Organic Semiconducting Nanoparticles for Biosensor: A Review
Abstract
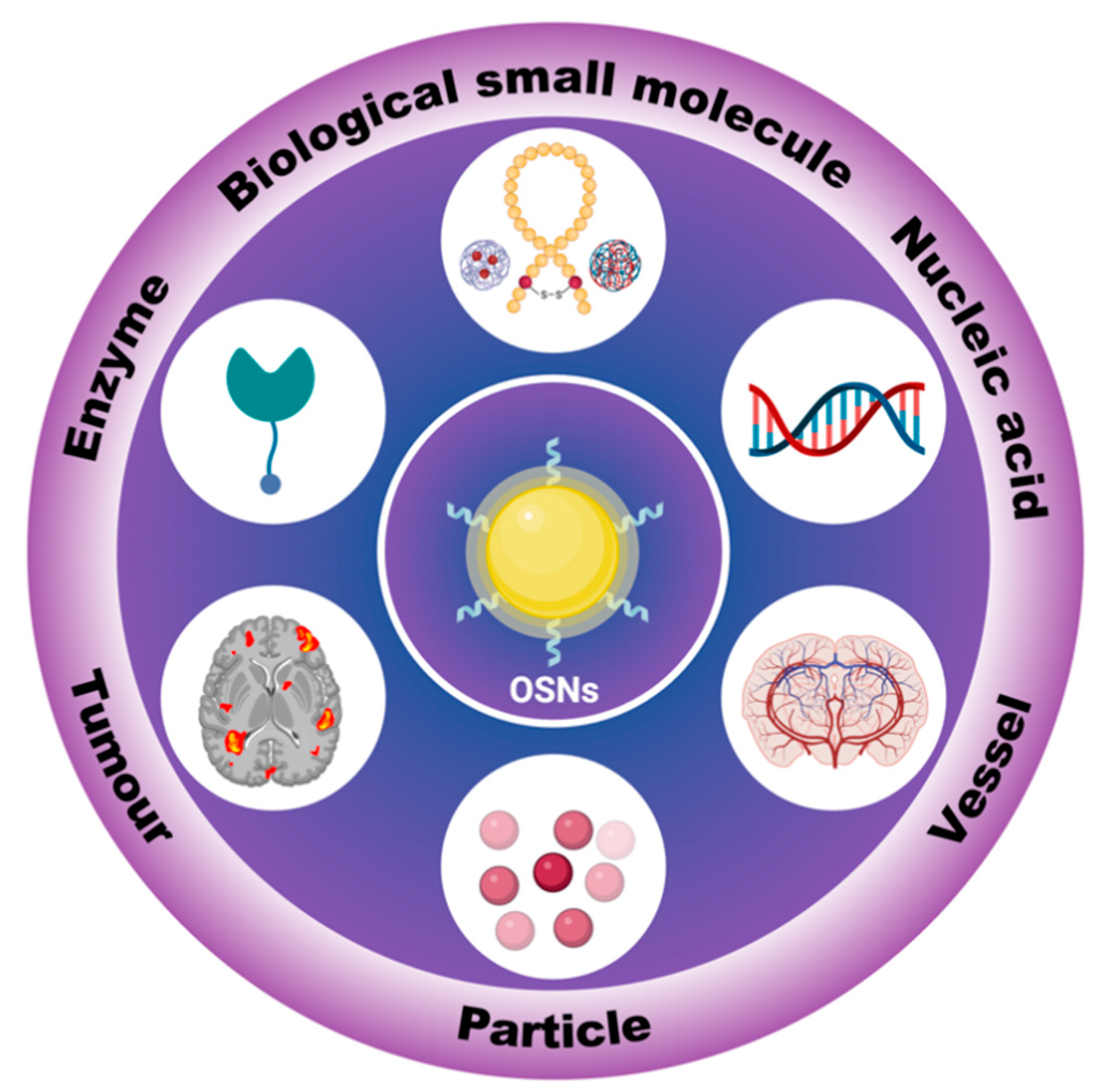
1. Introduction
2. In Vitro Tracking
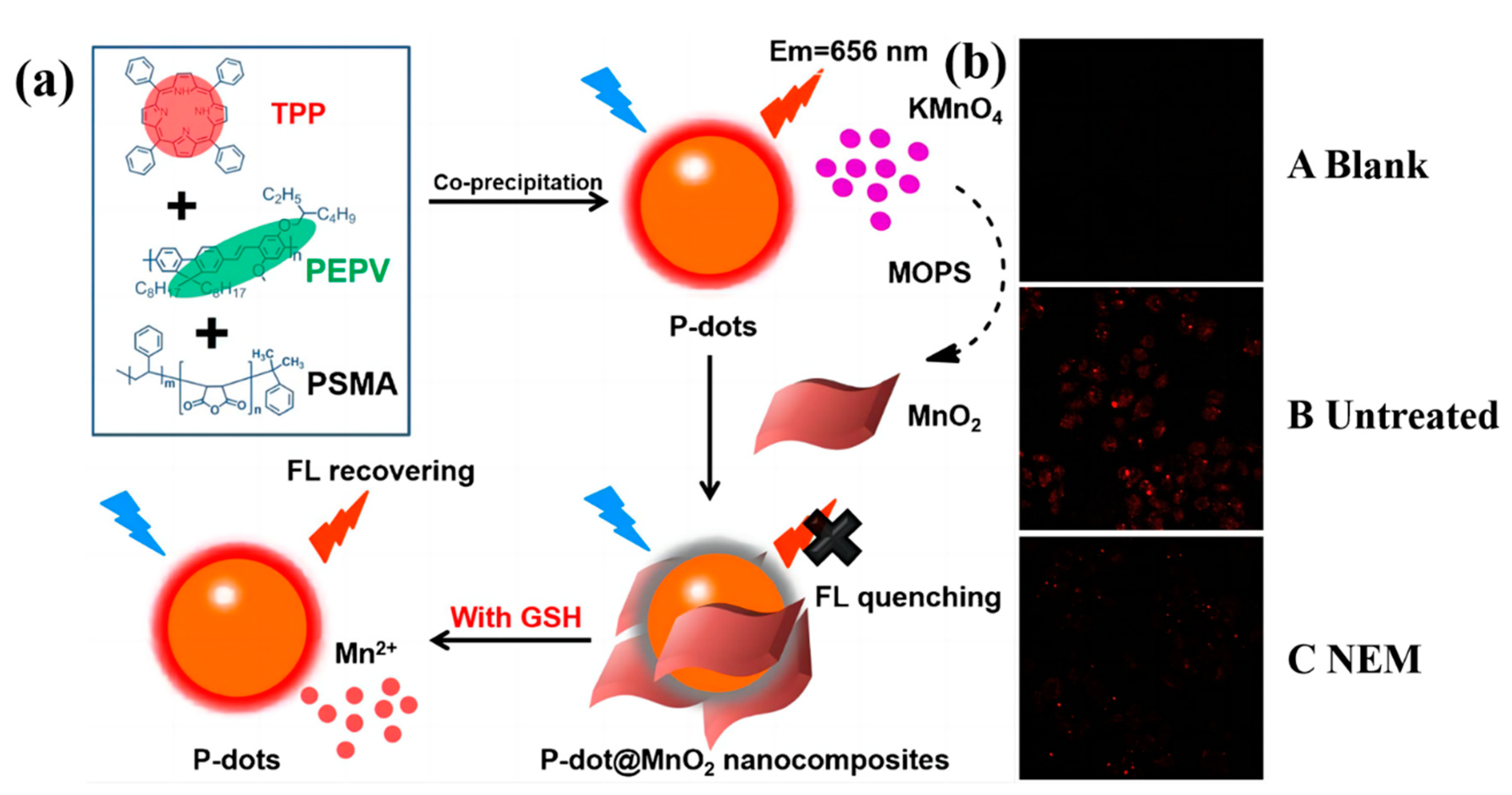
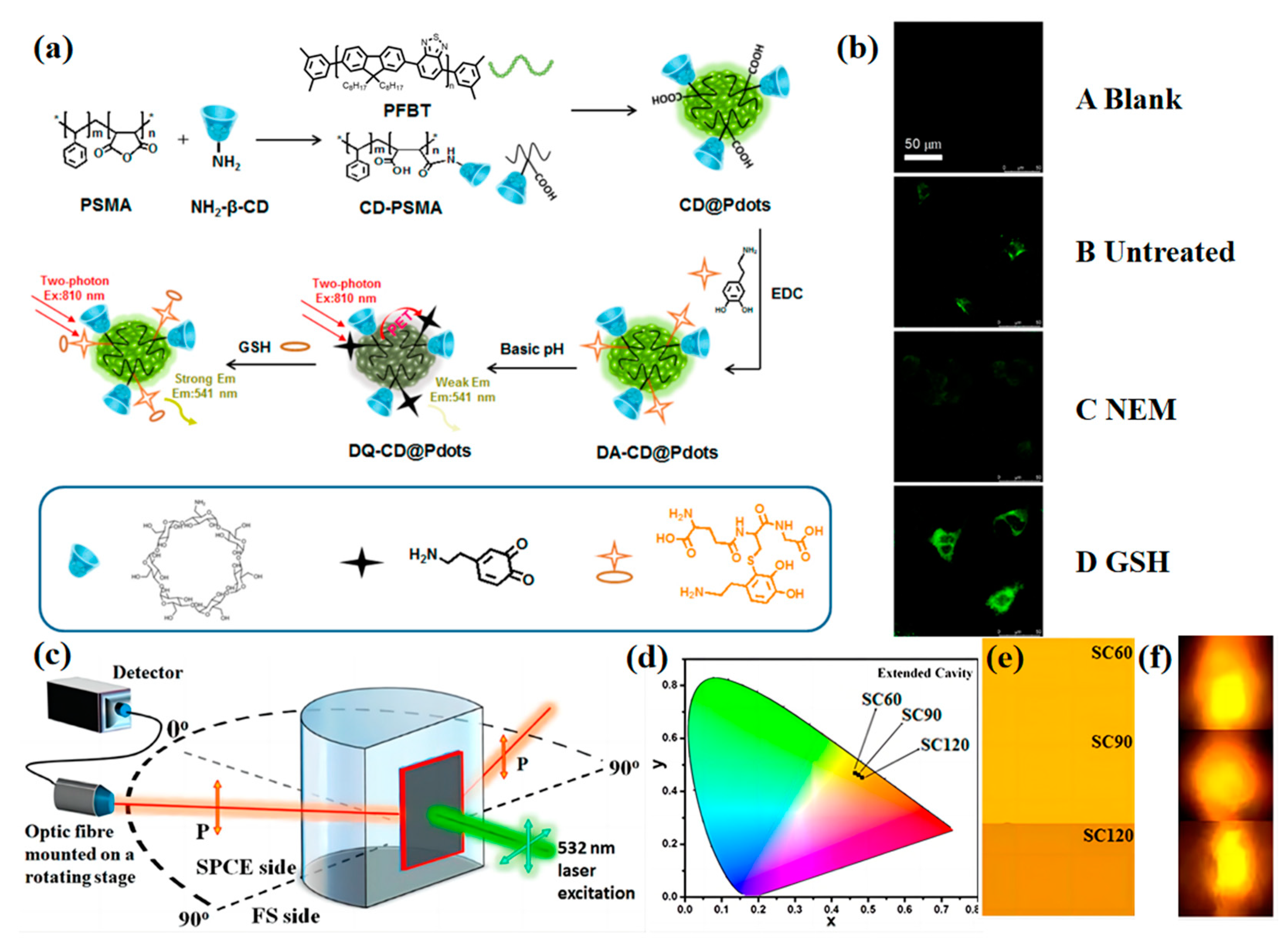
2.1. Biological Small Molecules Tracking
2.2. Enzyme Concentration Measurement
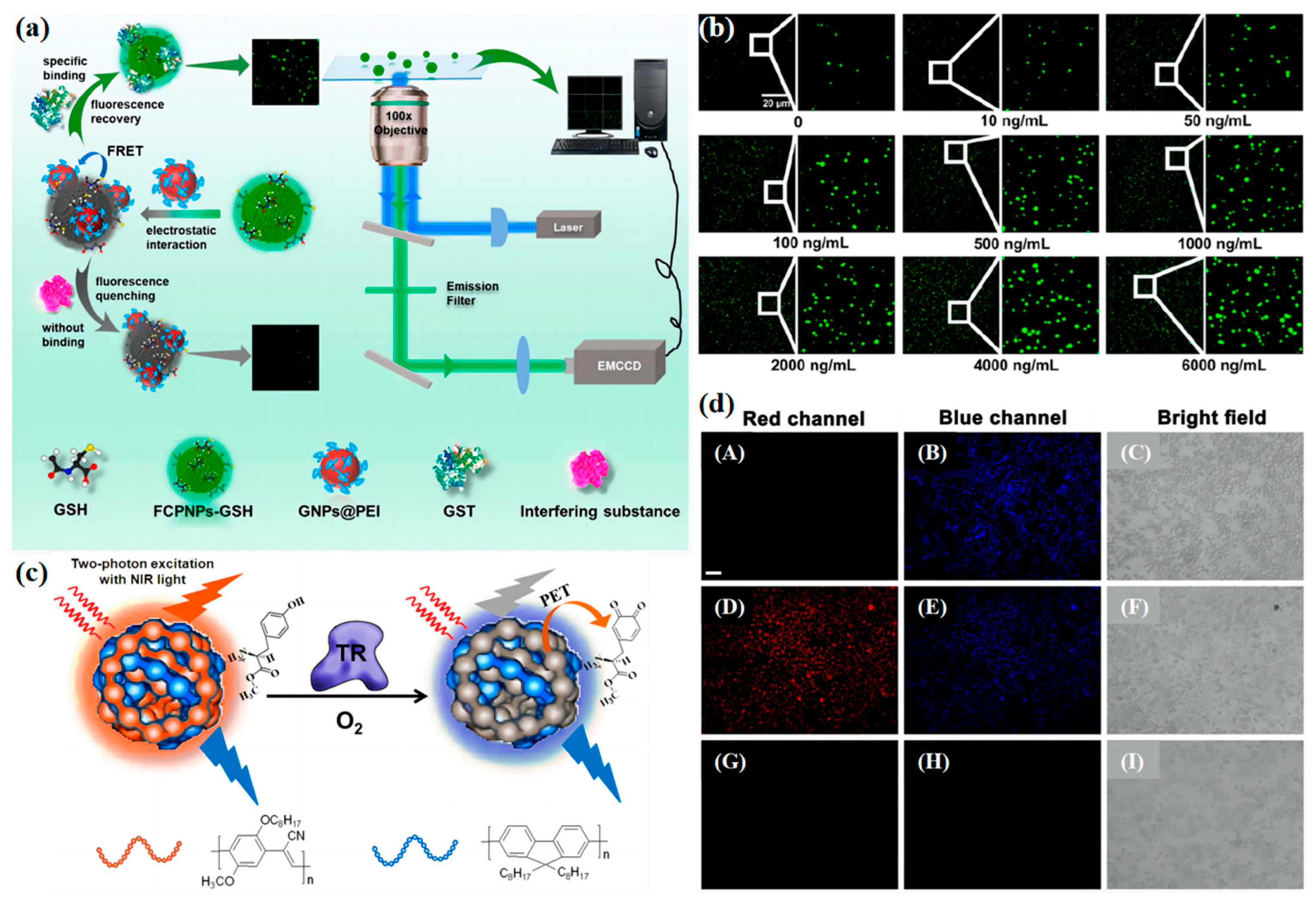
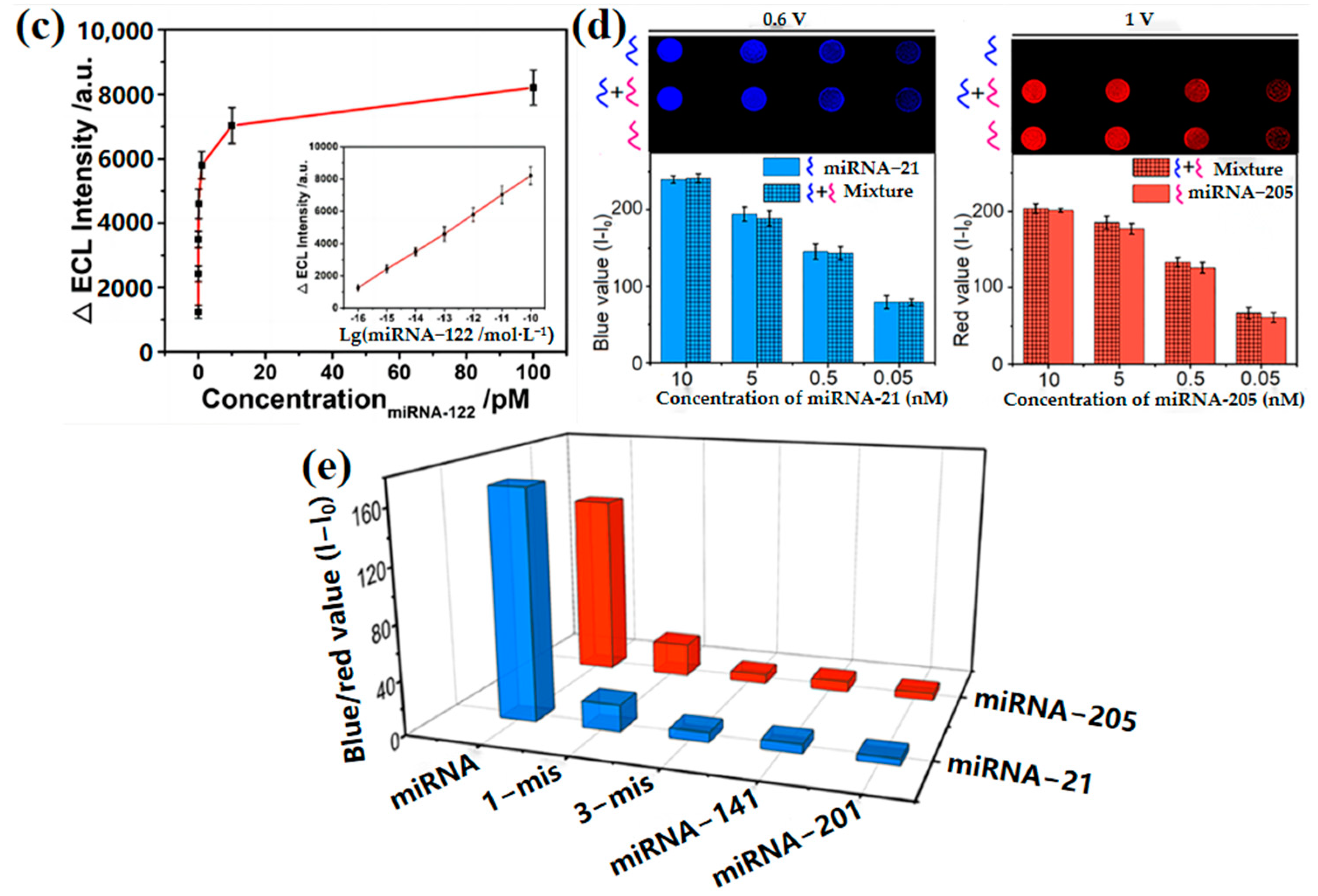
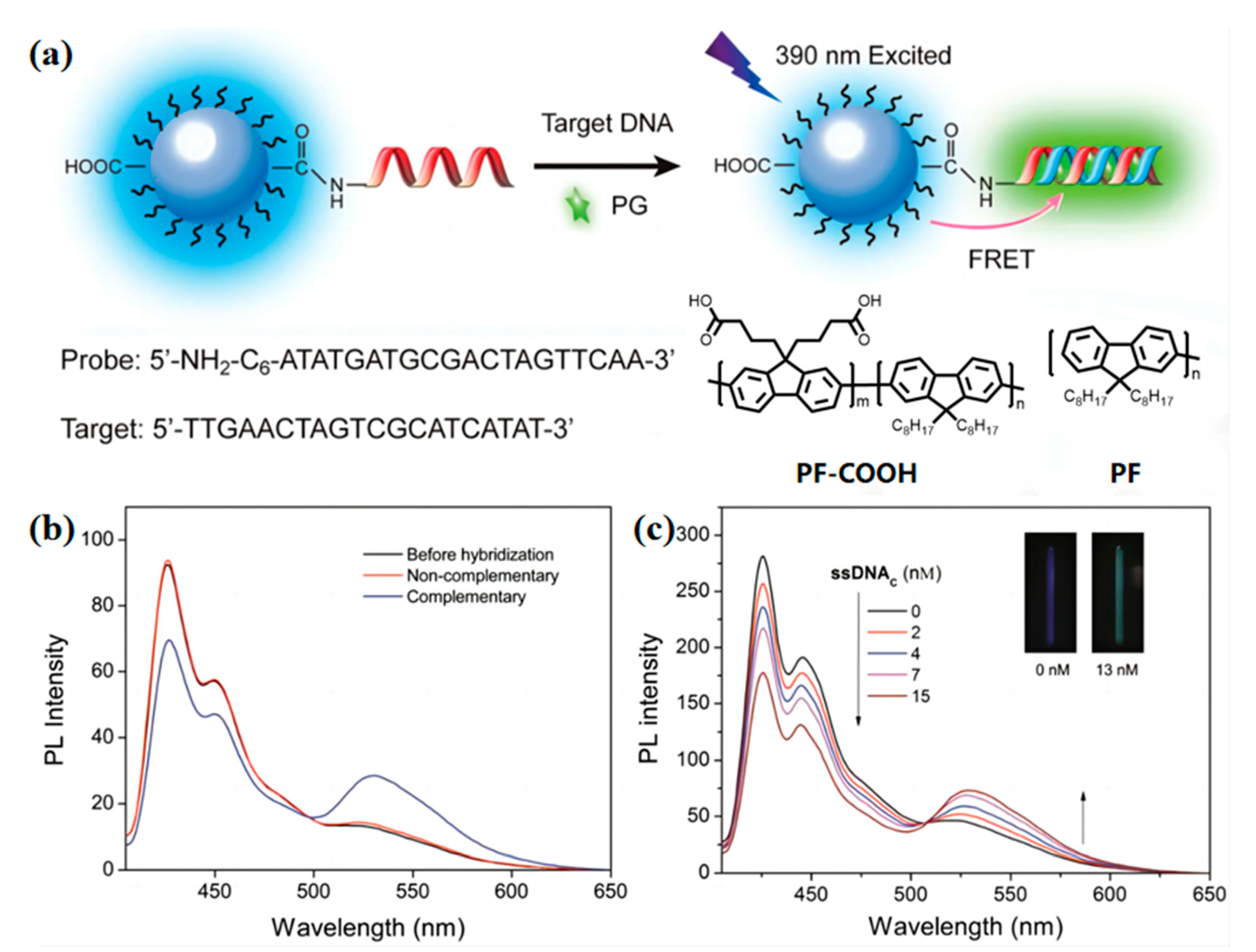
2.3. Nucleic Acid Concentration Measurement
3. In Vivo Tracking
3.1. Tumor Localization

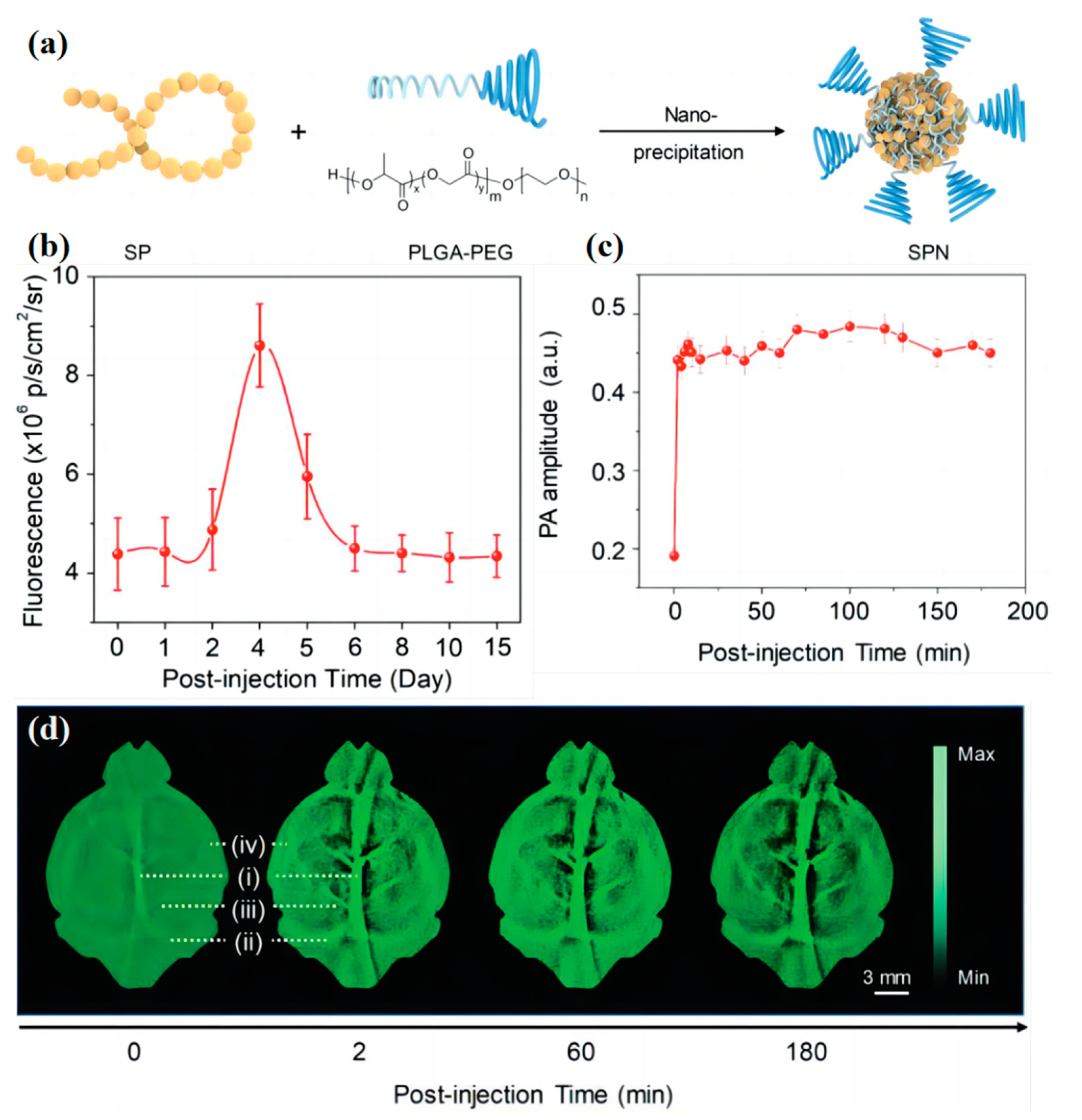
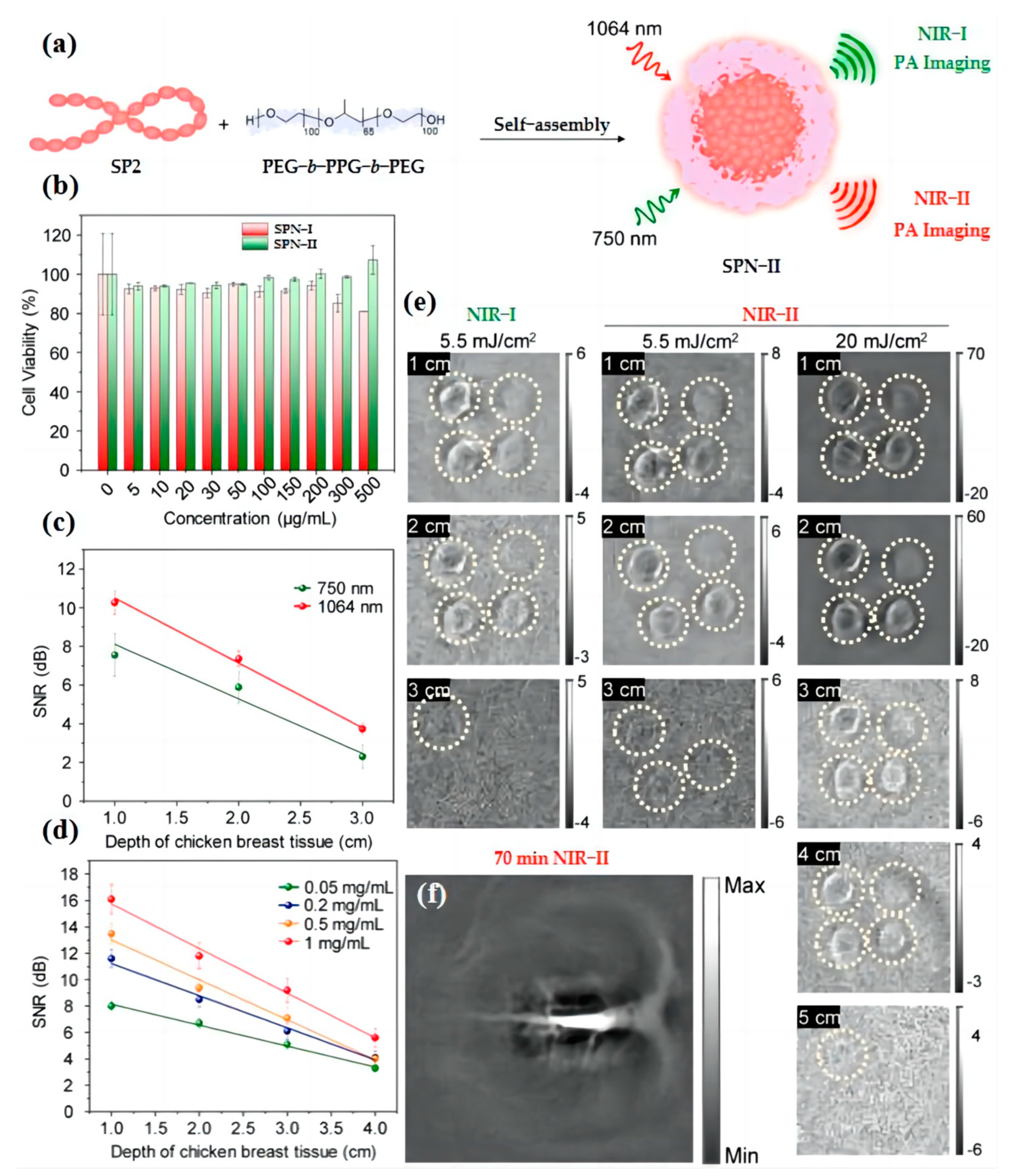
3.2. Blood Vessel Imaging
3.3. Particles Tracking
4. Conclusions and Outlook
| Probe | Target | Emission Spectra (λem) (nm) | Excitation Spectra (λex) (nm) | Ref. |
|---|---|---|---|---|
| P-dot@MnO2 | GSH | 510 | 458 | [44] |
| DQ-CD@Pdots | GSH | 541 | 450 | [45] |
| FCPNPs-GSH, GNPs@PEI | GST | 500–600 | 470 | [53] |
| PFO/CN-PPV@Tyr-OMe | TR | 586 | 380 | [60] |
| MiRNA-122 Probe | MiRNA-122 | 543 | - | [72] |
| L-Pdots | MiRNA-21 | 425 | - | [73] |
| N-Pdots | MiRNA-205 | 672 | - | [73] |
| PF-DNAP CPNs | ssDNAC | 530 | 390 | [80] |
| MMPF NPs | tumor | 810 | 680 | [107] |
| ASO | tumor | 790 | 600 | [111] |
| SPN-PT | brain vasculature | 820 | 710 | [125] |
| SPN-II | vessels | 1064 | - | [132] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gong, S.; Qin, A.; Zhang, Y.; Li, M.; Chen, X.; Liang, Y.; Xu, X.; Wang, Z.; Wang, S. A New Ratiometric AIE Fluorescent Probe for Detecting Cysteine in Food Samples and Imaging in the Biological System. Food Chem. 2023, 400, 134108. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ma, M.; Zai, H.; Zhang, L.; Fu, N.; Huang, W.; Wang, L. Conjugated Polymer Nanoparticles for Label-Free and Bioconjugate-Recognized DNA Sensing in Serum. Adv. Sci. 2015, 2, 1400009. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bai, H.; Yang, Z.; Shen, Q.; Li, M.; Huang, Y.; Lv, F.; Wang, S. Conjugated Polymers for Biomedical Applications. Chem. Commun. 2022, 58, 7232–7244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, R.; Wang, Q.; Li, X.; Hu, X.; Yuan, Y.; Lu, X.; Wang, W.; Huang, W.; Fan, Q. Semiconducting Polymer Nanotheranostics for NIR-II/Photoacoustic Imaging-Guided Photothermal Initiated Nitric Oxide/Photothermal Therapy. Biomaterials 2019, 217, 119304. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Kam, C.; Chou, T.Y.; Wu, M.-Y.; Zhao, X.; Chen, S. A Simple yet Effective AIE-Based Fluorescent Nano-Thermometer for Temperature Mapping in Living Cells Using Fluorescence Lifetime Imaging Microscopy. Nanoscale Horiz. 2020, 5, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, X.; Dong, Y.; Pan, Y.; Li, J.; Zang, Y.; Li, X. A Tandemly Activated Fluorescence Probe for Detecting Senescent Cells with Improved Selectivity by Targeting a Biomarker Combination. ACS Sens. 2022, 7, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In Vivo Gastrointestinal Drug-Release Monitoring through Second near-Infrared Window Fluorescent Bioimaging with Orally Delivered Microcarriers. Nat. Commun. 2017, 8, 14702. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, J.; Qin, Y.; Li, Z.; Pan, C.; Huo, Y.; Zhang, H. A Novel Quinolinyl-Tetraphenylethene-Based Fluorescence “Turn-on” Sensor for Zn2+ with a Large Stokes Shift and Its Applications for Portable Test Strips and Biological Imaging. Mater. Chem. Front. 2020, 4, 3338–3348. [Google Scholar] [CrossRef]
- Enbanathan, S.; Iyer, S.K. A Novel Phenanthridine and Terpyridine Based D-π-A Fluorescent Probe for the Ratiometric Detection of Cd2+ in Environmental Water Samples and Living Cells. Ecotoxicol. Environ. Saf. 2022, 247, 114272. [Google Scholar] [CrossRef]
