MoS2-Nanoflower and Nanodiamond Co-Engineered Surface Plasmon Resonance for Biosensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of MNF
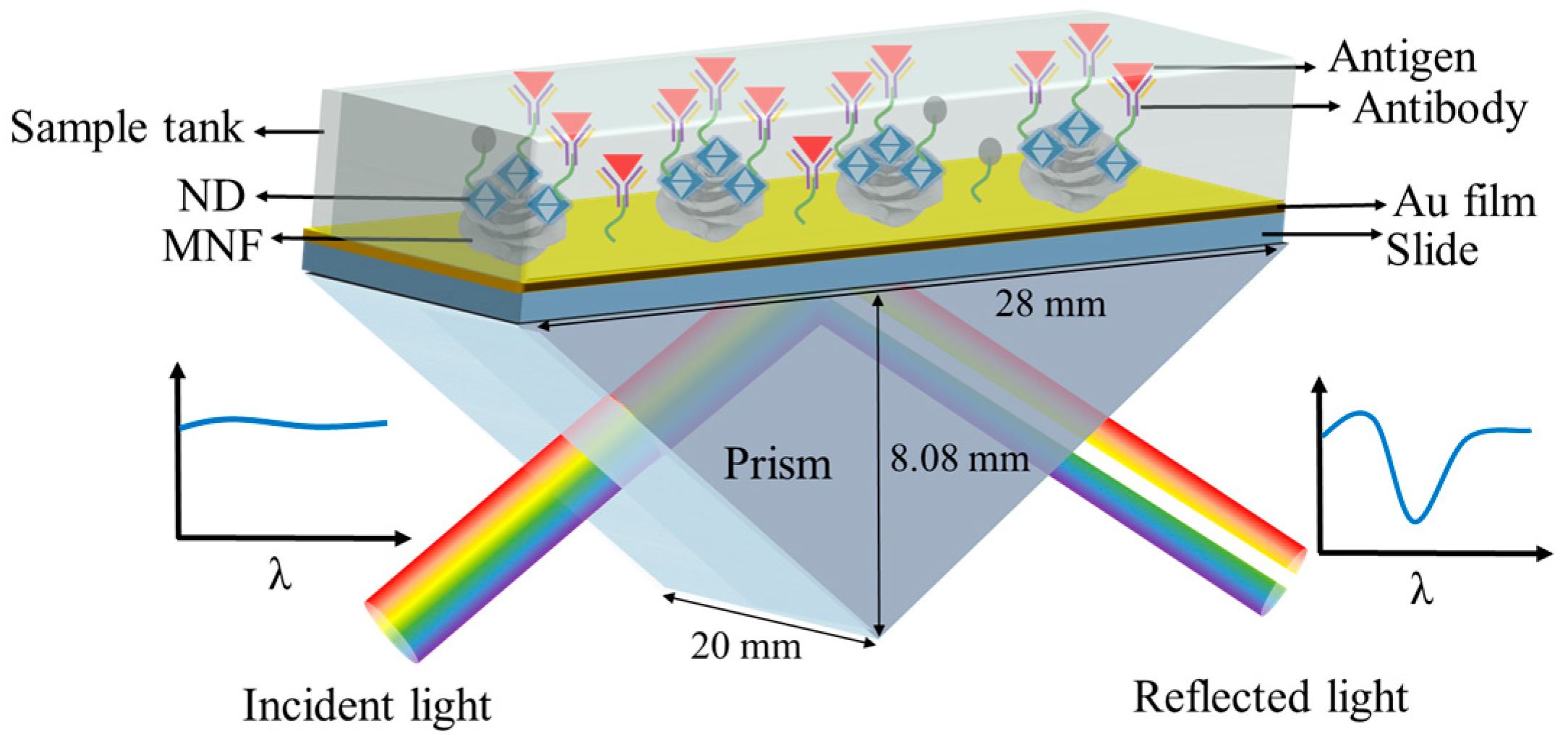
2.3. SPR Chips Preparation
2.4. Specific Modification on Chip Surface
2.5. IgG Immunoassay
2.6. PRV Immunoassay
3. Results and Discussions
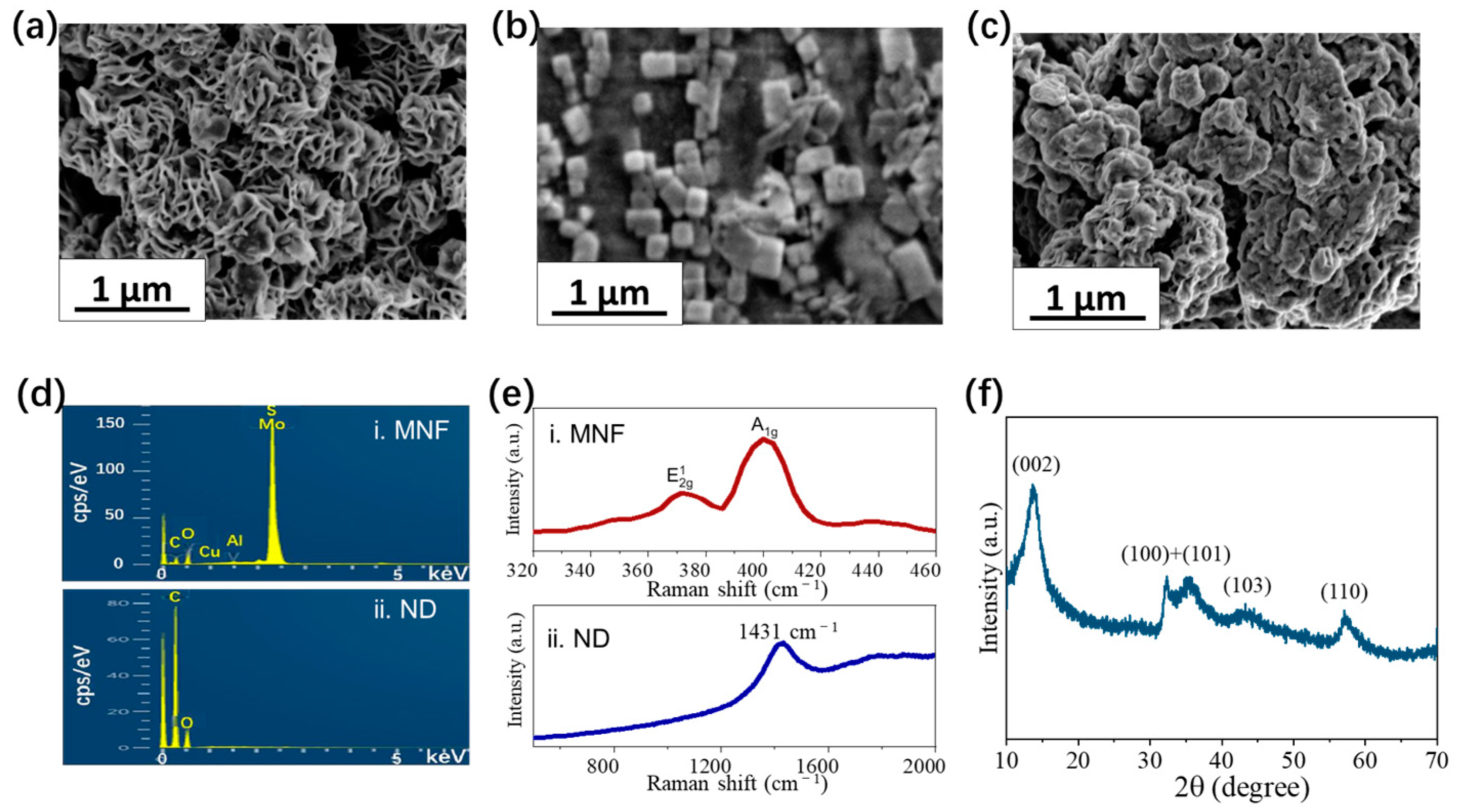
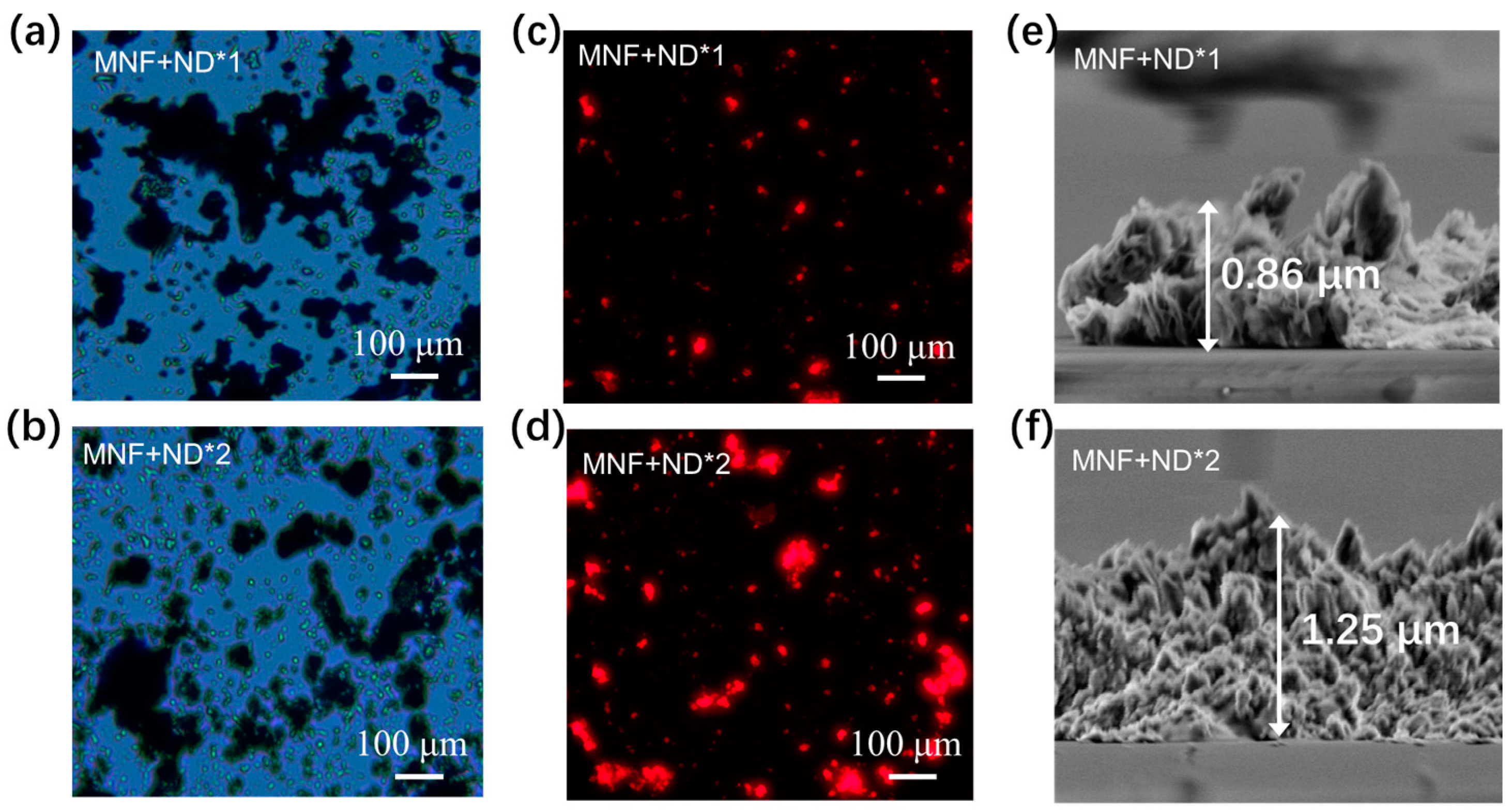
3.1. Materials and Chips Characterization
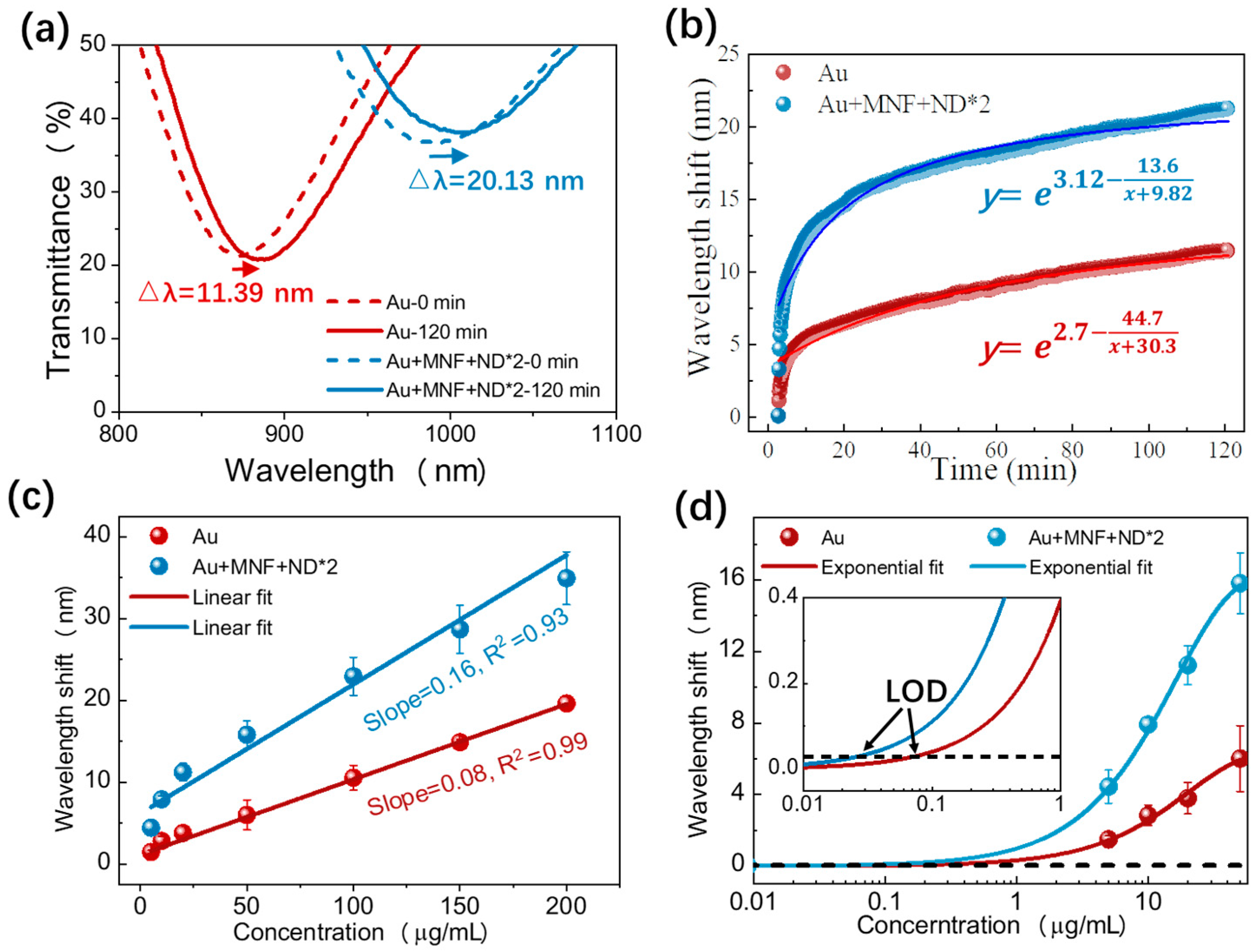
3.2. Bulk RI Sensitivity
3.3. IgG Immunoassay Performance
3.4. PRV Immunoassay Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guha, A.; Ahmad, O.S.; Guerreiro, A.; Karim, K.; Sandström, N.; Ostanin, V.P.; van der Wijngaart, W.; Piletsky, S.A.; Ghosh, S.K. Direct detection of small molecules using a nano-molecular imprinted polymer receptor and a quartz crystal resonator driven at a fixed frequency and amplitude. Biosens. Bioelectron. 2020, 158, 112176. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, C.; Wang, Y.; Luo, Y.; Rao, Y.-J.; Peng, G.-D.; Gong, Y. A sequentially bioconjugated optofluidic laser for wash-out-free and rapid biomolecular detection. Lab A Chip 2021, 21, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Zavatski, S.; Popov, A.I.; Chemenev, A.; Dauletbekova, A.; Bandarenka, H. Wet Chemical Synthesis and Characterization of Au Coatings on Meso-and Macroporous Si for Molecular Analysis by SERS Spectroscopy. Crystals 2022, 12, 1656. [Google Scholar] [CrossRef]
