Fiber Laser-Based Lasso-Shaped Biosensor for High Precision Detection of Cancer Biomarker-CEACAM5 in Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Antibody Immobilization
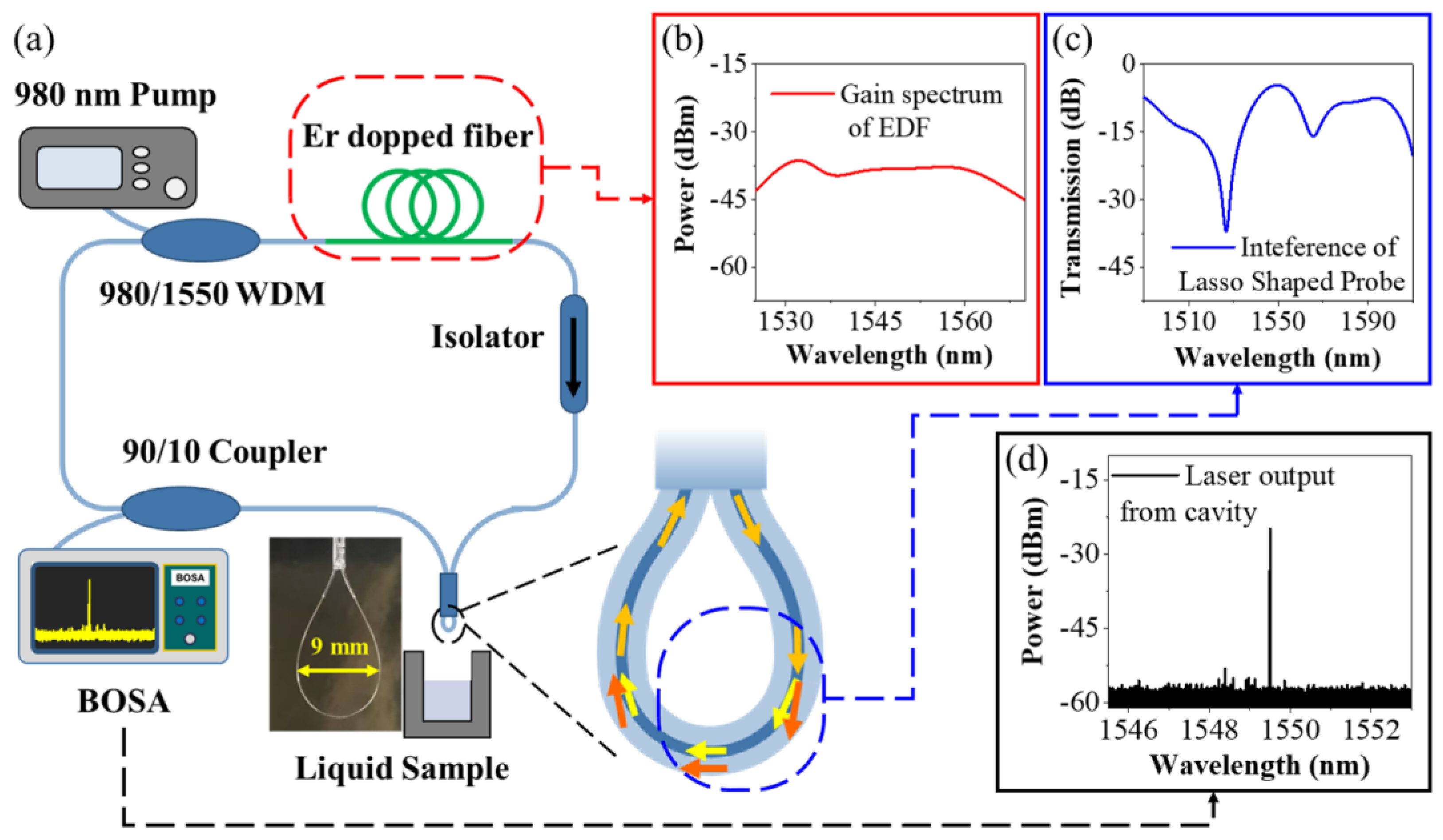
2.3. Sensor Preparation and Interrogation Setup
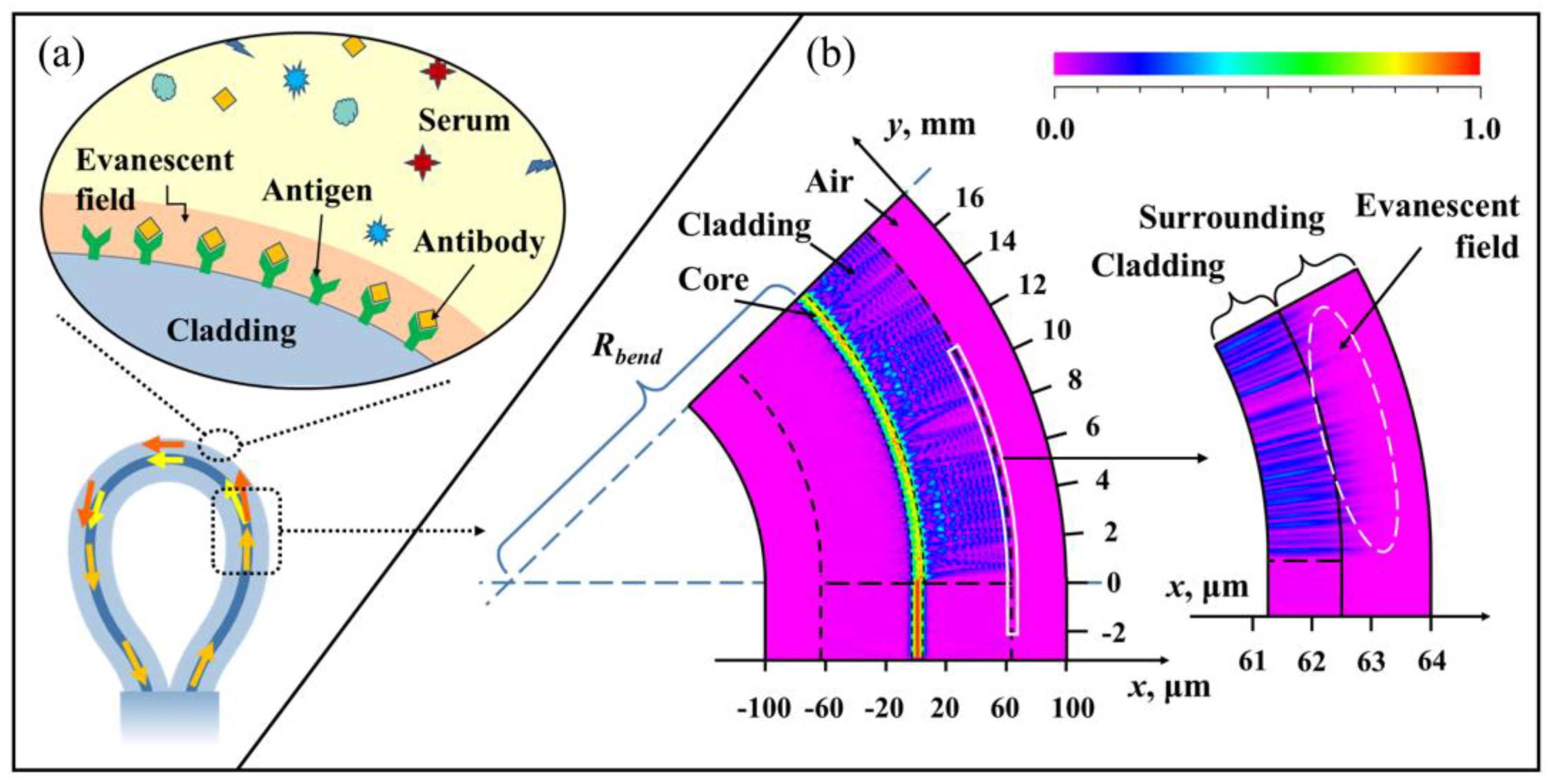
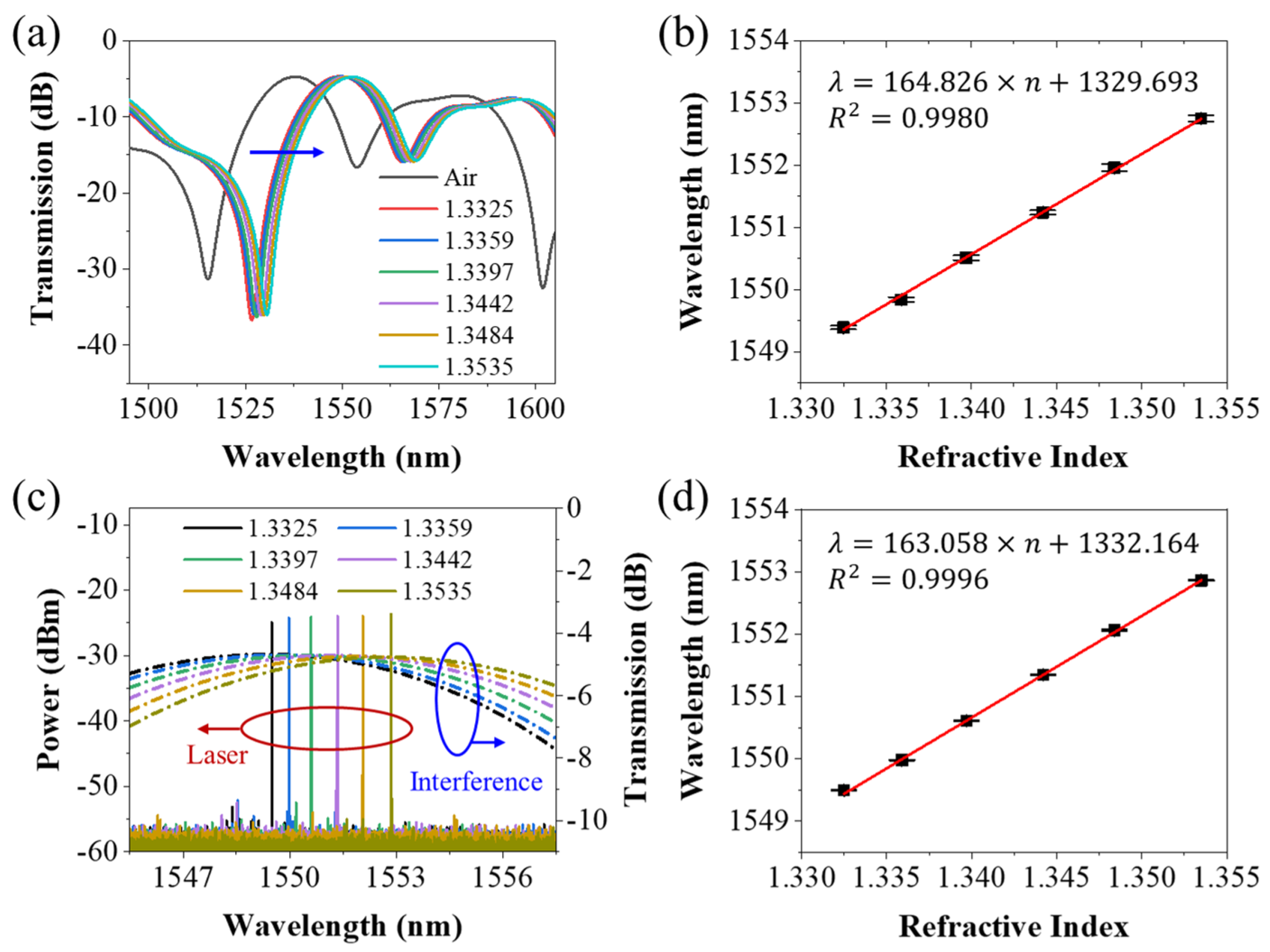
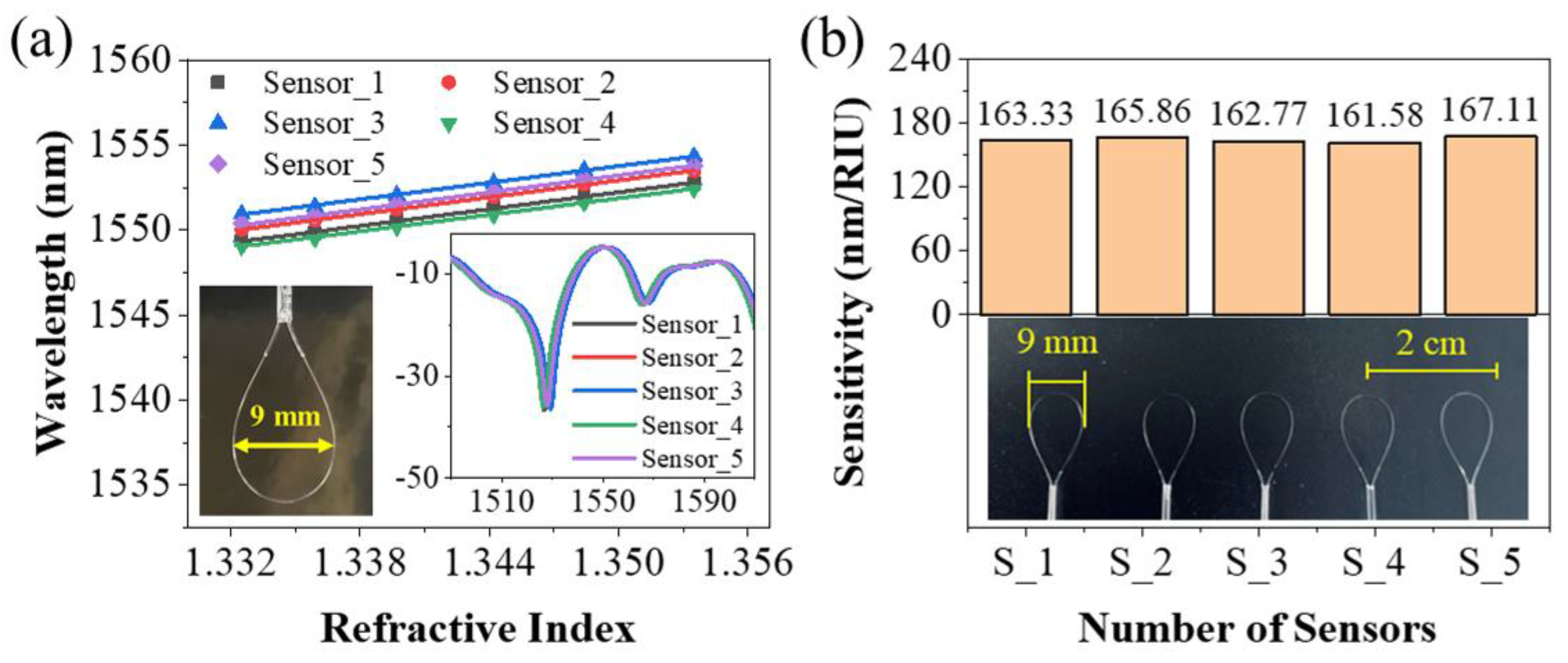
3. Results and Discussion
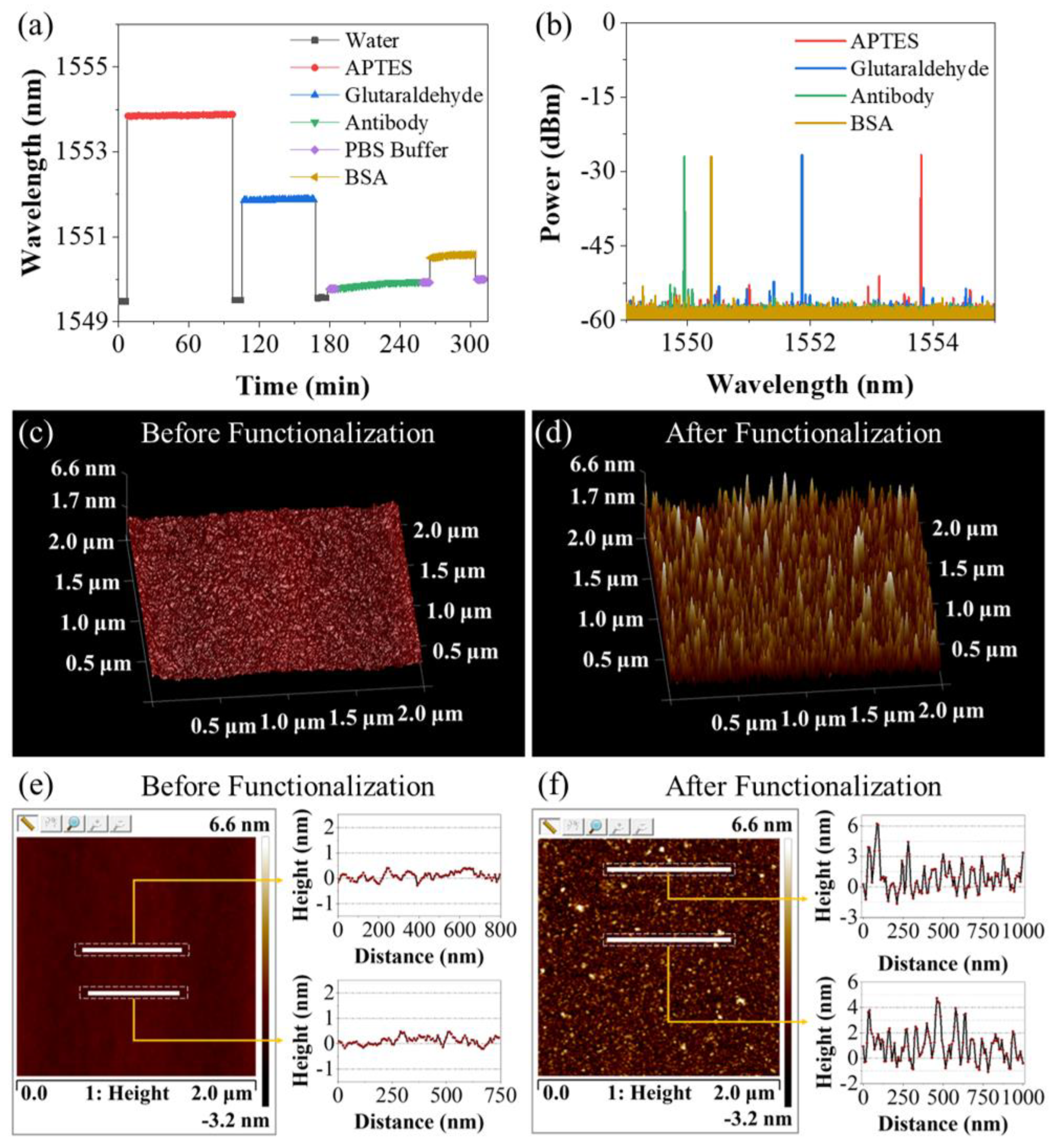
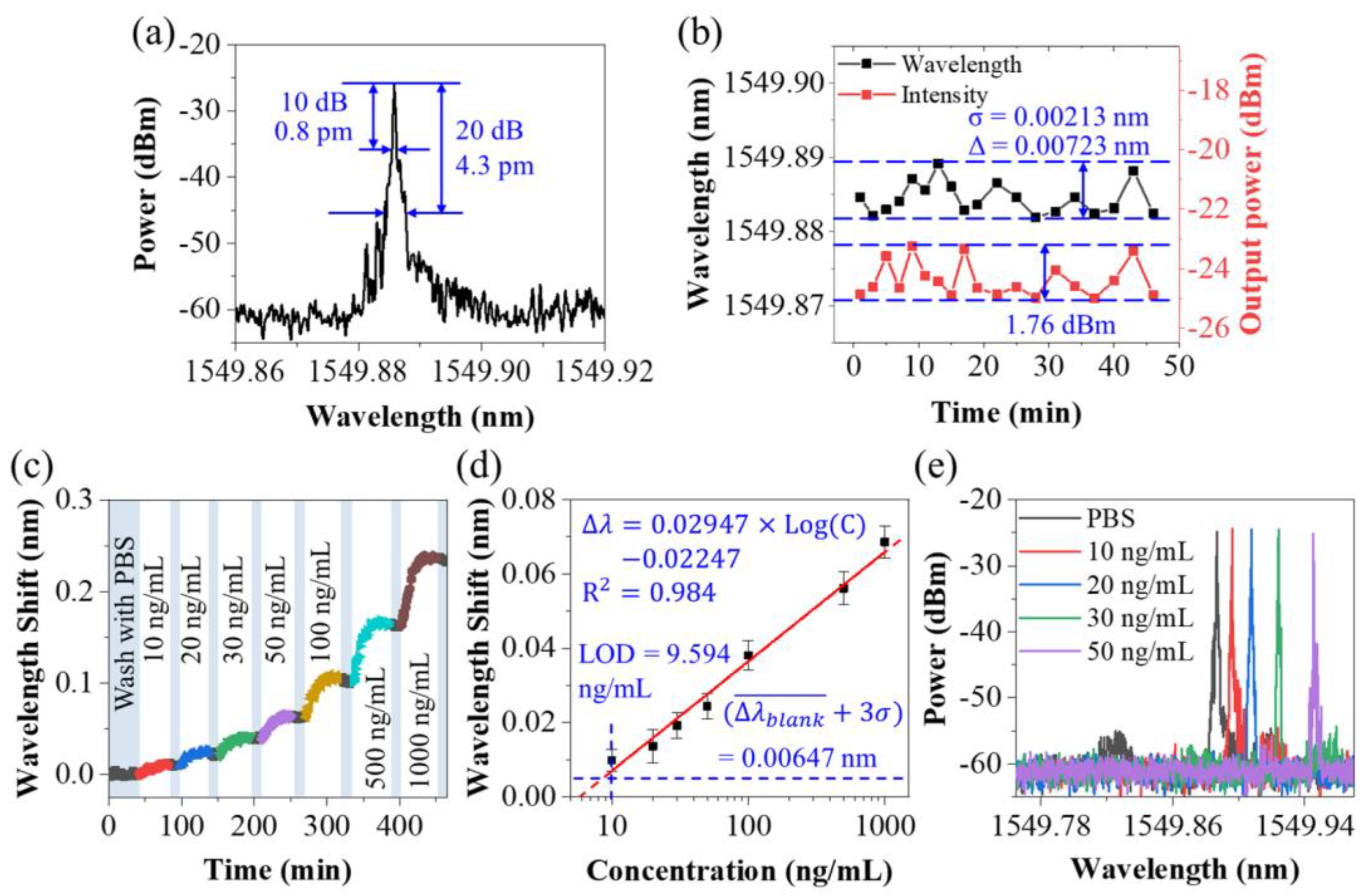
3.1. Sensing Characteristics of the Sensing Probe
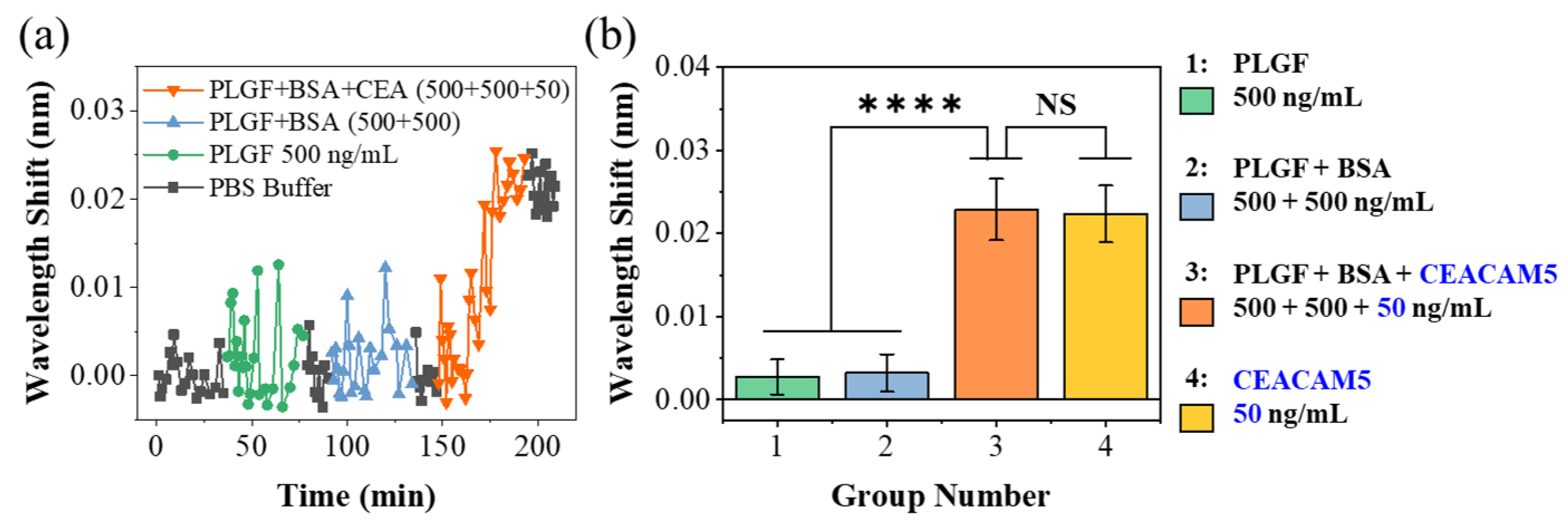
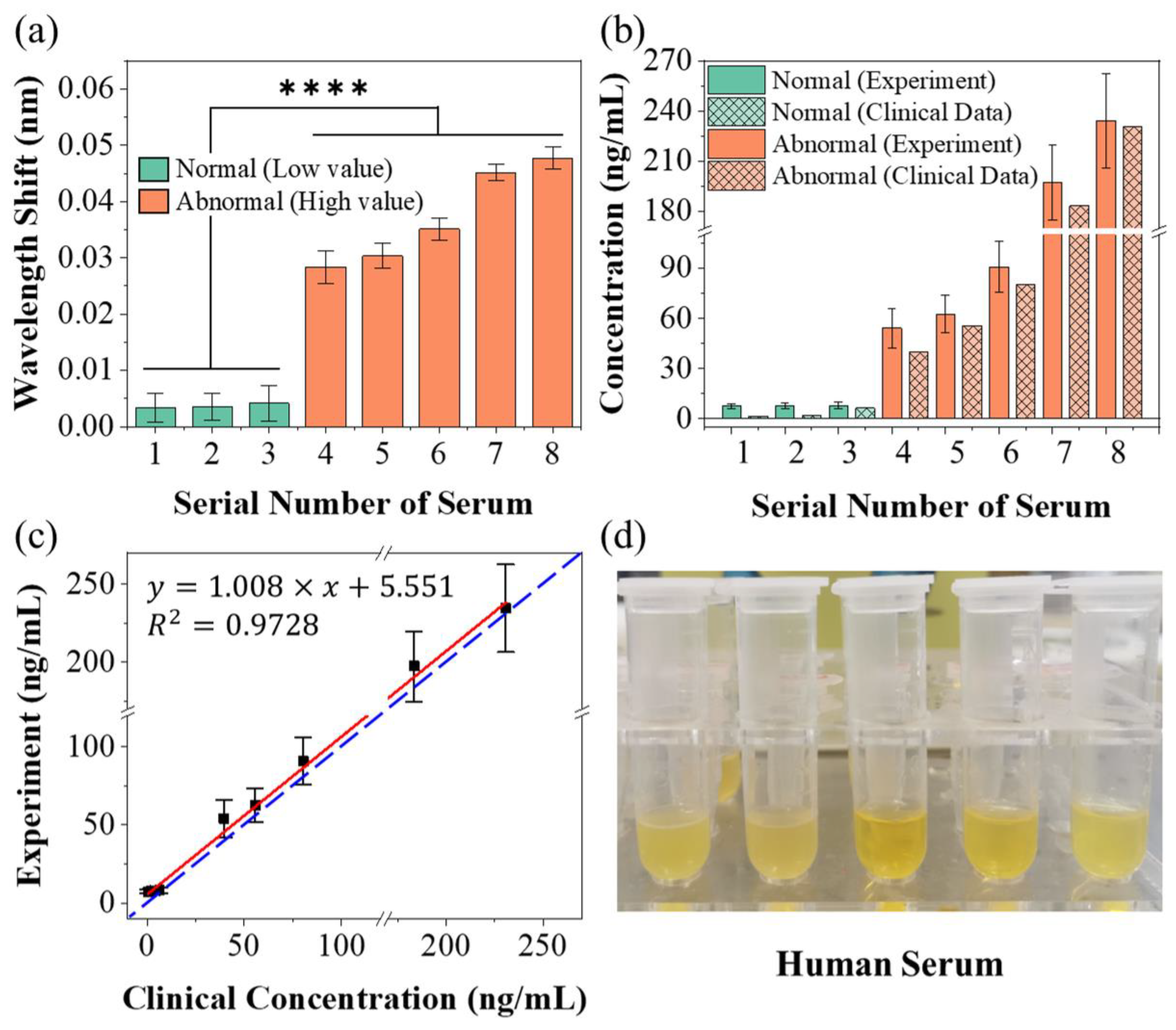
3.2. Protein Detection Using the Sensing System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kearney, A.J.; Murray, M. Breast cancer screening recommendations: Is mammography the only answer? J. Midwifery Women’s Health 2009, 54, 393–400. [Google Scholar] [CrossRef]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jalali, M.; Mahshid, S.; Wachsmann-Hogiu, S. Are plasmonic optical biosensors ready for use in point-of-need applications? Analyst 2020, 145, 364–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuteja, S.K.; Chen, R.; Kukkar, M.; Song, C.K.; Mutreja, R.; Singh, S.; Paul, A.K.; Lee, H.; Kim, K.-H.; Deep, A.; et al. A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens. Bioelectron. 2016, 86, 548–556. [Google Scholar] [CrossRef]
- Baaske, M.D.; Foreman, M.R.; Vollmer, F. Single-molecule nucleic acid interactions monitored on a label-free microcavity biosensor platform. Nat. Nanotechnol. 2014, 9, 933–939. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef]
- Rezapour Sarabi, M.; Jiang, N.; Ozturk, E.; Yetisen, A.K.; Tasoglu, S. Biomedical optical fibers. Lab Chip 2021, 21, 627–640. [Google Scholar] [CrossRef]