- Jiang, N.; Gong, X.; Zhong, T.; Zheng, Y.; Wang, G. A Highly Selective and Sensitive “Turn-on” Fluorescent Probe for Rapid Recognition and Detection of Cu2+ in Aqueous Solution and in Living Cells. J. Mol. Struct. 2020, 1219, 128573. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Q.; Li, Y.; Zeng, X.; Liu, Y.; Lu, S.; Zhou, H.; Wang, X.; Wu, J.; Meng, X.; et al. Novel Small-Molecule Fluorophores for in Vivo NIR-IIa and NIR-IIb Imaging. Chem. Commun. 2020, 56, 3289–3292. [Google Scholar] [CrossRef] [PubMed]
- Fortibui, M.M.; Jang, M.; Lee, S.; Ryoo, I.-J.; Ahn, J.S.; Ko, S.-K.; Kim, J. Near-Infrared Fluorescence Probe for Specific Detection of Acetylcholinesterase and Imaging in Live Cells and Zebrafish. ACS Appl. Bio Mater. 2022, 5, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Srinivasan, V.; Ramamurthy, S.S. Nd2O3-Ag Nanostructures for Plasmonic Biosensing, Antimicrobial, and Anticancer Applications. ACS Appl. Nano Mater. 2023, 6, 1129–1145. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Z.; Zhou, X.; Wang, R.; Yu, F. Emergence of Surface-Enhanced Raman Scattering Probes in Near-Infrared Windows for Biosensing and Bioimaging. Anal. Chem. 2022, 94, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, S.T.H.; Lam, J.W.Y.; Kwok, R.T.K.; Qian, J.; Tang, B.Z. Design of AIEgens for Near-Infrared IIb Imaging through Structural Modulation at Molecular and Morphological Levels. Nat. Commun. 2020, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Albani, J.R. Principles and Applications of Fluorescence Spectroscopy, 1st ed.; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-1-4051-3891-8. [Google Scholar]
- Feng, G.; Zhang, G.-Q.; Ding, D. Design of Superior Phototheranostic Agents Guided by Jablonski Diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef]
- Han, W.; Du, Y.; Song, M.; Sun, K.; Xu, B.; Yan, F.; Tian, W. Fluorescent Nanorods Based on 9,10-Distyrylanthracene (DSA) Derivatives for Efficient and Long-Term Bioimaging. J. Mater. Chem. B 2020, 8, 9544–9554. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Wu, P.; Xiong, H. Highly Sensitive D–A–D-Type Near-Infrared Fluorescent Probe for Nitric Oxide Real-Time Imaging in Inflammatory Bowel Disease. Anal. Chem. 2021, 93, 4975–4983. [Google Scholar] [CrossRef]
- Deng, S.; Li, L.; Zhang, J.; Wang, Y.; Huang, Z.; Chen, H. Semiconducting Polymer Dots for Point-of-Care Biosensing and In Vivo Bioimaging: A Concise Review. Biosensors 2023, 13, 137. [Google Scholar] [CrossRef]
- Xiong, Y.; Shepherd, S.; Tibbs, J.; Bacon, A.; Liu, W.; Akin, L.D.; Ayupova, T.; Bhaskar, S.; Cunningham, B.T. Photonic Crystal Enhanced Fluorescence: A Review on Design Strategies and Applications. Micromachines 2023, 14, 668. [Google Scholar] [CrossRef]
- Zhao, Z.; Bi, X.; Mao, W.; Xu, X. A Novel HPQ-Based Turn-on Fluorescent Probe for Detection of Fluoride Ions in Living Cells. Tetrahedron Lett. 2017, 58, 4129–4132. [Google Scholar] [CrossRef]
- Li, K.; Lyu, Y.; Huang, Y.; Xu, S.; Liu, H.-W.; Chen, L.; Ren, T.-B.; Xiong, M.; Huan, S.; Yuan, L.; et al. A de Novo Strategy to Develop NIR Precipitating Fluorochrome for Long-Term in Situ Cell Membrane Bioimaging. Proc. Natl. Acad. Sci. USA 2021, 118, e2018033118. [Google Scholar] [CrossRef]
- Li, B.; Chen, T.; Wang, Z.; Guo, Z.; Peña, J.; Zeng, L.; Xing, J. A Novel Cross-Linked Nanoparticle with Aggregation-Induced Emission Properties for Cancer Cell Imaging. J. Mater. Chem. B 2020, 8, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.Y.; Zhang, W.; Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. A Photostable AIEgen for Nucleolus and Mitochondria Imaging with Organelle-Specific Emission. J. Mater. Chem. B 2016, 4, 2614–2619. [Google Scholar] [CrossRef]
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.; Liu, J.; Manghnani, P.N.; Hu, F.; Ma, D.; Teh, C.; Wang, B.; Liu, B. Cancer-Cell-Activated Photodynamic Therapy Assisted by Cu(II)-Based Metal–Organic Framework. ACS Nano 2019, 13, 6879–6890. [Google Scholar] [CrossRef]
