Graphene-Based Sensors for the Detection of Microorganisms in Food: A Review
Abstract
:1. Introduction
2. Graphene-Based Sensors
3. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y. Post-CMOS and post-MEMS compatible flexible skin technologies: A review. IEEE Sens. J. 2013, 13, 3962–3975. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Jaaniso, R.; Tan, O.K. Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Tilli, M.; Paulasto-Krockel, M.; Petzold, M.; Theuss, H.; Motooka, T.; Lindroos, V. Handbook of Silicon Based MEMS Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bhatt, G.; Manoharan, K.; Chauhan, P.S.; Bhattacharya, S. MEMS sensors for automotive applications: A review. In Sensors for Automotive and Aerospace Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 223–239. [Google Scholar]
- Al-Salman, H.S.; Abdullah, M. Preparation of ZnO nanostructures by RF-magnetron sputtering on thermally oxidized porous silicon substrate for VOC sensing application. Measurement 2015, 59, 248–257. [Google Scholar] [CrossRef]
- Orofeo, C.M.; Ago, H.; Yoshihara, N.; Tsuji, M. Top-down approach to align single-walled carbon nanotubes on silicon substrate. Appl. Phys. Lett. 2009, 94, 053113. [Google Scholar] [CrossRef]
- Nag, A.; Zia, A.I.; Li, X.; Mukhopadhyay, S.C.; Kosel, J. Novel sensing approach for LPG leakage detection: Part I—Operating mechanism and preliminary results. IEEE Sens. J. 2015, 16, 996–1003. [Google Scholar] [CrossRef]
- Nag, A.; Zia, A.I.; Li, X.; Mukhopadhyay, S.C.; Kosel, J. Novel sensing approach for LPG leakage detection—Part II: Effects of particle size, composition, and coating layer thickness. IEEE Sens. J. 2015, 16, 1088–1094. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C.; Burkitt, L. Imprinted polymer coated impedimetric nitrate sensor for real-time water quality monitoring. Sens. Actuators B Chem. 2018, 259, 753–761. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Xie, L.; Mukhopadhyay, S.; Burkitt, L. A temperature compensated smart nitrate-sensor for agricultural industry. IEEE Trans. Ind. Electron. 2017, 64, 7333–7341. [Google Scholar] [CrossRef]
- Advantages and Disadvantages of Silicon Sensors. Available online: https://www.radiation-dosimetry.org/what-is-advantage-and-disadvantage-of-silicon-detectors-definition/ (accessed on 12 May 2023).
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable flexible sensors: A review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef]
- Khan, S.; Lorenzelli, L.; Dahiya, R.S. Technologies for printing sensors and electronics over large flexible substrates: A review. IEEE Sens. J. 2014, 15, 3164–3185. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Afsarimanesh, N.; Mukhopadhyay, S.C.; Kundu, S.; Xu, Y. Laser-Assisted Printed Flexible Sensors: A Review. Sensors 2019, 19, 1462. [Google Scholar] [CrossRef]
