li, X.; Zeng, Y.; Sun, W.; Huang, X. Direct electrochemistry and electrocatalysis of hemoglobin with carbon nanotube-ionic liquid-chitosan composite materials modified carbon ionic liquid electrode. Electrochim. Acta 2010, 55, 5959–5965. [Google Scholar] [CrossRef]
- Shan, W.; He, P.; Hu, N. Electrocatalytic reduction of nitric oxide and other substrates on hydrogel triblock copolymer Pluronic films containing hemoglobin or myoglobin based on protein direct electrochemistry. Electrochim. Acta 2005, 51, 432–440. [Google Scholar] [CrossRef]
- Mimica, D.; Zagal, J.H.; Bedioui, F. Electrocatalysis of nitric oxide reduction by hemoglobin entrapped in surfactant films. Electrochem. Commun. 2001, 3, 435–438. [Google Scholar] [CrossRef]
- Shimizu, K.; Sepunaru, L.; Compton, R.G. Innovative catalyst design for the oxygen reduction reaction for fuel cells. Chem. Sci. 2016, 7, 3364–3369. [Google Scholar] [CrossRef] [Green Version]





| Biconjugate | Ec/V | Ea/V | E°’/V | ΔE/mV | ks/s−1 |
|---|---|---|---|---|---|
| Hb-AuNT | −0.300 | −0.237 | −0.268 | 63 | 138.0 |
| Mb-AuNT | −0.280 | −0.237 | −0.258 | 43 | 104.1 |
| Cyt c-AuNT | −0.290 | −0.252 | −0.271 | 38 | 148.4 |
| Platform | Hb-AuNT | Mb-AuNT | Cyt c-AuNT | |
|---|---|---|---|---|
| H2O2 | KMapp/mM | 0.808 | 0.602 | 0.195 |
| Jmax/μA | 50.2 | 38.0 | 15.5 | |
| S/μA·mM−1 | 48.7 | 45.6 | 45.4 | |
| LOD/µM | 18 | 27 | 39 | |
| NO2− | KMapp/mM | 49 | 5.3 | 2.9 |
| Jmax/μA | 17.8 | 7.7 | 3.2 | |
| S/μA·mM−1 | 0.2 | 0.3 | 0.3 | |
| LOD/mM | 1.7 | 0.8 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, M.; Fernandez-Merino, Á.; del Caño, R.; Sánchez-Obrero, G.; Madueño, R.; Blázquez, M.; Pineda, T. Behind the Optimization of the Sensor Film: Bioconjugation of Triangular Gold Nanoparticles with Hemoproteins for Sensitivity Enhancement of Enzymatic Biosensors. Biosensors 2023, 13, 467. https://doi.org/10.3390/bios13040467
Chávez M, Fernandez-Merino Á, del Caño R, Sánchez-Obrero G, Madueño R, Blázquez M, Pineda T. Behind the Optimization of the Sensor Film: Bioconjugation of Triangular Gold Nanoparticles with Hemoproteins for Sensitivity Enhancement of Enzymatic Biosensors. Biosensors. 2023; 13(4):467. https://doi.org/10.3390/bios13040467
Chicago/Turabian StyleChávez, Miriam, Ángela Fernandez-Merino, Rafael del Caño, Guadalupe Sánchez-Obrero, Rafael Madueño, Manuel Blázquez, and Teresa Pineda. 2023. "Behind the Optimization of the Sensor Film: Bioconjugation of Triangular Gold Nanoparticles with Hemoproteins for Sensitivity Enhancement of Enzymatic Biosensors" Biosensors 13, no. 4: 467. https://doi.org/10.3390/bios13040467
APA StyleChávez, M., Fernandez-Merino, Á., del Caño, R., Sánchez-Obrero, G., Madueño, R., Blázquez, M., & Pineda, T. (2023). Behind the Optimization of the Sensor Film: Bioconjugation of Triangular Gold Nanoparticles with Hemoproteins for Sensitivity Enhancement of Enzymatic Biosensors. Biosensors, 13(4), 467. https://doi.org/10.3390/bios13040467