- Ran, Y.; Long, J.; Xu, Z.; Yin, Y.; Hu, D.; Long, X.; Zhang, Y.; Liang, L.; Liang, H.; Guan, B.-O. Harmonic optical microfiber Bragg grating immunosensor for the accelerative test of cardiac biomarker (cTn-I). Biosens. Bioelectron. 2021, 179, 113081. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Bock, W.J.; Mikulic, P.; Chinnappan, R.; Ng, A.; Tolba, M.; Zourob, M. Long period grating based biosensor for the detection of Escherichia coli bacteria. Biosens. Bioelectron. 2012, 35, 308–312. [Google Scholar] [CrossRef]
- Quero, G.; Consales, M.; Severino, R.; Vaiano, P.; Boniello, A.; Sandomenico, A.; Ruvo, M.; Borriello, A.; Diodato, L.; Zuppolini, S.; et al. Long period fiber grating nano-optrode for cancer biomarker detection. Biosens. Bioelectron. 2016, 80, 590–600. [Google Scholar] [CrossRef]
- Zuppolini, S.; Quero, G.; Consales, M.; Diodato, L.; Vaiano, P.; Venturelli, A.; Santucci, M.; Spyrakis, F.; Costi, M.P.; Giordano, M.; et al. Label-free fiber optic optrode for the detection of class C β-lactamases expressed by drug resistant bacteria. Biomed. Opt. Express 2017, 8, 5191–5205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, A.; Hanash, S.M. Unleashing the power of proteomics to develop blood-based cancer markers. Clin. Chem. 2013, 59, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekmurzayeva, A.; Ashikbayeva, Z.; Assylbekova, N.; Myrkhiyeva, Z.; Dauletova, A.; Ayupova, T.; Shaimerdenova, M.; Tosi, D. Ultra-wide, attomolar-level limit detection of CD44 biomarker with a silanized optical fiber biosensor. Biosens. Bioelectron. 2022, 208, 114217. [Google Scholar] [CrossRef]
- Chen, L.; Leng, Y.-K.; Liu, B.; Liu, J.; Wan, S.-P.; Wu, T.; Yuan, J.; Shao, L.; Gu, G.; Fu, Y.Q.; et al. Ultrahigh-sensitivity label-free optical fiber biosensor based on a tapered singlemode- no core-singlemode coupler for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128283. [Google Scholar] [CrossRef]
- Zhang, N.; Li, K.; Cui, Y.; Wu, Z.; Shum, P.P.; Auguste, J.-L.; Dinh, X.Q.; Humbert, G.; Wei, L. Ultra-sensitive chemical and biological analysis via specialty fibers with built-in microstructured optofluidic channels. Lab Chip 2018, 18, 655–661. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Trinh, K.T.L.; Yoon, W.J.; Lee, N.Y.; Ju, H. Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sens. Actuators B Chem. 2017, 242, 1–8. [Google Scholar] [CrossRef]