- Su, Y.; Ma, C.; Chen, J.; Wu, H.; Luo, W.; Peng, Y.; Luo, Z.; Li, L.; Tan, Y.; Omisore, O.M. Printable, Highly Sensitive Flexible Temperature Sensors for Human Body Temperature Monitoring: A Review. Nanoscale Res. Lett. 2020, 15, 200. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Chen, P.-Y.; Ren, H. A review of printable flexible and stretchable tactile sensors. Research 2019, 2019, 3018568. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Printing conductive nanomaterials for flexible and stretchable electronics: A review of materials, processes, and applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Gao, J.; He, S.; Nag, A.; Wong, J.W.C. A Review of the Use of Carbon Nanotubes and Graphene-Based Sensors for the Detection of Aflatoxin M1 Compounds in Milk. Sensors 2021, 21, 3602. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Pham, T.; Ramnani, P.; Mulchandani, A. Carbon allotropes as sensors for environmental monitoring. Curr. Opin. Electrochem. 2017, 3, 106–113. [Google Scholar] [CrossRef]
- He, S.; Hong, Y.; Liao, M.; Li, Y.; Qiu, L.; Peng, H. Flexible sensors based on assembled carbon nanotubes. Aggregate 2021, 2, e143. [Google Scholar] [CrossRef]
- Thanh, C.T.; Binh, N.H.; Duoc, P.N.D.; Thu, V.T.; Van Trinh, P.; Anh, N.N.; Van Tu, N.; Tuyen, N.V.; Van Quynh, N.; Tu, V.C. Electrochemical sensor based on reduced graphene oxide/double-walled carbon nanotubes/octahedral Fe3O4/chitosan composite for glyphosate detection. Bull. Environ. Contam. Toxicol. 2021, 106, 1017–1023. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.-X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Zhang, H.; He, R.; Niu, Y.; Han, F.; Li, J.; Zhang, X.; Xu, F. Graphene-enabled wearable sensors for healthcare monitoring. Biosens. Bioelectron. 2022, 197, 113777. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, W.; Liang, Y.; Wu, Z.; Zhong, B.; Zhou, Z.; Ye, J.; Tao, K.; Zhou, Y.; Xie, X. Self-Calibrated, Sensitive, and Flexible Temperature Sensor Based on 3D Chemically Modified Graphene Hydrogel. Adv. Electron. Mater. 2021, 7, 2001084. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.-N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar]
