The Sensitivity of a Hexagonal Au Nanohole Array under Different Incident Angles
Abstract
:1. Introduction
2. Methods
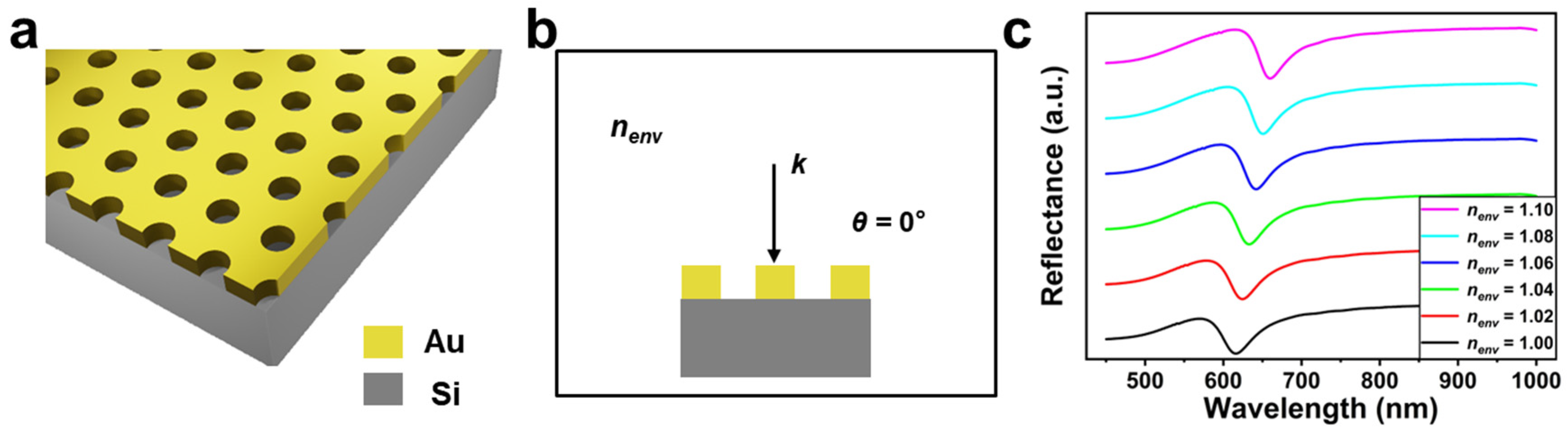
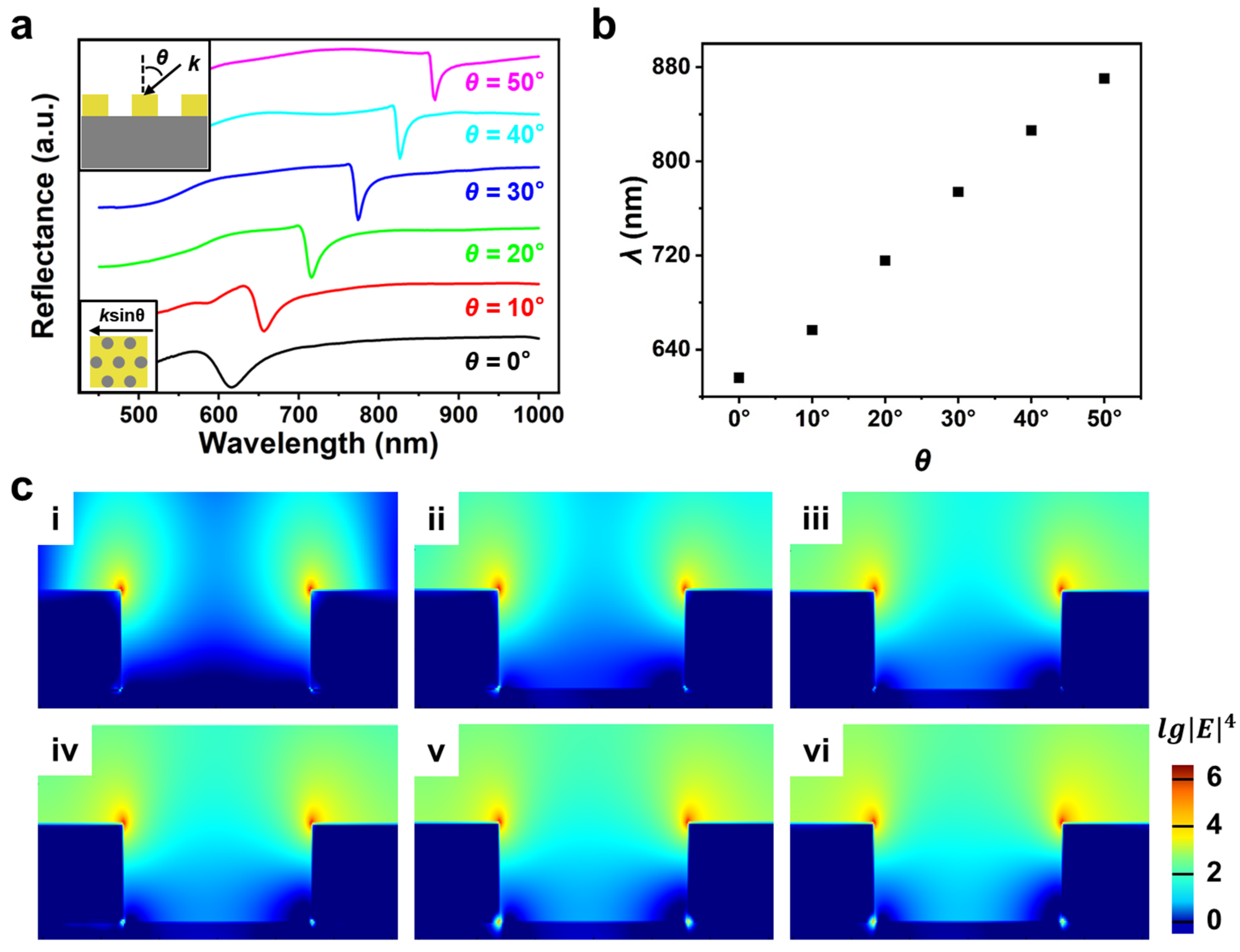
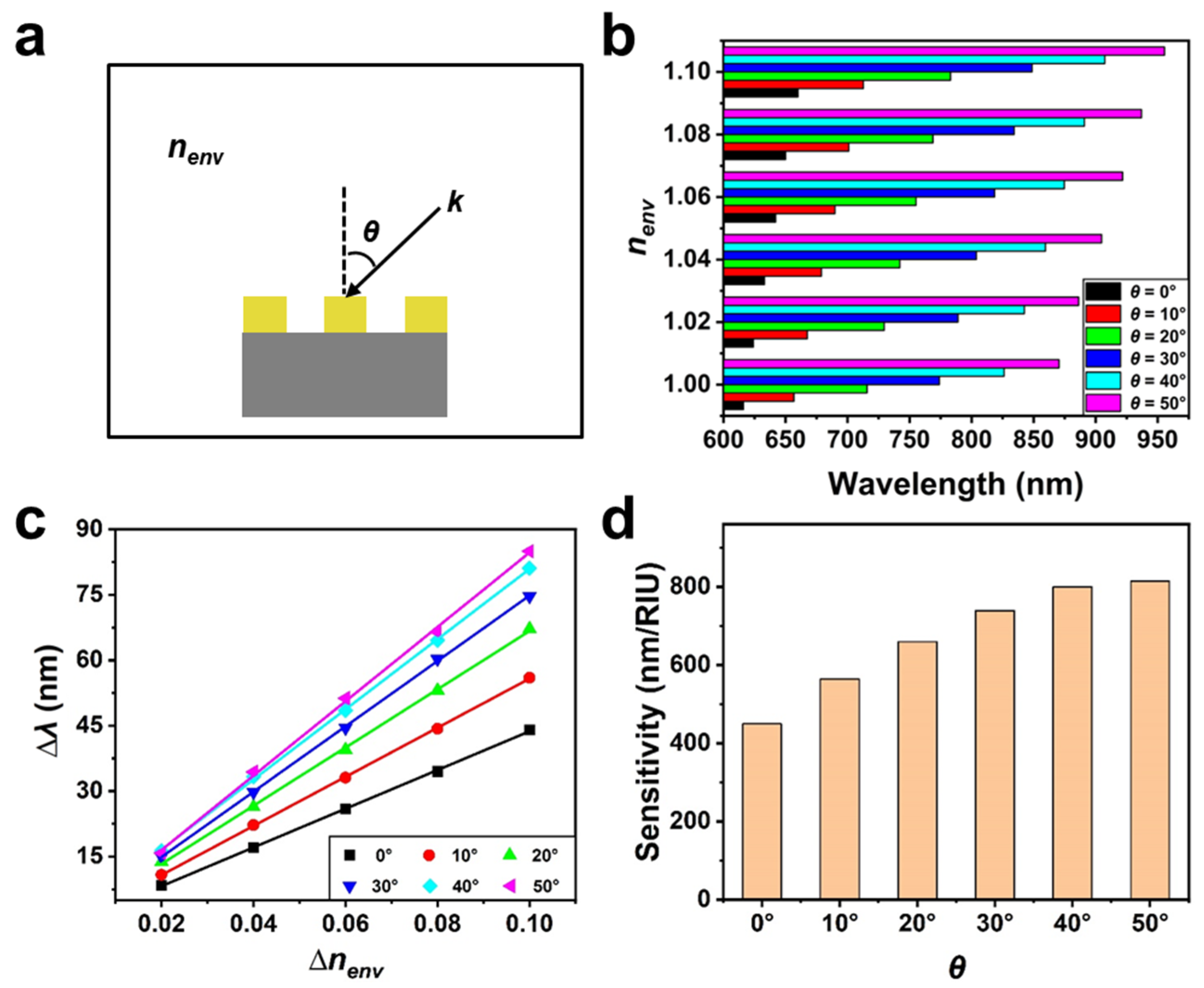
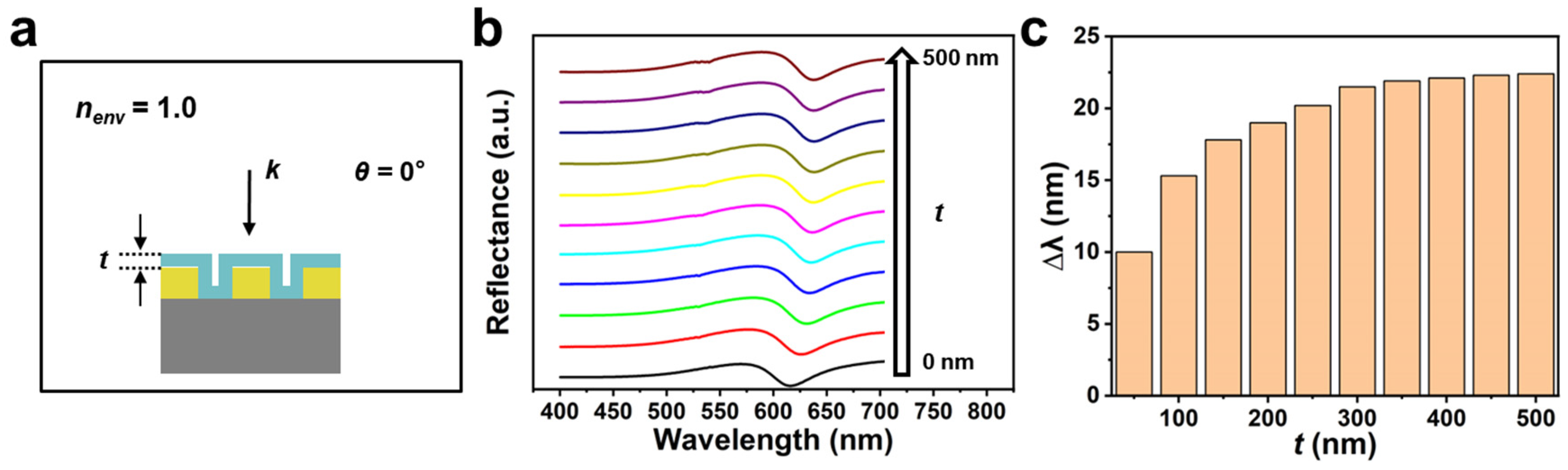
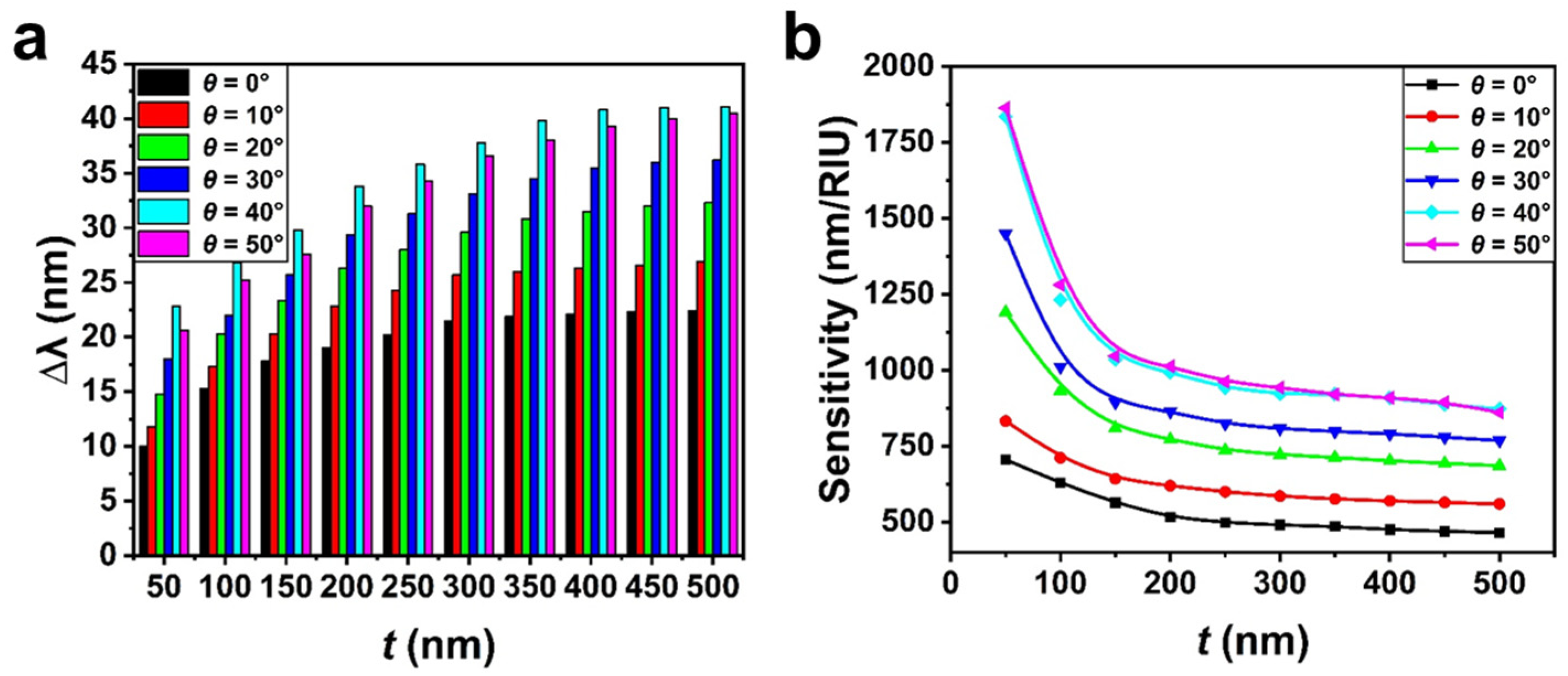
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gramotnev, D.K.; Bozhevolnyi, S.I. Plasmonics beyond the diffraction limit. Nat. Photonics 2010, 4, 83–91. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef]
- Yang, K.; Yao, X.; Liu, B.; Ren, B. Metallic plasmonic array structures: Principles, fabrications, properties, and applications. Adv. Mater. 2021, 33, e2007988. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Valsecchi, C.; Brolo, A.G. Periodic metallic nanostructures as plasmonic chemical sensors. Langmuir 2013, 29, 5638–5649. [Google Scholar] [CrossRef]
- Mejia-Salazar, J.R.; Oliveira, O.N., Jr. Plasmonic biosensing. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Brolo, A.G. Plasmonics for future biosensors. Nat. Photonics 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Tokel, O.; Inci, F.; Demirci, U. Advances in plasmonic technologies for point of care applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Soler, M.; Ozdemir, C.I.; Belushkin, A.; Yesilkoy, F.; Altug, H. Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab. Chip 2017, 17, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Son, T.; Hong, J.S.; Liu, A.Q.; Skog, J.; Castro, C.M.; Weissleder, R.; Lee, H.; Im, H. Plasmonic sensors for extracellular vesicle analysis: From scientific development to translational research. ACS Nano 2020, 14, 14528–14548. [Google Scholar] [CrossRef]
- Masterson, A.N.; Muhoberac, B.B.; Gopinadhan, A.; Wilde, D.J.; Deiss, F.T.; John, C.C.; Sardar, R. Multiplexed and high-throughput label-free detection of RNA/spike protein/IgG/IgM biomarkers of SARS-CoV-2 infection utilizing nanoplasmonic biosensors. Anal. Chem. 2021, 93, 8754–8763. [Google Scholar] [CrossRef]
- Nangare, S.N.; Patil, P.O. Affinity-based nanoarchitectured biotransducer for sensitivity enhancement of surface plasmon resonance sensors for in vitro diagnosis: A review. ACS Biomater. Sci. Eng. 2021, 7, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cheng, Y.; He, L.; Feng, Y.; Tian, Y.; Chen, Z.; Feng, Y.; Li, Y.; Xie, W.; Huang, W.; et al. T-shaped aptamer-based LSPR biosensor using omega-shaped fiber optic for rapid detection of SARS-CoV-2. Anal. Chem. 2023, 95, 1599–1607. [Google Scholar] [PubMed]
- Soler, M.; Belushkin, A.; Cavallini, A.; Kebbi-Beghdadi, C.; Greub, G.; Altug, H. Multiplexed nanoplasmonic biosensor for one-step simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine. Biosens. Bioelectron. 2017, 94, 560. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Yao, X.; Lyu, D.; Zhu, J.; Yang, Z.; Liu, B.; Ren, B. Large-area plasmonic metamaterial with thickness-dependent absorption. Adv. Optical Mater. 2021, 9, 2001375. [Google Scholar] [CrossRef]
- Sannomiya, T.; Scholder, O.; Jefimovs, K.; Hafner, C.; Dahlin, A.B. Investigation of plasmon resonances in metal films with nanohole arrays for biosensing applications. Small 2011, 7, 1653–1663. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Ye, S.; Wu, S.; Zhang, J.; Cui, L.; Li, A.; Wang, T.; Li, S.; Yang, B. Elevated Ag nanohole arrays for high performance plasmonic sensors based on extraordinary optical transmission. J. Mater. Chem. 2012, 22, 8903. [Google Scholar] [CrossRef]
- Couture, M.; Liang, Y.; Poirier Richard, H.P.; Faid, R.; Peng, W.; Masson, J.F. Tuning the 3D plasmon field of nanohole arrays. Nanoscale 2013, 5, 12399–12408. [Google Scholar] [CrossRef]
- Belushkin, A.; Yesilkoy, F.; Altug, H. Nanoparticle-enhanced plasmonic biosensor for digital biomarker detection in a microarray. ACS Nano 2018, 12, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.M.; Alegret, J.; Kall, M.; Sutherland, D.S. Sensing characteristics of NIR localized surface plasmon resonances in gold nanorings for application as ultrasensitive biosensors. Nano Lett. 2007, 7, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Stakenborg, T.; Lagae, L. Gold nanoring as a sensitive plasmonic biosensor for on-chip DNA detection. Appl. Phys. Lett. 2012, 100, 173114. [Google Scholar] [CrossRef] [Green Version]