- Hu, F.; Mao, D.; Kenry; Wang, Y.; Wu, W.; Zhao, D.; Kong, D.; Liu, B. Metal–Organic Framework as a Simple and General Inert Nanocarrier for Photosensitizers to Implement Activatable Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1707519. [Google Scholar] [CrossRef]
- Cai, X.; Mao, D.; Wang, C.; Kong, D.; Cheng, X.; Liu, B. Multifunctional Liposome: A Bright AIEgen-Lipid Conjugate with Strong Photosensitization. Angew. Chem. Int. Ed. 2018, 57, 16396–16400. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef]
- Liang, B.; Hu, X.; Ding, Y.; Liu, M. Tumor-derived Exosomes in the PD-1/PD-L1 Axis: Significant Regulators as Well as Promising Clinical Targets. J. Cell. Physiol. 2021, 236, 4138–4151. [Google Scholar] [CrossRef]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530. [Google Scholar] [CrossRef] [PubMed]
- Kuno, M.; Fromm, D.P.; Hamann, H.F.; Gallagher, A.; Nesbitt, D.J. Nonexponential “Blinking” Kinetics of Single CdSe Quantum Dots: A Universal Power Law Behavior. J. Chem. Phys. 2000, 112, 3117–3120. [Google Scholar] [CrossRef]
- Smith, W.E.; Brownell, J.; White, C.C.; Afsharinejad, Z.; Tsai, J.; Hu, X.; Polyak, S.J.; Gao, X.; Kavanagh, T.J.; Eaton, D.L. In Vitro Toxicity Assessment of Amphiphillic Polymer-Coated CdSe/ZnS Quantum Dots in Two Human Liver Cell Models. ACS Nano 2012, 6, 9475–9484. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, X.; Hu, X.; Shao, Z.; Zhu, J.; Dai, L.; Man, Z.; Yuan, L.; Chen, H.; Zhou, C.; et al. A Functional Biphasic Biomaterial Homing Mesenchymal Stem Cells for in Vivo Cartilage Regeneration. Biomaterials 2014, 35, 9608–9619. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Law, W.-C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity Assessment of Quantum Dots: From Cellular to Primate Studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef]
- Hopkins, F.G.; Morgan, E.J. Some relations between ascorbic acid and glutathione. Biochem. J. 1936, 30, 1446–1462. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The Role of Glutathione in Cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef]
- Mulay, S.V.; Kim, Y.; Choi, M.; Lee, D.Y.; Choi, J.; Lee, Y.; Jon, S.; Churchill, D.G.; Estrela, J.M.; Ortega, A.; et al. Enhanced Doubly Activated Dual Emission Fluorescent Probes for Selective Imaging of Glutathione or Cysteine in Living Systems. Anal. Chem. 2018, 90, 2648–2654. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Mulay, S.V.; Kim, Y.; Choi, M.; Lee, D.Y.; Choi, J.; Lee, Y.; Jon, S.; Churchill, D.G.; Estrela, J.M.; Ortega, A.; et al. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Zheng, C.; Ding, L.; Wu, Y.; Tan, X.; Zeng, Y.; Zhang, X.; Liu, X.; Liu, J. A Near-Infrared Turn-on Fluorescence Probe for Glutathione Detection Based on Nanocomposites of Semiconducting Polymer Dots and MnO2 Nanosheets. Anal. Bioanal. Chem. 2020, 412, 8167–8176. [Google Scholar] [CrossRef]
- Sun, J.; Chen, N.; Chen, X.; Zhang, Q.; Gao, F. Two-Photon Fluorescent Nanoprobe for Glutathione Sensing and Imaging in Living Cells and Zebrafish Using a Semiconducting Polymer Dots Hybrid with Dopamine and β-Cyclodextrin. Anal. Chem. 2019, 91, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Moronshing, M.; Srinivasan, V.; Badiya, P.K.; Subramaniam, C.; Ramamurthy, S.S. Silver Soret Nanoparticles for Femtomolar Sensing of Glutathione in a Surface Plasmon-Coupled Emission Platform. ACS Appl. Nano Mater. 2020, 3, 4329–4341. [Google Scholar] [CrossRef]
- McDonald, A.G.; Tipton, K.F. Enzyme Nomenclature and Classification: The State of the Art. FEBS J. 2022, febs.16274. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Glutathione Transferases, Regulators of Cellular Metabolism and Physiology. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef]