- Kausar, A. Poly (methyl methacrylate) nanocomposite reinforced with graphene, graphene oxide, and graphite: A review. Polym. -Plast. Technol. Mater. 2019, 58, 821–842. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Wang, W.; Guo, X.; Hao, C.; Pan, W.; Liu, P.; Liu, C.; Ma, Y.; Zhang, Y. High-resolution flexible temperature sensor based graphite-filled polyethylene oxide and polyvinylidene fluoride composites for body temperature monitoring. Sens. Actuators A Phys. 2018, 278, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, Q.; Jian, M.; Zhang, Y. Sheath–core graphite/silk fiber made by dry-meyer-rod-coating for wearable strain sensors. ACS Appl. Mater. Interfaces 2016, 8, 20894–20899. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: London, UK, 2010; pp. 11–19. [Google Scholar]
- Mansuriya, B.D.; Altintas, Z. Applications of graphene quantum dots in biomedical sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Zhang, H.; Duan, X. Temperature Sensing at the Robot Fingertip Using Reduced Graphene Oxide-based Sensor on a Flexible Substrate. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Liu, G.; Tan, Q.; Kou, H.; Zhang, L.; Wang, J.; Lv, W.; Dong, H.; Xiong, J. A flexible temperature sensor based on reduced graphene oxide for robot skin used in internet of things. Sensors 2018, 18, 1400. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Gan, D.; Guo, L.; Liu, M.; Chen, J.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A facile strategy for preparation of magnetic graphene oxide composites and their potential for environmental adsorption. Ceram. Int. 2018, 44, 18571–18577. [Google Scholar] [CrossRef]
- Ren, J.; Wang, C.; Zhang, X.; Carey, T.; Chen, K.; Yin, Y.; Torrisi, F. Environmentally-friendly conductive cotton fabric as flexible strain sensor based on hot press reduced graphene oxide. Carbon 2017, 111, 622–630. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Xian-Lun, L.; Sathishkumar, N.; Chen, C.-Y.; Santiago, K.S.; Chen, H.-T.; Lin, Y.-F.; Yeh, J.-M. Detection of hydrogen sulfide using polyaniline incorporated with graphene oxide aerogel. Synth. Met. 2021, 282, 116934. [Google Scholar] [CrossRef]
- Xiong, S.; Zhou, J.; Wu, J.; Li, H.; Zhao, W.; He, C.; Liu, Y.; Chen, Y.; Fu, Y.; Duan, H. High performance acoustic wave nitrogen dioxide sensor with ultraviolet activated 3D porous architecture of Ag-decorated reduced graphene oxide and polypyrrole aerogel. ACS Appl. Mater. Interfaces 2021, 13, 42094–42103. [Google Scholar] [CrossRef]
- Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.A.; Szkudlarek, A.; Pisarek, M.; Kanak, J.; Rydosz, A. Nitrogen dioxide sensing using multilayer structure of reduced graphene oxide and α-Fe2O3. Sensors 2021, 21, 1011. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, C.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; He, L.; Chu, X. Growth of ZnFe2O4 nanosheets on reduced graphene oxide with enhanced ethanol sensing properties. Sens. Actuators B Chem. 2021, 330, 129280. [Google Scholar] [CrossRef]