- Masson, J.F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Shalabney, A.; Abdulhalim, I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photonics Rev. 2011, 5, 571–606. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.-Y.; Wang, X.-Z.; Niu, L.-Y.; Zhao, W.-M. A D-Shaped Fiber Long-Range Surface Plasmon Resonance Sensor With High Q-Factor and Temperature Self-Compensation. IEEE Trans. Instrum. Meas. 2020, 69, 2218–2224. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Jing, J.; Liu, K.; Jiang, J.; Xu, T.; Wang, S.; Ma, J.; Zhang, Z.; Zhang, W.; Liu, T. Performance improvement approaches for optical fiber SPR sensors and their sensing applications. Photonics Res. 2022, 10, 126–147. [Google Scholar] [CrossRef]
- Cai, H.; Wang, M.; Liu, J.; Wang, X. Theoretical and experimental study of a highly sensitive SPR biosensor based on Au grating and Au film coupling structure. Opt. Express 2022, 30, 26136. [Google Scholar] [CrossRef]
- Yan, R.; Wang, T.; Yue, X.; Wang, H.; Zhang, Y.-H.; Xu, P.; Wang, L.; Wang, Y.; Zhang, J. Highly sensitive plasmonic nanorod hyperbolic metamaterial biosensor. Photonics Res. 2022, 10, 84–95. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Thomas, P.A.; Kravets, V.G.; Arola, H.O.; Soikkeli, M.; Iljin, K.; Kim, G.; Kim, M.; Shin, H.S.; Andreeva, D.V.; et al. Layered material platform for surface plasmon resonance biosensing. Sci. Rep. 2019, 9, 20286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A.; Kaźmierczak, A.; Piramidowicz, R. A numerical investigation of a plasmonic sensor based on a metal-insulator-metal waveguide for simultaneous detection of biological analytes and ambient temperature. Nanomaterials 2021, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, J.; Shafi, M.; Liu, R.; Zha, Z.; Feng, D.; Liu, M.; Du, X.; Yue, W.; Jiang, S. Optical fiber SPR biosensor complying with a 3D composite hyperbolic metamaterial and a graphene film. Photonics Res. 2021, 9, 379–388. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Chen, Y.; Chen, L.; Zheng, H.; Azeman, N.H.; Liu, M.X.; Liu, G.-S.; Luo, Y.; Chen, Z. High-performance fiber plasmonic sensor by engineering the dispersion of hyperbolic metamaterials composed of Ag/TiO2. Opt. Express 2020, 28, 25562–25573. [Google Scholar] [CrossRef]
- Hu, S.; Shi, W.; Chen, Y.; Chen, Y.; Liu, G.-S.; Chen, L.; Luo, Y.; Chen, Z. Dispersion Management for Hyperbolic-Metamaterials Based Surface Plasmon Resonance Sensor Towards Extremely High Sensitivity. J. Light. Technol. 2022, 40, 887–893. [Google Scholar] [CrossRef]
- Hu, S.; Shi, W.; Chen, Y.; Yuan, J.; Xiong, X.; Liu, T.; Ding, S.; Xiao, W.; Chen, Y.; Liu, G.S.; et al. Universal and flexible design for high-sensitivity and wide-ranging surface plasmon resonance sensors based on a three-dimensional tuning hypersurface. Sens. Actuators B Chem. 2023, 380, 133284. [Google Scholar] [CrossRef]
- Mao, Z.; Peng, X.; Zhou, Y.; Liu, Y.; Koh, K.; Chen, H. Review of Interface Modification Based on 2D Nanomaterials for Surface Plasmon Resonance Biosensors. ACS Photonics 2022, 9, 3807–3823. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct quantification of cancerous exosomes via surface plasmon resonance with dual gold nanoparticle-assisted signal amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, H.; Hu, S.; Xia, K.; Xiong, X.; Huang, W.; Lu, H.; Yu, J.; Guan, H.; et al. Titanium dioxide nanoparticle modified plasmonic interface for enhanced refractometric and biomolecular sensing. Opt. Express 2018, 26, 33226–33237. [Google Scholar] [CrossRef]