- Janik, M.; Hamidi, S.V.; Koba, M.; Perreault, J.; Walsh, R.; Bock, W.J.; Śmietana, M. Real-time isothermal DNA amplification monitoring in picoliter volumes using an optical fiber sensor. Lab Chip 2021, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, Y. High-Sensitivity Detection of IgG Operating near the Dispersion Turning Point in Tapered Two-Mode Fibers. Micromachines 2020, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Zhang, J.; Wang, Y.; Chen, J.; Li, Y.; Duan, Y. An aptamer based method for small molecules detection through monitoring salt-induced AuNPs aggregation and surface plasmon resonance (SPR) detection. Sens. Actuators B Chem. 2016, 236, 474–479. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ning, W.; Yang, E.; Li, Y.; Luo, Z.; Duan, Y. Sandwich method-based sensitivity enhancement of Ω-shaped fiber optic LSPR for time-flexible bacterial detection. Biosens. Bioelectron. 2022, 201, 113911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Huang, Y.; Zheng, J.; Chen, P.; Guan, B.-O. An optical microfiber biosensor for CEACAM5 detection in serum: Sensitization by a nanosphere interface. ACS Appl. Mater. Interfaces 2019, 12, 1799–1805. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, H.-J.; Park, J.-H.; Lee, S.-K. High-performance biosensor using a sandwich assay via antibody-conjugated gold nanoparticles and fiber-optic localized surface plasmon resonance. Anal. Chim. Acta 2022, 1213, 339960. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Sansone, L.; Srivastava, A.; Baldini, F.; Campopiano, S.; Chiavaioli, F.; Giordano, M.; Giannetti, A.; Iadicicco, A. Long period grating in double cladding fiber coated with graphene oxide as high-performance optical platform for biosensing. Biosens. Bioelectron. 2021, 172, 112747. [Google Scholar] [CrossRef] [PubMed]