- Gupta, M.; Hawari, H.F.; Kumar, P.; Burhanudin, Z.A.; Tansu, N. Functionalized reduced graphene oxide thin films for ultrahigh CO2 gas sensing performance at room temperature. Nanomaterials 2021, 11, 623. [Google Scholar] [CrossRef]
- Kedambaimoole, V.; Kumar, N.; Shirhatti, V.; Nuthalapati, S.; Kumar, S.; Nayak, M.M.; Sen, P.; Akinwande, D.; Rajanna, K. Reduced graphene oxide tattoo as wearable proximity sensor. Adv. Electron. Mater. 2021, 7, 2001214. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Chemical and biological sensors for food-quality monitoring and smart packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent food packaging: A review of smart sensing technologies for monitoring food quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-based smart materials for food packaging and sensors—A review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef]
- Mikalauskiene, A.; Narutaviciute-Cikanauske, R.; Sarkiunaite, I.; Streimikiene, D.; Zlateva, R. Social aspect of sustainable development: Issues of poverty and food shortage. Montenegrin J. Econ. 2018, 14, 59–78. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; dos Santos Gomes, M.G. COVID-19 outbreak: What should be done to avoid food shortages? Trends Food Sci. Technol. 2020, 102, 291. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Ferguson, J.D.; Galligan, D.T.; Kelly, A.M.; Finn, S.M.; Giegengack, R. Assessing US food wastage and opportunities for reduction. Glob. Food Secur. 2016, 8, 19–26. [Google Scholar] [CrossRef]
- Sundramoorthy, A.K.; Gunasekaran, S. Applications of graphene in quality assurance and safety of food. TrAC Trends Anal. Chem. 2014, 60, 36–53. [Google Scholar] [CrossRef]
- Arreguin-Campos, R.; Eersels, K.; Rogosic, R.; Cleij, T.J.; Diliën, H.; van Grinsven, B. Imprinted Polydimethylsiloxane-Graphene oxide composite receptor for the biomimetic thermal sensing of Escherichia coli. ACS Sens. 2022, 7, 1467–1475. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Yazdian, F.; Hojjati, S.; Khosravi-Darani, K. Detection of microorganisms using graphene-based nanobiosensors. Food Technol. Biotechnol. 2021, 59, 496–506. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, B.; Xu, J.; Qing, T.; Zhang, P.; Qing, Z. Graphene biosensors for bacterial and viral pathogens. Biosens. Bioelectron. 2020, 166, 112471. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Hernández, R.; Vallés, C.; Benito, A.M.; Maser, W.K.; Rius, F.X.; Riu, J. Graphene-based potentiometric biosensor for the immediate detection of living bacteria. Biosens. Bioelectron. 2014, 54, 553–557. [Google Scholar] [CrossRef]