- Board, P.G.; Anders, M.W. Glutathione Transferase Omega 1 Catalyzes the Reduction of S-(Phenacyl)Glutathiones to Acetophenones. Chem. Res. Toxicol. 2007, 20, 149–154. [Google Scholar] [CrossRef]
- Fahmi, O.A.; Ripp, S.L. Evaluation of Models for Predicting Drug–Drug Interactions Due to Induction. Expert Opinion on Drug Metab. Toxicol. 2010, 6, 1399–1416. [Google Scholar] [CrossRef]
- Kolodziej, C.M.; Chang, C.-W.; Maynard, H.D. GlutathioneS-Transferase as a General and Reversible Tag for Surface Immobilization of Proteins. J. Mater. Chem. 2011, 21, 1457–1461. [Google Scholar] [CrossRef]
- Singh, S. Cytoprotective and Regulatory Functions of Glutathione S-Transferases in Cancer Cell Proliferation and Cell Death. Cancer Chemother. Pharmacol. 2015, 75, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, T.; Li, Y.; Chen, L.; Wei, L.; Xiao, L. Single-Particle Enumeration-Based Sensitive Glutathione S-Transferase Assay with Fluorescent Conjugated Polymer Nanoparticle. Anal. Chem. 2019, 91, 11146–11153. [Google Scholar] [CrossRef] [PubMed]
- VEDRINE, C. Amperometric Tyrosinase Based Biosensor Using an Electrogenerated Polythiophene Film as an Entrapment Support. Talanta 2003, 59, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.-F.; Zeng, C.; Hu, L.; Liang, X.-J. Ultrasensitive Tyrosinase-Activated Turn-On Near-Infrared Fluorescent Probe with a Rationally Designed Urea Bond for Selective Imaging and Photodamage to Melanoma Cells. Anal. Chem. 2018, 90, 3666–3669. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, K.; Abe, J. Photochromism of a Water-Soluble Vesicular [2.2]Paracyclophane-Bridged Imidazole Dimer. Chem. Commun. 2011, 47, 8868. [Google Scholar] [CrossRef]
- Wang, C.; Yan, S.; Huang, R.; Feng, S.; Fu, B.; Weng, X.; Zhou, X. A Turn-on Fluorescent Probe for Detection of Tyrosinase Activity. Analyst 2013, 138, 2825. [Google Scholar] [CrossRef]
- Yan, S.; Huang, R.; Wang, C.; Zhou, Y.; Wang, J.; Fu, B.; Weng, X.; Zhou, X. A Two-Photon Fluorescent Probe for Intracellular Detection of Tyrosinase Activity. Chem. Asian J. 2012, 7, 2782–2785. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, J.; Zhao, Z.; Sun, M.; Chi, X.; Wang, X.; Gao, J. Kinetic and Sensitive Analysis of Tyrosinase Activity Using Electron Transfer Complexes: In Vitro and Intracellular Study. Small 2015, 11, 862–870. [Google Scholar] [CrossRef]
- Sun, J.; Mei, H.; Wang, S.; Gao, F. Two-Photon Semiconducting Polymer Dots with Dual-Emission for Ratiometric Fluorescent Sensing and Bioimaging of Tyrosinase Activity. Anal. Chem. 2016, 88, 7372–7377. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, Z.; Cai, Y.; Gong, J.; Kwok, R.T.K.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. An Easily Accessible Ionic Aggregation-Induced Emission Luminogen with Hydrogen-Bonding-Switchable Emission and Wash-Free Imaging Ability. Angew. Chem. Int. Ed. 2018, 57, 5011–5015. [Google Scholar] [CrossRef]
- Todorov, T.I.; Morris, M.D. Comparison of RNA, Single-Stranded DNA and Double-Stranded DNA Behavior during Capillary Electrophoresis in Semidilute Polymer Solutions. Electrophoresis 2002, 23, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Dahm, R. Discovering DNA: Friedrich Miescher and the Early Years of Nucleic Acid Research. Hum. Genet. 2008, 122, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.G.; Barlow, K.F.; McLay, K.E.; Kaul, R.; Swarbreck, D.; Dunham, A.; Scott, C.E.; Howe, K.L.; Woodfine, K.; Spencer, C.C.A.; et al. The DNA Sequence and Biological Annotation of Human Chromosome 1. Nature 2006, 441, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Yeh, H.-C.; Kuroki, M.T.; Wang, T.-H. Single-Quantum-Dot-Based DNA Nanosensor. Nat. Mater. 2005, 4, 826–831. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Jopling, C. Liver-Specific MicroRNA-122: Biogenesis and Function. RNA Biol. 2012, 9, 137–142. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and Energy Metabolism. ABBS 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhang, W.; Zhang, L. MicroRNAs Play an Essential Role in Autophagy Regulation in Various Disease Phenotypes. BioFactors 2019, 45, 844–856. [Google Scholar] [CrossRef]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic MicroRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Annotating High Confidence MicroRNAs Using Deep Sequencing Data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Z.-Y.; Gao, H.; Yu, Y.; Pan, J.-B.; Chen, H.-Y.; Xu, J.-J. An Ultrasensitive Electrochemiluminescence Assay for Nucleic Acid Detection Based on Carboxyl Functionalized Polymer Dots. J. Electroanal. Chem. 2021, 900, 115743. [Google Scholar] [CrossRef]
- Wang, N.; Chen, L.; Chen, W.; Ju, H. Potential- and Color-Resolved Electrochemiluminescence of Polymer Dots for Array Imaging of Multiplex MicroRNAs. Anal. Chem. 2021, 93, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. DNA Replication and Recombination. Nature 2003, 421, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Frock, R.L.; Hu, J.; Meyers, R.M.; Ho, Y.-J.; Kii, E.; Alt, F.W. Genome-Wide Detection of DNA Double-Stranded Breaks Induced by Engineered Nucleases. Nat. Biotechnol. 2015, 33, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical Detection of Nucleic Acids, Proteins, Small Molecules and Cells Using a DNA-Nanostructure-Based Universal Biosensing Platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Angell, C.; Xie, S.; Zhang, L.; Chen, Y. DNA Nanotechnology for Precise Control over Drug Delivery and Gene Therapy. Small 2016, 12, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. DNA in a Material World. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef]
- Isam, Z.; Al-jelawi, R.O.; Mahmood, A.H. Detection Single Nucleotide Polymorphisms in Uromodulin Promoter Region Associated with Renal Diseases Using Sin-gle-Strand Conformation Polymorphism-Polymerase Chain Polymorphisms Technique. Asian J. Pharm. Clin. Res. 2003, 11, 205. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Filichev, V.V.; Vester, B.; Hansen, L.H.; Abdel Aal, M.T.; Babu, B.R.; Wengel, J.; Pedersen, E.B. Enhanced Inhibition of Transcription Start by Targeting with 2′-OMe Pentaribonucleotides Comprising Locked Nucleic Acids and Intercalating Nucleic Acids. ChemBioChem 2005, 6, 1181–1184. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2020, 6, 7–26. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, X.; Huang, J.; Li, J.; Upputuri, P.K.; Sun, H.; Han, X.; Pramanik, M.; Miao, Y.; Duan, H.; et al. Transformable Hybrid Semiconducting Polymer Nanozyme for Second Near-Infrared Photothermal Ferrotherapy. Nat. Commun. 2020, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Xie, C.; Pu, K. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat. Nanotechnol. 2014, 9, 233–239. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, L.; Sun, Y.; Wang, Y.; Wang, J.; Ye, D. Ratiometric Imaging of MMP-2 Activity Facilitates Tumor Detection Using Activatable Near-Infrared Fluorescent Semiconducting Polymer Nanoparticles. Small 2021, 17, 2101924. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene Quantum Dot Photodynamic Therapy Agent with High Singlet Oxygen Generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, C.; Han, X.; Zhong, H.; Cheng, C. Viral Nanoparticle System: An Effective Platform for Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 1728. [Google Scholar] [CrossRef]