- Yang, M.; Xiong, X.; He, R.; Luo, Y.; Tang, J.; Dong, J.; Lu, H.; Yu, J.; Guan, H.; Zhang, J.; et al. Halloysite nanotube-modified plasmonic interface for highly sensitive refractive index sensing. ACS Appl. Mater. Interfaces 2018, 10, 5933–5940. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chen, Y.; Wang, H.; Hu, S.; Luo, Y.; Dong, J.; Zhu, W.; Qiu, W.; Guan, H.; Lu, H.; et al. Plasmonic interface modified with graphene oxide sheets overlayer for sensitivity enhancement. ACS Appl. Mater. Interfaces 2018, 10, 34916–34923. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.M.; Camilo, D.E.; Shimizu, F.M.; Ferreira, M. Improved antibody loading on self-assembled graphene oxide films for using in surface plasmon resonance immunosensors. Appl. Surf. Sci. 2019, 490, 502–509. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, S.; Wang, H.; Chen, Y.; Dong, J.; Jiang, Z.; Xiong, X.; Zhu, W.; Qiu, W.; Lu, H.; et al. Sensitivity-enhanced surface plasmon sensor modified with MoSe2 overlayer. Opt. Express 2018, 26, 34250–34258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, S.; Wu, L.; Zhu, J.; Xiang, Y.; Dai, X. GeSe nanosheets modified surface plasmon resonance sensors for enhancing sensitivity. Nanophotonics 2019, 9, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Li, N.; Wang, Y.; Liu, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; et al. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef]
- Shi, X.; Posysaev, S.; Huttula, M.; Pankratov, V.; Hoszowska, J.; Dousse, J.C.; Zeeshan, F.; Niu, Y.; Zakharov, A.; Cao, W.; et al. Metallic contact between MoS2 and Ni via Au nanoglue. Small 2018, 14, 1704526. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Chen, Y.; Chen, Y.; Hu, S.; Chen, H.; Xiao, W.; Liu, G.; Tang, Y.; Shi, J.; He, Z.; et al. A MoS2 nanoflower and gold nanoparticle-modified surface plasmon resonance biosensor for a sensitivity-improved immunoassay. J. Mater. Chem. C 2020, 8, 6861–6868. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, W.; Hu, S.; Huang, Q.; Liu, G.S.; Shi, J.; Chen, L.; Azeman, N.H.; Bakar, A.A.A.; et al. MoS2-nanoflower enhanced programmable adsorption/desorption plasmonic detection for bipolar-molecules with high sensitivity. Biosens. Bioelectron. 2022, 198, 113787. [Google Scholar] [CrossRef]
- Mao, P.; Luo, Y.; Chen, C.; Peng, S.; Feng, X.; Tang, J.; Fang, J.; Zhang, J.; Lu, H.; Yu, J.; et al. Design and optimization of surface plasmon resonance sensor based on multimode fiber. Opt. Quantum Electron. 2015, 47, 1495–1502. [Google Scholar] [CrossRef]
- Ye, M.; Winslow, D.; Zhang, D.; Pandey, R.; Yap, Y.K. Recent advancement on the optical properties of two-dimensional molybdenum disulfide (MoS2) thin films. Photonics 2015, 2, 288–307. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ding, J.; Yao, S.; Wu, X.; Feng, Q.; Wang, Z.; Geng, B. High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures. J. Mater. Chem. A 2014, 2, 15958–15963. [Google Scholar] [CrossRef]
- Huang, Q.Z.; Fang, Y.; Shi, J.; Liang, Y.L.; Zhu, Y.Q.; Xu, G. Flower-like molybdenum disulfide for polarity-triggered accumulation/release of small molecules. ACS Appl. Mater. Interfaces 2017, 9, 36431–36437. [Google Scholar] [CrossRef] [PubMed]
- Torelli, M.D.; Nunn, N.A.; Shenderova, O.A. A perspective on fluorescent nanodiamond bioimaging. Small 2019, 15, 1902151. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cai, Q.; Xu, B.; Zhu, W.; Zhang, L.; Zhao, J.; Chen, X. Graphene oxide functionalized long period grating for ultrasensitive label-free immunosensing. Biosens. Bioelectron. 