- Kaladevi, G.; Wilson, P.; Pandian, K. Silver nanoparticle–decorated PANI/reduced graphene oxide for sensing of hydrazine in water and inhibition studies on microorganism. Ionics 2020, 26, 3123–3133. [Google Scholar] [CrossRef]
- Li, B.; Tan, H.; Anastasova, S.; Power, M.; Seichepine, F.; Yang, G.-Z. A bio-inspired 3D micro-structure for graphene-based bacteria sensing. Biosens. Bioelectron. 2019, 123, 77–84. [Google Scholar] [CrossRef]
- Zhu, F.; Zhao, G.; Dou, W. A non-enzymatic electrochemical immunoassay for quantitative detection of Escherichia coli O157: H7 using Au@ Pt and graphene. Anal. Biochem. 2018, 559, 34–43. [Google Scholar] [CrossRef]
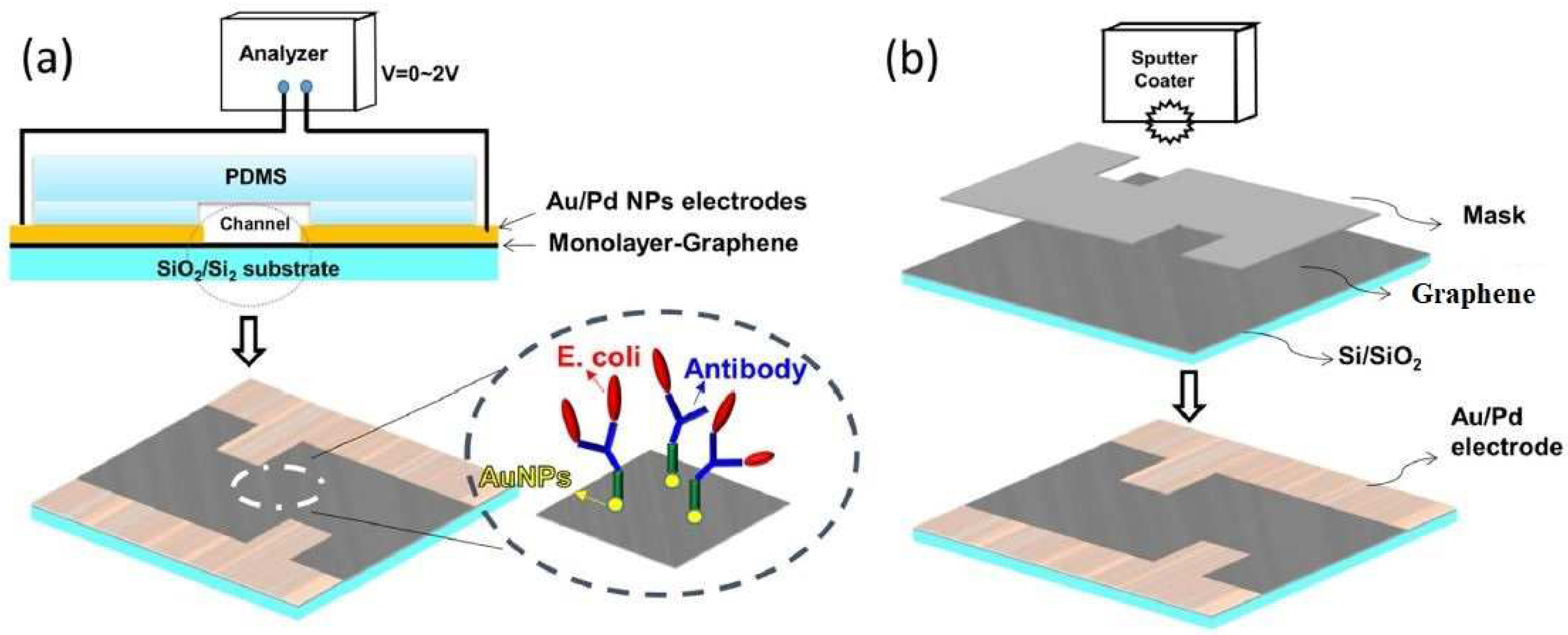
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.-H.; Magruder, M.; Chen, J. Rapid detection of single E. coli bacteria using a graphene-based field-effect transistor device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Liu, Y.; Li, L.-J.; Chen, P. Graphene-based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem. 2011, 21, 12358–12362. [Google Scholar] [CrossRef]
- Pandit, S.; Li, M.; Chen, Y.; Rahimi, S.; Mokkapati, V.; Merlo, A.; Yurgens, A.; Mijakovic, I. Graphene-Based Sensor for Detection of Bacterial Pathogens. Sensors 2021, 21, 8085. [Google Scholar] [CrossRef]
- Pandey, A.; Gurbuz, Y.; Ozguz, V.; Niazi, J.H.; Qureshi, A. Graphene-interfaced electrical biosensor for label-free and sensitive detection of foodborne pathogenic E. coli O157: H7. Biosens. Bioelectron. 2017, 91, 225–231. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-based biosensors for detection of biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Nano silver embedded starch hybrid graphene oxide sandwiched poly (ethylmethacrylate) for packaging application. Nano-Struct. Nano-Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Del Aguila, E.M.; Paschoalin, V.M.; Andrade, C.T. Extruded hybrids based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and reduced graphene oxide composite for active food packaging. Food Packag. Shelf Life 2018, 16, 77–85. [Google Scholar] [CrossRef]
- Kotsilkov, S.; Ivanov, E.; Vitanov, N.K. Release of graphene and carbon nanotubes from biodegradable poly (lactic acid) films during degradation and combustion: Risk associated with the end-of-life of nanocomposite food packaging materials. Materials 2018, 11, 2346. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; Morsy, M.; Ateia, M.A.; Abdel-Haleem, F.M. Ultrafast response humidity sensors based on polyvinyl chloride/graphene oxide nanocomposites for intelligent food packaging. Sens. Actuators A Phys. 2021, 331, 112918. [Google Scholar] [CrossRef]