- Shivran, N.; Tyagi, M.; Mula, S.; Gupta, P.; Saha, B.; Patro, B.S.; Chattopadhyay, S. Syntheses and Photodynamic Activity of Some Glucose-Conjugated BODIPY Dyes. Eur. J. Med. Chem. 2016, 122, 352–365. [Google Scholar] [CrossRef]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-Photon Excitation Nanoparticles for Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-Based Nanoparticles Entrapping Water-Insoluble Photosensitizing Anticancer Drugs: A Novel Drug−Carrier System for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.; Lee, A.K.; Scott, T.; Sproull, M.; Ménard, C.; Tofilon, P.J.; Camphausen, K. In Vivo Tumor Imaging in Mice with near-Infrared Labeled Endostatin. Mol. Cancer Ther. 2004, 3, 481–488. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Hu, X.; Zou, H.; Luo, Q.; Lee, M.M.S.; Xu, W.; Lam, J.W.Y.; Tang, B.Z. Rational Design of a Water-Soluble NIR AIEgen, and Its Application in Ultrafast Wash-Free Cellular Imaging and Photodynamic Cancer Cell Ablation. Chem. Sci. 2018, 9, 3685–3693. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Nolting, D.D.; Gore, J.C.; Pham, W. Near-Infrared Dyes: Probe Development and Applications in Optical Molecular Imaging. Curr. Org. Synth. 2011, 8, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent Progress in the Development of Near-Infrared Fluorescent Probes for Bioimaging Applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-Red to near Infrared Analyte-Responsive Fluorescent Probes Based on Organic Fluorophore Platforms for Fluorescence Imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef]
- Patel, N.; Pera, P.; Joshi, P.; Dukh, M.; Tabaczynski, W.A.; Siters, K.E.; Kryman, M.; Cheruku, R.R.; Durrani, F.; Missert, J.R.; et al. Highly Effective Dual-Function Near-Infrared (NIR) Photosensitizer for Fluorescence Imaging and Photodynamic Therapy (PDT) of Cancer. J. Med. Chem. 2016, 59, 9774–9787. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, B.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. Fabrication of Aggregation Induced Emission Dye-Based Fluorescent Organic Nanoparticles via Emulsion Polymerization and Their Cell Imaging Applications. Polym. Chem. 2014, 5, 399–404. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, H.; Jiang, Z.; Guan, Y.; Zou, J.; Wang, X.; Cheng, R.; Gao, H. Low-Power White Light Triggered AIE Polymer Nanoparticles with High ROS Quantum Yield for Mitochondria-Targeted and Image-Guided Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 6277–6281. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, C.-J.; Kwok, R.T.K.; Xu, S.; Zhang, R.; Wu, J.; Tang, B.Z.; Liu, B. Light-Up Probe for Targeted and Activatable Photodynamic Therapy with Real-Time In Situ Reporting of Sensitizer Activation and Therapeutic Responses. Adv. Funct. Mater. 2015, 25, 6586–6595. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Dong, S.; Xiong, J.; Du, Z.; Cheng, X. A PH-Responsive AIE Nanoprobe as a Drug Delivery System for Bioimaging and Cancer Therapy. J. Mater. Chem. B 2015, 3, 7401–7407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yong, T.-Y.; Wan, J.; Li, Z.-H.; Zhao, H.; Zhao, Y.; Gan, L.; Yang, X.-L.; Xu, H.-B.; Zhang, C. Temperature-Sensitive Fluorescent Organic Nanoparticles with Aggregation-Induced Emission for Long-Term Cellular Tracing. ACS Appl. Mater. Interfaces 2015, 7, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.G.; Lytle, N.K.; Jaquish, D.V.; Park, F.D.; Ito, T.; Bajaj, J.; Koechlein, C.S.; Zimdahl, B.; Yano, M.; Kopp, J.L.; et al. Image-Based Detection and Targeting of Therapy Resistance in Pancreatic Adenocarcinoma. Nature 2016, 534, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zheng, X.; Wang, Y.; Xia, X.; Chu, S.; Rao, J. A Magneto-Optical Nanoplatform for Multimodality Imaging of Tumors in Mice. ACS Nano 2019, 13, 7750–7758. [Google Scholar] [CrossRef]
- Rijcken, C.J.; Snel, C.J.; Schiffelers, R.M.; van Nostrum, C.F.; Hennink, W.E. Hydrolysable Core-Crosslinked Thermosensitive Polymeric Micelles: Synthesis, Characterisation and in Vivo Studies. Biomaterials 2007, 28, 5581–5593. [Google Scholar] [CrossRef]