- Nemiroski, A.; Gonidec, M.; Fox, J.M.; Jean-Remy, P.; Turnage, E.; Whitesides, G.M. Engineering shadows to fabricate optical metasurfaces. ACS Nano 2014, 8, 11061–11070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Lan, C.; Bian, H.; Gao, R.; Zhou, J. Flexible, all-dielectric metasurface fabricated via nanosphere lithography and its applications in sensing. Opt. Express 2017, 25, 22038–22045. [Google Scholar] [CrossRef]
- Pisco, M.; Galeotti, F.; Quero, G.; Grisci, G.; Micco, A.; Mercaldo, L.V.; Veneri, P.D.; Cutolo, A.; Cusano, A. Nanosphere lithography for optical fiber tip nanoprobes. Light Sci. Appl. 2017, 6, e16229. [Google Scholar] [CrossRef] [Green Version]
- Calafiore, G.; Koshelev, A.; Allen, F.I.; Dhuey, S.; Sassolini, S.; Wong, E.; Lum, P.; Munechika, K.; Cabrini, S. Nanoimprint of a 3D structure on an optical fiber for light wavefront manipulation. Nanotechnology 2016, 27, 375301. [Google Scholar] [CrossRef] [Green Version]
- Juhl, M.; Mueller, J.P.B.; Leosson, K. Metasurface polarimeter on optical fiber facet by nano-transfer to UV-curable hybrid polymer. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 4500107. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Ureña, E.; Steiner, U. Self-rolled multilayer metasurfaces. ACS Photonics 2019, 6, 2198–2204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, H.; Wang, H.; Yin, B.; Wong, S.H.D.; Zhang, A.P.; Tam, H.-Y. Ultraminiature optical fiber-tip directly-printed plasmonic biosensors for label-free biodetection. Biosens. Bioelectron. 2022, 218, 114761. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, S.; Quero, G.; Managò, S.; Zito, G.; Terracciano, D.; Macchia, P.; Galeotti, F.; Pisco, M.; De Luca, A.; Cusano, A. SERS assisted sandwich immunoassay platforms for ultrasensitive and selective detection of human Thyroglobulin. Biosens. Bioelectron. 2023, 233, 115322. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Guo, T.; Ran, Y.; Feng, X.; Guan, B.-O.; Yao, J. High-resolution and temperature-compensational HER2 antigen detection based on microwave photonic interrogation. Sens. Actuators B Chem. 2017, 245, 583–589. [Google Scholar] [CrossRef]