- Barra, A.; Ferreira, N.M.; Martins, M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.; Neumayer, S.M.; Rodriguez, B.J.; Enăchescu, M.; Ferreira, P. Eco-friendly preparation of electrically conductive chitosan-reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60. [Google Scholar] [CrossRef]
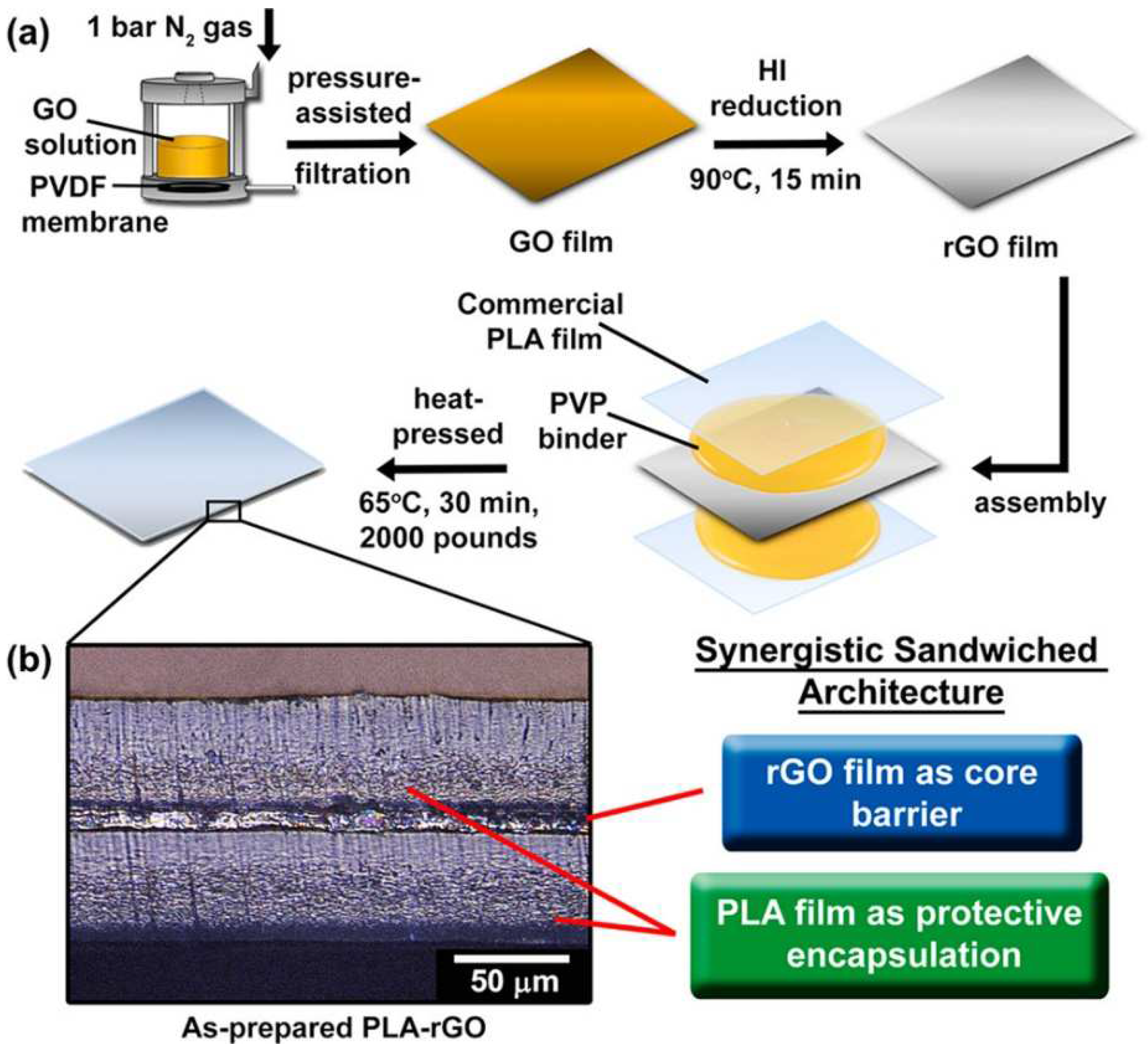
- Goh, K.; Heising, J.K.; Yuan, Y.; Karahan, H.E.; Wei, L.; Zhai, S.; Koh, J.-X.; Htin, N.M.; Zhang, F.; Wang, R. Sandwich-architectured poly (lactic acid)–graphene composite food packaging films. ACS Appl. Mater. Interfaces 2016, 8, 9994–10004. [Google Scholar] [CrossRef]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2017; p. 1600114. [Google Scholar]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Huang, H.; Feng, Z.; Li, Y.; Liu, Z.; Zhang, L.; Ma, Y.; Tong, J. Highly sensitive detection of bisphenol A in food packaging based on graphene quantum dots and peroxidase. Anal. Methods 2015, 7, 2928–2935. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Siralertmukul, K.; Rodthongkum, N. Colorimetric sensor and LDI-MS detection of biogenic amines in food spoilage based on porous PLA and graphene oxide. Food Chem. 2020, 329, 127165. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Detection of bacterial metabolic volatile indole using a graphene-based field-effect transistor biosensor. Nanomaterials 2021, 11, 1155. [Google Scholar] [CrossRef]
- Zhao, W.; Xing, Y.; Lin, Y.; Gao, Y.; Wu, M.; Xu, J. Monolayer graphene chemiresistive biosensor for rapid bacteria detection in a microchannel. Sens. Actuators Rep. 2020, 2, 100004. [Google Scholar] [CrossRef]
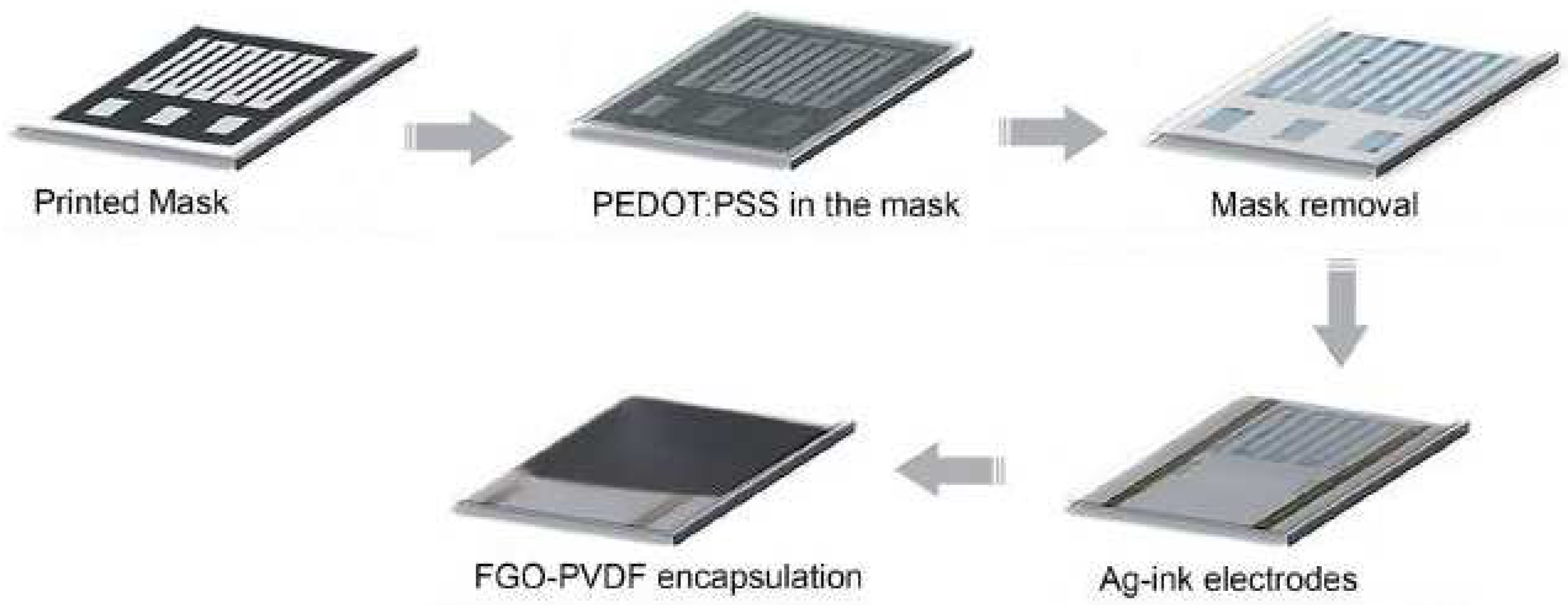
- Maskey, B.B.; Shrestha, K.; Sun, J.; Park, H.; Park, J.; Parajuli, S.; Shrestha, S.; Jung, Y.; Ramasundaram, S.; Koirala, G.R. Proving the robustness of a PEDOT: PSS-based thermistor via functionalized graphene oxide–poly (vinylidene fluoride) composite encapsulation for food logistics. RSC Adv. 2020, 10, 12407–12414. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Kim, M. Development of an immuno-electrochemical glass carbon electrode sensor based on graphene oxide/gold nanocomposite and antibody for the detection of patulin. Food Chem. 2021, 342, 128257. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Haldorai, Y.; Khan, I.; Kang, S.-M.; Kwak, C.H.; Gandhi, S.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Bioreceptor-free, sensitive and rapid electrochemical detection of patulin fungal toxin, using a reduced graphene oxide@ SnO2 nanocomposite. Mater. Sci. Eng. C 2020, 113, 110916. [Google Scholar] [CrossRef] [PubMed]