- Halpern, A.R.; Corn, R.M. Lithographically patterned electrodeposition of gold, silver, and nickel nanoring arrays with widely tunable near-infrared plasmonic resonances. ACS Nano 2013, 7, 1755–1762. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Zhu, W.; Agrawal, A.; Lezec, H.; Li, L.; Peng, W.; Zou, Y.; Lu, Y.; Xu, T. Subradiant dipolar interactions in plasmonic nanoring resonator array for integrated label-free biosensing. ACS Sens. 2017, 2, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, K.S.; Ahn, J.; Lee, J.J.; Kim, M.G.; Shin, Y.B. Highly sensitive biosensing using arrays of plasmonic Au nanodisks realized by nanoimprint lithography. ACS Nano 2011, 5, 897–904. [Google Scholar] [CrossRef]
- Horrer, A.; Krieg, K.; Freudenberger, K.; Rau, S.; Leidner, L.; Gauglitz, G.; Kern, D.P.; Fleischer, M. Plasmonic vertical dimer arrays as elements for biosensing. Anal. Bioanal. Chem. 2015, 407, 8225–8231. [Google Scholar] [CrossRef]
- Zhan, Y.; Lei, D.Y.; Li, X.; Maier, S.A. Plasmonic Fano resonances in nanohole quadrumers for ultra-sensitive refractive index sensing. Nanoscale 2014, 6, 4705–4715. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Ameling, R.; Giessen, H. Microcavity plasmonics: Strong coupling of photonic cavities and plasmons. Laser Photonics Rev. 2013, 7, 141–169. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.K.; Wang, X.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Q.; Chen, C.; Lagae, L.; Van Dorpe, P. Nanoplasmonic sensors with various photonic coupling effects for detecting different targets. J. Phys. Chem. C 2015, 119, 29116–29122. [Google Scholar] [CrossRef]
- Cetin, A.E.; Altug, H. Fano resonant ring/disk plasmonic nanocavities on conducting substrates for advanced biosensing. ACS Nano 2012, 6, 9989–9995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, J. Highly controllable double Fano resonances in plasmonic metasurfaces. Nanoscale 2016, 8, 17665–17674. [Google Scholar] [CrossRef]
- Doiron, B.; Mota, M.; Wells, M.P.; Bower, R.; Mihai, A.; Li, Y.; Cohen, L.F.; Alford, N.M.; Petrov, P.K.; Oulton, R.F.; et al. Quantifying figures of merit for localized surface plasmon resonance applications: A materials survey. ACS Photonics 2019, 6, 240–259. [Google Scholar] [CrossRef]
- Agharazy Dormeny, A.; Abedini Sohi, P.; Kahrizi, M. Design and simulation of a refractive index sensor based on SPR and LSPR using gold nanostructures. Results Phys. 2020, 16, 102869. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Huang, H.; Ouyang, F. Multi-path remote-excited surface plasmon resonance of a hollow gold nanosheet studied using FDTD simulations. Results Phys. 2022, 40, 105871. [Google Scholar] [CrossRef]