- Niu, P.; Jiang, J.; Liu, K.; Wang, S.; Jing, J.; Xu, T.; Wang, T.; Liu, Y.; Liu, T. Fiber-integrated WGM optofluidic chip enhanced by microwave photonic analyzer for cardiac biomarker detection with ultra-high resolution. Biosens. Bioelectron. 2022, 208, 114238. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bandyopadhyay, S.; Shao, L.-Y.; Xiao, D.; Gu, G.; Song, Z. Side-polished DBR fiber laser with enhanced sensitivity for axial force and refractive index measurement. IEEE Photonics J. 2019, 11, 7101910. [Google Scholar] [CrossRef]
- Guo, K.; He, J.; Li, H.; Xu, X.; Du, B.; Liu, S.; Chen, Y.; Ma, D.; Wang, Y.; Xu, G.; et al. Polarimetric fiber laser for relative humidity sensing based on graphene oxide-coated D-shaped fiber and beat frequency demodulation. Opt. Express 2022, 30, 15998–16008. [Google Scholar] [CrossRef]
- Mansor, M.; Bakar, M.A.; Omar, M.; Kamil, Y.M.; Abidin, N.Z.; Mustafa, F.; Mahdi, M. Taper biosensor in fiber ring laser cavity for protein detection. Opt. Laser Technol. 2020, 125, 106033. [Google Scholar] [CrossRef]
- Martinez-Ríos, A.; Anzueto-Sanchez, G.; Selvas-Aguilar, R.; Guzman, A.A.C.; Toral-Acosta, D.; Guzman-Ramos, V.; Duran-Ramirez, V.M.; Guerrero-Viramontes, J.A.; Calles-Arriaga, C.A. High sensitivity fiber laser temperature sensor. IEEE Sens. J. 2014, 15, 2399–2402. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, X.; Zhou, Y.; Tan, X.; Chen, Y.C. Distinguishing Small Molecules in Microcavity with Molecular Laser Polarization. ACS Photonics 2020, 7, 1908–1914. [Google Scholar] [CrossRef]
- Yang, X.; Shu, W.; Wang, Y.; Gong, Y.; Gong, C.; Chen, Q.; Tan, X.; Peng, G.-D.; Fan, X.; Rao, Y.-J. Turbidimetric inhibition immunoassay revisited to enhance its sensitivity via an optofluidic laser. Biosens. Bioelectron. 2019, 131, 60–66. [Google Scholar] [CrossRef]