- Tyrrell, Z.L.; Shen, Y.; Radosz, M. Fabrication of Micellar Nanoparticles for Drug Delivery through the Self-Assembly of Block Copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Kang, N.; Perron, M.-È.; Prud’homme, R.E.; Zhang, Y.; Gaucher, G.; Leroux, J.-C. Stereocomplex Block Copolymer Micelles: Core−Shell Nanostructures with Enhanced Stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef]
- Yin, C.; Zhen, X.; Zhao, H.; Tang, Y.; Ji, Y.; Lyu, Y.; Fan, Q.; Huang, W.; Pu, K. Amphiphilic Semiconducting Oligomer for Near-Infrared Photoacoustic and Fluorescence Imaging. ACS Appl. Mater. Interfaces 2017, 9, 12332–12339. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Wu, K.-Y.; Chen, Y.-M.; Deng, R.; Li, X.; Jin, H.; Li, S.; Chuang, S.S.C.; Wang, C.-L.; et al. Hydrogen-Bonding-Mediated Solid-State Self-Assembled Isoepindolidiones (IsoEpi) Crystal for Organic Field-Effect Transistor. J. Phys. Chem. C 2018, 122, 5888–5895. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, R.; Wang, J.; Li, X.; Chen, Y.-M.; Liu, K.; Taubert, C.J.; Cheng, S.Z.D.; Zhu, Y. Crystalline Organic Pigment-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2017, 9, 21891–21899. [Google Scholar] [CrossRef] [PubMed]
- Ocheje, M.U.; Charron, B.P.; Cheng, Y.-H.; Chuang, C.-H.; Soldera, A.; Chiu, Y.-C.; Rondeau-Gagné, S. Amide-Containing Alkyl Chains in Conjugated Polymers: Effect on Self-Assembly and Electronic Properties. Macromolecules 2018, 51, 1336–1344. [Google Scholar] [CrossRef]
- Li, R.; Li, C.; Liu, M.; Vivo, P.; Zheng, M.; Dai, Z.; Zhan, J.; He, B.; Li, H.; Yang, W.; et al. Hydrogen-Bonded Dopant-Free Hole Transport Material Enables Efficient and Stable Inverted Perovskite Solar Cells. CCS Chem. 2022, 4, 3084–3094. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, K. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Acc. Chem. Res. 2018, 51, 1840–1849. [Google Scholar] [CrossRef]
- Urade, M.; Shinbo, T. Barium Appendicitis 1 Month After a Barium Meal. Int. Surg. 2013, 97, 296–298. [Google Scholar] [CrossRef]
- Kurer, M.A.; Chintapatla, S. Intestinal Obstruction Due to Inspissated Barium. N. Engl. J. Med. 2007, 356, 1656. [Google Scholar] [CrossRef]
- Cumberland, D.C. Optimum Viscosity of Barium Suspension for Use in the Double Contrast Barium Meal. Gastrointest. Radiol. 1977, 2, 169–174. [Google Scholar] [CrossRef]
- Stringer, D.A.; Hassall, E.; Ferguson, A.C.; Cairns, R.; Nadel, H.; Sargent, M. Hypersensitivity Reaction to Single Contrast Barium Meal Studies in Children. Pediatr. Radiol. 1993, 23, 587–588. [Google Scholar] [CrossRef]
- Nijhawan, S.; Kumpawat, S.; Mallikarjun, P.; Bansal, R.; Singla, D.; Ashdhir, P.; Mathur, A.; Rai, R.R. Barium Meal Follow through with Pneumocolon: Screening Test for Chronic Bowel Pain. World J. Gastroenterol. 2008, 14, 6694. [Google Scholar] [CrossRef]
- Tada, S.; Iida, M.; Fuchigami, T.; Matsui, T.; Iwashita, A.; Yao, T.; Fujishima, M. Barium Meal Study for Amyloidosis of the Small Intestine: Measurements on Radiograph. Gastrointest. Radiol. 1990, 15, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Wang, W.; Huang, Y.-M. Barium Meal Peritonitis, Fatal Outcome in Unsuspected Small Bowel Perforation. Indian J. Surg. 2021, 83, 1589–1590. [Google Scholar] [CrossRef]
- Elman, R.; MacLeod, J.W. Studies on the Neutralization of Gastric Acidity: Ewald Test Meal and X-Ray (Barium Meal) Studies in Patients with Duodenal Ulcer, Gastro-Jejunostomy and Gastric Resection. Am. J. Dig. Dis. Nutr. 1935, 2, 21–26. [Google Scholar] [CrossRef]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Zeng, Z.; Sharma, A.; Zhen, X.; Li, J.; Huang, J.; Pramanik, M.; Pu, K. Metabolizable Semiconducting Polymer Nanoparticles for Second Near-Infrared Photoacoustic Imaging. Adv. Mater. 2019, 31, 1808166. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, C.; He, S.; Huang, J.; Huang, J.; Liew, S.S.; Zeng, Z.; Pu, K. A Dual-Locked Activatable Phototheranostic Probe for Biomarker-Regulated Photodynamic and Photothermal Cancer Therapy. Angew. Chem. Int. Ed. 2022, 61, e202202966. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.; Shi, Q.; Cai, X.; Huang, H. Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements. Adv. Mater. 2022, 34, 2108146. [Google Scholar] [CrossRef]