- Elfadil, D.; Silveri, F.; Palmieri, S.; Della Pelle, F.; Sergi, M.; Del Carlo, M.; Amine, A.; Compagnone, D. Liquid-phase exfoliated 2D graphene nanoflakes electrochemical sensor coupled to molecularly imprinted polymers for the determination of citrinin in food. Talanta 2023, 253, 124010. [Google Scholar] [CrossRef]
- Savas, S.; Altintas, Z. Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocolitica in milk and human serum. Materials 2019, 12, 2189. [Google Scholar] [CrossRef]
- Soares, R.R.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-induced graphene electrochemical immunosensors for rapid and label-free monitoring of Salmonella enterica in chicken broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]

| Materials | Target Analyte | Detection Technique | Analytical Parameters | Application to Real Samples | Ref. |
|---|---|---|---|---|---|
| Graphene | E. coli | Field-effect transistors |
| Milk, meats and seafood | [77] |
| Graphene, PDMS | E. coli | Chemisensitive measurement |
| Meat, milk | [78] |
| rGO, poly (lactic) acid | E. coli | Resistance measurement |
| Oil and potato chips | [71] |
| Chitosan, Graphene oxide | E. coli (Gram-negative) and B. subtillis (Gram-positve) | Differential scanning calorimetry |
| Food packaging, water treatment | [72] |
| Polyhydroxybutyrate, graphene nanoplatelets | Polyhydroxybutyrate | Differential thermogravimetric analysis |
| Potato chips and milk | [73] |
| Graphene oxide, gold nanoaparticles | Patulin | Cyclic voltammetry and differential pulse voltammetry |
| Fruits, grains, cheese | [80] |
| Reduced graphene oxide, tin oxide | Patulin | High-performance liquid chromatography |
| Apple juice | [81] |
| Graphene nanoflakes | Citrinin | Molecular Imprinting polymer |
| Rice, blueberry, corn, wheat, germ, rice starch | [82] |
| Graphene quantum dots | Yersinia enterocolitica | Immunoassay |
| Milk | [83] |
| Laser induced graphene | Salmonella enterica | Cyclic voltammetry and impedance spectroscopy |
| Chicken broth | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Chakraborthy, A.; He, S.; Yang, S.; Afsarimanesh, N.; Nag, A.; Deng, S. Graphene-Based Sensors for the Detection of Microorganisms in Food: A Review. Biosensors 2023, 13, 579. https://doi.org/10.3390/bios13060579
Gao J, Chakraborthy A, He S, Yang S, Afsarimanesh N, Nag A, Deng S. Graphene-Based Sensors for the Detection of Microorganisms in Food: A Review. Biosensors. 2023; 13(6):579. https://doi.org/10.3390/bios13060579
Chicago/Turabian StyleGao, Jingrong, Aniket Chakraborthy, Shan He, Song Yang, Nasrin Afsarimanesh, Anindya Nag, and Shanggui Deng. 2023. "Graphene-Based Sensors for the Detection of Microorganisms in Food: A Review" Biosensors 13, no. 6: 579. https://doi.org/10.3390/bios13060579