- Yang, X.; Gong, C.; Zhang, C.; Wang, Y.; Yan, G.-F.; Wei, L.; Chen, Y.-C.; Rao, Y.-J.; Gong, Y. Fiber Optofluidic Microlasers: Structures, Characteristics, and Applications. Laser Photonics Rev. 2022, 16, 2100171. [Google Scholar] [CrossRef]
- Hu, X.-G.; Zhao, Y.; Peng, Y.; Tong, R.-J.; Zheng, H.-K.; Zhao, J.; Hu, S. In-fiber optofluidic michelson interferometer for detecting small volume and low concentration chemicals with a fiber ring cavity laser. Sens. Actuators B Chem. 2022, 370, 132467. [Google Scholar] [CrossRef]
- Warren-Smith, S.C.; André, R.M.; Dellith, J.; Eschrich, T.; Becker, M.; Bartelt, H. Sensing with ultra-short Fabry-Perot cavities written into optical micro-fibers. Sens. Actuators B Chem. 2017, 244, 1016–1021. [Google Scholar] [CrossRef] [Green Version]
- Eftimov, T.; Janik, M.; Koba, M.; Śmietana, M.; Mikulic, P.; Bock, W. Long-period gratings and microcavity in-line Mach Zehnder interferometers as highly sensitive optical fiber platforms for bacteria sensing. Sensors 2020, 20, 3772. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Farrell, G.; Wang, X.; Lewis, E.; Wang, P. Highly sensitive displacement sensor based on composite interference established within a balloon-shaped bent multimode fiber structure. Appl. Opt. 2018, 57, 9662–9668. [Google Scholar] [CrossRef] [PubMed]
- Dyshlyuk, A.V. Tunable Fano-like resonances in a bent single-mode waveguide-based Fabry–Perot resonator. Opt. Lett. 2019, 44, 231–234. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Lv, R.-Q.; Wang, Q. Enhancement of RI sensitivity through bending a tapered-SMF-based balloon-like interferometer. J. Light. Technol. 2016, 34, 3293–3299. [Google Scholar] [CrossRef]