- Liu, M.; Cao, J.; Tu, Y.; Huang, C.; Zhang, M.; Zheng, J. An Ultra-Sensitive near-Infrared Fluorescent Probe Based on Triphenylamine with High Selectivity Detecting the Keratin. Anal. Biochem. 2022, 646, 114638. [Google Scholar] [CrossRef]
- Li, S.; Deng, X.; Cheng, H.; Li, X.; Wan, Y.; Cao, C.; Yu, J.; Liu, Y.; Yuan, Y.; Wang, K.; et al. Bright Near-Infrared π-Conjugated Oligomer Nanoparticles for Deep-Brain Three-Photon Microscopy Excited at the 1700 Nm Window in Vivo. ACS Nano 2022, 16, 12480–12487. [Google Scholar] [CrossRef]
- Alifu, N.; Zebibula, A.; Qi, J.; Zhang, H.; Sun, C.; Yu, X.; Xue, D.; Lam, J.W.Y.; Li, G.; Qian, J.; et al. Single-Molecular Near-Infrared-II Theranostic Systems: Ultrastable Aggregation-Induced Emission Nanoparticles for Long-Term Tracing and Efficient Photothermal Therapy. ACS Nano 2018, 12, 11282–11293. [Google Scholar] [CrossRef]
- Tang, F.; Liu, J.-Y.; Wu, C.-Y.; Liang, Y.-X.; Lu, Z.-L.; Ding, A.-X.; Xu, M.-D. Two-Photon Near-Infrared AIE Luminogens as Multifunctional Gene Carriers for Cancer Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 23384–23395. [Google Scholar] [CrossRef]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Lyu, Y.; Zhang, L.; Xiong, Q.; Pramanik, M.; Pu, K. Broadband Absorbing Semiconducting Polymer Nanoparticles for Photoacoustic Imaging in Second Near-Infrared Window. Nano Lett. 2017, 17, 4964–4969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; West, J.J.; Mathur, R.; Xing, J.; Hogrefe, C.; Roselle, S.J.; Bash, J.O.; Pleim, J.E.; Gan, C.-M.; Wong, D.C. Long-term trends in the ambient PM2.5- and O3-related mortality burdens in the United States under emission reductions from 1990 to 2010. Atmos. Chem. Phys. 2018, 18, 15003–15016. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Kan, H. Air pollution: A global problem needs local fixes. Nature 2019, 570, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Li, G.; Zhang, Y.; Li, J.; Chen, H. Fluorescent Reconstitution on Deposition of PM2.5 in Lung and Extrapulmonary Organs. Proc. Natl. Acad. Sci. USA 2019, 116, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, A.; Kanno, S.; Kobayashi, T.; Hirano, S. Extrapulmonary Translocation of Intratracheally Instilled Fine and Ultrafine Particles via Direct and Alveolar Macrophage-Associated Routes. Arch. Toxicol. 2009, 83, 429–437. [Google Scholar] [CrossRef]
- Hopkins, L.E.; Patchin, E.S.; Chiu, P.-L.; Brandenberger, C.; Smiley-Jewell, S.; Pinkerton, K.E. Nose-to-Brain Transport of Aerosolised Quantum Dots Following Acute Exposure. Nanotoxicology 2014, 8, 885–893. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Han, D.; Wang, H.; Zheng, M.; Xu, Y.; Zhang, H. Organic Semiconducting Nanoparticles for Biosensor: A Review. Biosensors 2023, 13, 494. https://doi.org/10.3390/bios13040494
Wang Z, Han D, Wang H, Zheng M, Xu Y, Zhang H. Organic Semiconducting Nanoparticles for Biosensor: A Review. Biosensors. 2023; 13(4):494. https://doi.org/10.3390/bios13040494
Chicago/Turabian StyleWang, Zheng, Dongyang Han, Hongzhen Wang, Meng Zheng, Yanyi Xu, and Haichang Zhang. 2023. "Organic Semiconducting Nanoparticles for Biosensor: A Review" Biosensors 13, no. 4: 494. https://doi.org/10.3390/bios13040494
APA StyleWang, Z., Han, D., Wang, H., Zheng, M., Xu, Y., & Zhang, H. (2023). Organic Semiconducting Nanoparticles for Biosensor: A Review. Biosensors, 13(4), 494. https://doi.org/10.3390/bios13040494