2017, 94, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Jiang, W.-S.; Xin, W.; Xun, S.; Chen, S.-N.; Gao, X.-G.; Liu, Z.-B.; Tian, J.-G. Reduced graphene oxide-based optical sensor for detecting specific protein. Sens. Actuators B Chem. 2017, 249, 142–148. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Gao, S.; Zhang, H.; Zhang, J.; Bai, Y.; Song, D. Studies of gold nanorod-iron oxide nanohybrids for immunoassay based on SPR biosensor. Talanta 2014, 125, 29–35. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.; Jiang, J.; Xu, T.; Wang, S.; Chang, P.; Zhang, Z.; Ma, J.; Liu, T. MoSe2-Au Based Sensitivity Enhanced Optical Fiber Surface Plasmon Resonance Biosensor for Detection of Goat-Anti-Rabbit IgG. IEEE Access 2019, 8, 660–668. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Wu, Q.; Ma, P.; Zhang, H.; Wang, Y.; Song, D. Enhancing sensitivity of surface plasmon resonance biosensor by Ag nanocubes/chitosan composite for the detection of mouse IgG. Talanta 2016, 146, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Zhang, J.; Chen, Y.; Wang, J.; Hong, X.; Su, Q.; Li, X. A disposable fiber optic SPR probe for immunoassay. Biosens. Bioelectron. 2019, 144, 111621. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Song, D.; Zhang, D.; Sun, Y. An enhanced SPR immunosensing platform for human IgG based on the use of silver nanocubes and carboxy-functionalized graphene oxide. Microchim. Acta 2016, 183, 2177–2184. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, W.; Xu, T.; Chen, H.; Jin, Z.; Zhang, Z.; Song, Q.; Tang, Y. Miniaturized Paper-Based Smartphone Biosensor for Differential Diagnosis of Wild-type Pseudorabies Virus Infection versus Vaccination Immunization. Sens. Actuators B Chem. 2021, 327, 128893. [Google Scholar] [CrossRef]