- Xie, W.-G.; Zhang, Y.-N.; Wang, P.-Z.; Wang, J.-Z. Optical fiber sensors based on fiber ring laser demodulation technology. Sensors 2018, 18, 505. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Gao, J.; Shafi, M.; Liu, R.; Zha, Z.; Feng, D.; Liu, M.; Du, X.; Yue, W.; Jiang, S. Optical fiber SPR biosensor complying with a 3D composite hyperbolic metamaterial and a graphene film. Photonics Res. 2021, 9, 379–388. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-N.; Zheng, W.; Li, X.; Zhao, Y. Optical fiber SPR biosensor based on gold nanoparticle amplification for DNA hybridization detection. Talanta 2022, 247, 123599. [Google Scholar] [CrossRef]
- Kumar, R.; Leng, Y.; Liu, B.; Zhou, J.; Shao, L.; Yuan, J.; Fan, X.; Wan, S.; Wu, T.; Liu, J.; et al. Ultrasensitive biosensor based on magnetic microspheres enhanced microfiber interferometer. Biosens. Bioelectron. 2019, 145, 111563. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, Y.; He, L.; He, F.; Wu, J.; Huang, Z.; Tian, Y.; Li, Y.; Duan, Y. Development of a rapid and ultra-sensitive cytosensor: Ω-shaped fiber optic LSPR integrated with suitable AuNPs coverage. Sens. Actuators B Chem. 2021, 336, 129706. [Google Scholar] [CrossRef]
- Xiao, P.; Xu, Z.; Hu, D.; Liang, L.; Sun, L.; Li, J.; Ran, Y.; Guan, B.-O. Efficiently writing Bragg grating in high-birefringence elliptical microfiber for label-free immunosensing with temperature compensation. Adv. Fiber Mater. 2021, 3, 321–330. [Google Scholar] [CrossRef]
- Ran, Y.; Xu, Z.; Chen, M.; Wang, W.; Wu, Y.; Cai, J.; Long, J.; Chen, Z.-S.; Zhang, D.; Guan, B.-O. Fiber-Optic Theranostics (FOT): Interstitial Fiber-Optic Needles for Cancer Sensing and Therapy. Adv. Sci. 2022, 9, 2200456. [Google Scholar] [CrossRef]
- He, L.; He, F.; Feng, Y.; Wang, X.; Li, Y.; Tian, Y.; Gao, A.; Zhang, P.; Qi, X.; Luo, Z.; et al. Hybridized nanolayer modified Ω-shaped fiber-optic synergistically enhances localized surface plasma resonance for ultrasensitive cytosensor and efficient photothermal therapy. Biosens. Bioelectron. 2021, 194, 113599. [Google Scholar] [CrossRef]
| Method | RI Sensitivity | FWHM | FOM | LOD | Reference |
|---|---|---|---|---|---|