- Zhou, W.; Odom, T.W. Tunable subradiant lattice plasmons by out-of-plane dipolar interactions. Nat. Nanotechnol. 2011, 6, 423–427. [Google Scholar] [CrossRef]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative Interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Li, J.; Ye, J.; Chen, C.; Li, Y.; Verellen, N.; Moshchalkov, V.V.; Lagae, L.; Van Dorpe, P. Revisiting the surface sensitivity of nanoplasmonic biosensors. ACS Photonics 2015, 2, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Shalabney, A.; Abdulhalim, I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photonics Rev. 2011, 5, 571–606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Li, M. The Sensitivity of a Hexagonal Au Nanohole Array under Different Incident Angles. Biosensors 2023, 13, 654. https://doi.org/10.3390/bios13060654
Yang K, Li M. The Sensitivity of a Hexagonal Au Nanohole Array under Different Incident Angles. Biosensors. 2023; 13(6):654. https://doi.org/10.3390/bios13060654
Chicago/Turabian StyleYang, Kang, and Meiying Li. 2023. "The Sensitivity of a Hexagonal Au Nanohole Array under Different Incident Angles" Biosensors 13, no. 6: 654. https://doi.org/10.3390/bios13060654
APA StyleYang, K., & Li, M. (2023). The Sensitivity of a Hexagonal Au Nanohole Array under Different Incident Angles. Biosensors, 13(6), 654. https://doi.org/10.3390/bios13060654