| Nanomaterial | RI Sensitivity (nm/RIU) | Analyte | LOD (μg/mL) | Reference |
|---|---|---|---|---|
| Graphene oxide | 3311 | Human IgG | 0.05 | [37] |
| Reduced graphene oxide | - | Rabbit IgG | 0.0625 | [38] |
| Gold nanorod-iron oxide | - | Mouse IgG | 0.15 | [39] |
| MoSe2 | 2793 | Rabbit IgG | 0.33 | [40] |
| Ag nanocubes | - | Mouse IgG | 0.6 | [41] |
| Half-antibody fragments | 1959 | Mouse IgG | 0.1 | [42] |
| Silver nanocubes | - | Human IgG | 0.075 | [43] |
| MNF + ND | 12,219 | Mouse IgG | 0.024 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xiong, X.; Chen, Y.; Chen, L.; Liu, G.; Xiao, W.; Shi, J.; Chen, Z.; Luo, Y. MoS2-Nanoflower and Nanodiamond Co-Engineered Surface Plasmon Resonance for Biosensing. Biosensors 2023, 13, 506. https://doi.org/10.3390/bios13050506
Chen Y, Xiong X, Chen Y, Chen L, Liu G, Xiao W, Shi J, Chen Z, Luo Y. MoS2-Nanoflower and Nanodiamond Co-Engineered Surface Plasmon Resonance for Biosensing. Biosensors. 2023; 13(5):506. https://doi.org/10.3390/bios13050506
Chicago/Turabian StyleChen, Yaofei, Xin Xiong, Yu Chen, Lei Chen, Guishi Liu, Wei Xiao, Jifu Shi, Zhe Chen, and Yunhan Luo. 2023. "MoS2-Nanoflower and Nanodiamond Co-Engineered Surface Plasmon Resonance for Biosensing" Biosensors 13, no. 5: 506. https://doi.org/10.3390/bios13050506
APA StyleChen, Y., Xiong, X., Chen, Y., Chen, L., Liu, G., Xiao, W., Shi, J., Chen, Z., & Luo, Y. (2023). MoS2-Nanoflower and Nanodiamond Co-Engineered Surface Plasmon Resonance for Biosensing. Biosensors, 13(5), 506. https://doi.org/10.3390/bios13050506