| hyperbolic metamaterial and graphene film on fiber | 4461 nm/RIU | >200 nm | ~20 | 10 pM | [49] |
| SPR and AuNPs Amplification (Sandwich model) | 1452 nm/RIU | ~100 nm | 14.5 | 1 pM | [50] |
| Microfiber Bragg grating (mFBG) | 60 nm/RIU | ~2 nm | 30 | 13.5 ng/mL (365 pM) | [9] |
| mFBG and microwave photonics filter (MPF) demodulation | 7 nm/RIU (OSA) 255 GHz/RIU (MPF) | ~0.2 nm 26 MHz | 35 (OSA) | 50 ng/mL (270 pM) | [33] |
| Lasso structure and laser demodulation | 164 nm/RIU | <0.8 pm | 9.6 ng/mL (48 pM) | This work |
| Category | Wavelength Shift (pm) | Concentration (ng/mL) | |||
|---|---|---|---|---|---|
| Mean Value 1 | SD 2 | Mean Value | SD | Clinical Data | |
| Sample 1 | 3.38 | 2.19 | 7.62 | 1.35 | 0.55 |
| Sample 2 | 3.96 | 2.54 | 8.01 | 1.59 | 1.91 |
| Sample 3 | 4.77 | 3.03 | 8.58 | 2.00 | 6.45 |
| Sample 4 | 28.36 | 2.88 | 54.08 | 12.02 | 39.72 |
| Sample 5 | 30.32 | 2.22 | 62.64 | 11.06 | 55.64 |
| Sample 6 | 35.09 | 1.99 | 90.72 | 15.01 | 80.43 |
| Sample 7 | 45.11 | 1.47 | 197.46 | 22.48 | 183.51 |
| Sample 8 | 47.38 | 1.94 | 235.99 | 30.42 | 230.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Song, E.; Liu, Y.; Yang, Q.; Sun, J.; Chen, J.; Meng, Y.; Jia, Y.; Yu, Z.; Ran, Y.; et al. Fiber Laser-Based Lasso-Shaped Biosensor for High Precision Detection of Cancer Biomarker-CEACAM5 in Serum. Biosensors 2023, 13, 674. https://doi.org/10.3390/bios13070674
Hu J, Song E, Liu Y, Yang Q, Sun J, Chen J, Meng Y, Jia Y, Yu Z, Ran Y, et al. Fiber Laser-Based Lasso-Shaped Biosensor for High Precision Detection of Cancer Biomarker-CEACAM5 in Serum. Biosensors. 2023; 13(7):674. https://doi.org/10.3390/bios13070674
Chicago/Turabian StyleHu, Jie, Enlai Song, Yuhui Liu, Qiaochu Yang, Junhui Sun, Jinna Chen, Yue Meng, Yanwei Jia, Zhiguang Yu, Yang Ran, and et al. 2023. "Fiber Laser-Based Lasso-Shaped Biosensor for High Precision Detection of Cancer Biomarker-CEACAM5 in Serum" Biosensors 13, no. 7: 674. https://doi.org/10.3390/bios13070674





