Recent Advances and Challenges in Textile Electrodes for Wearable Biopotential Signal Monitoring: A Comprehensive Review
Abstract
:1. Introduction
2. Biopotentials
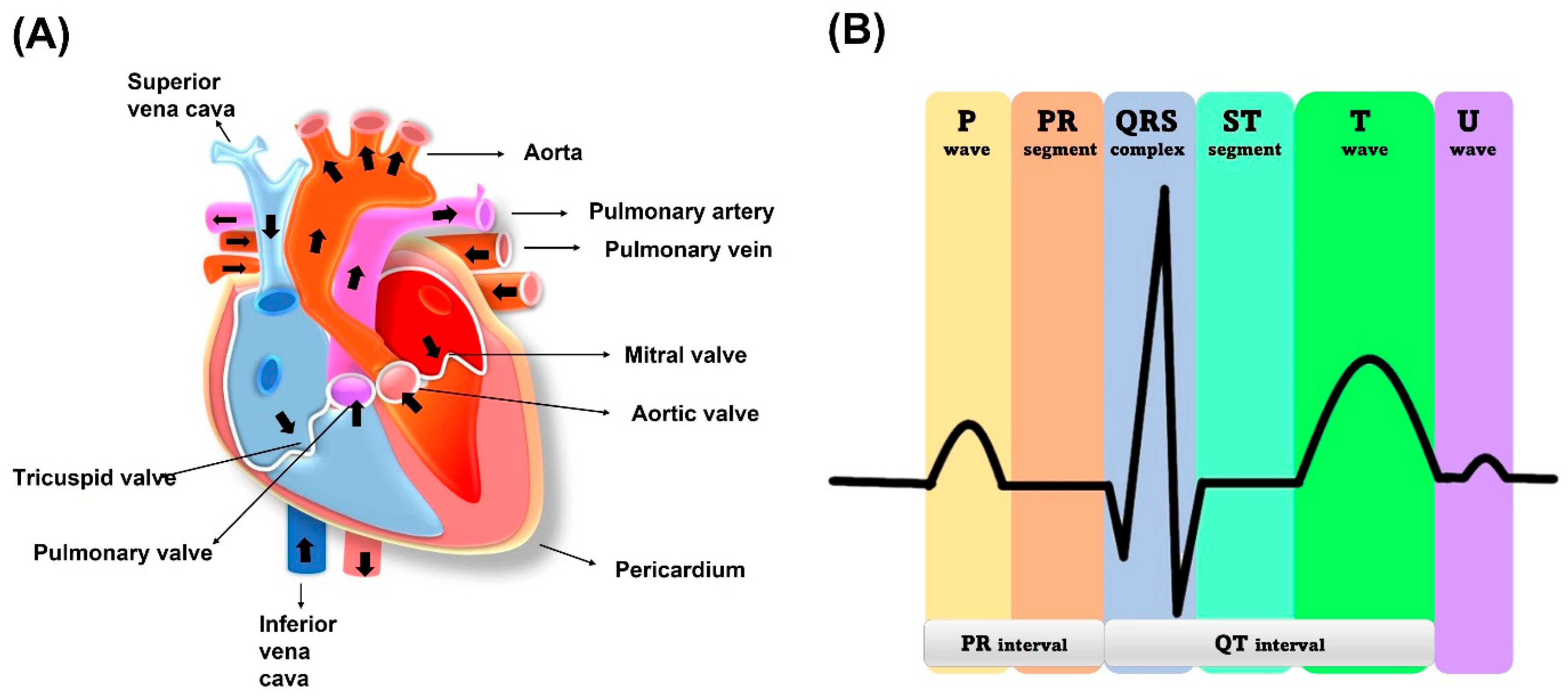
2.1. Electrocardiogram (ECG)
2.2. Electromyogram (EMG)
3. Biopotential Electrodes
3.1. Different Categories of Biopotential Electrodes
3.2. Wet Electrodes
3.3. Dry Electrodes
3.3.1. Direct Contact Electrodes
3.3.2. Capacitive Electrodes
4. Different Conductive Coatings on Textile
4.1. Metallic Coating
4.2. Conductive Polymer Coating
4.3. Carbon and Its Derivatives
4.4. Direct Laser Writing
4.5. Knitting, Weaving, and Embroidering with Conductive Threads, Yarns
5. Fabrication Methods of Conductive Textiles
5.1. Electrospinning
5.2. Screen/Inkjet Printing
5.3. Drop/Dye/Dip Coating
6. Essential Characteristics for a Wearable Textile Electrode
- Biocompatibility: The electrode should be made of materials that are safe for the skin and do not cause any irritation or allergic reactions;
- Flexibility: Textile electrodes should be flexible and able to conform to the contours of the body for comfortable and accurate placement;
- High conductivity: The electrode should have low impedance to ensure good signal quality and reduce noise;
- Stability: The electrode should be stable over time and not deteriorate or lose its conductivity during use;
- Durability: The electrode should be able to withstand repeated use and washing without damage;
- Cost-effectiveness: The electrode should be affordable and cost-effective for widespread use in healthcare and research applications;
- Ease of use: The electrode should be easy to apply and remove and not require specialized skills or training;
- Compatibility: The electrode should be compatible with standard ECG and EMG equipment for seamless integration into existing systems;
- Signal selectivity: The electrode should have high signal selectivity for specific biopotential signals, such as ECG or EMG, to avoid crosstalk and interference from other sources;
- Washability: The electrode should be washable to allow for repeated use and maintain good signal quality after washing.
6.1. Electrode Design
6.2. Effect of Pressure on the Electrode
6.3. Effect of Noise and Motion Artifacts
6.4. Effect of Washing and Stretching
7. Challenges and Limitations
- Poor signal quality: Dry textile electrodes have higher skin–electrode impedance than wet electrodes, resulting in poor signal quality and increased noise in the recorded signals;
- Limited electrode-skin contact area: Dry electrodes rely on mechanical pressure to maintain good contact with the skin, which can be challenging to achieve consistently over time. This limited electrode–skin contact area can result in poor signal quality and inconsistent recording of biopotential signals;
- Sensitivity to motion artifacts: Dry electrodes are more sensitive to motion artifacts than wet electrodes, as they rely solely on skin–electrode contact for signal acquisition. Movement or muscle contractions can cause the electrode to lose contact with the skin, leading to signal loss or artifacts;
- Lack of standardization: Currently, there is no standardized design or manufacturing process for dry textile electrodes, resulting in variability in electrode performance and compatibility with existing ECG/EMG equipment;
- Limited lifespan: for some dry electrodes have a limited lifespan due to the wear and tear of the conductive materials and the adhesive used to attach them to the skin. This can result in decreased signal quality over time and increased replacement costs;
- Crosstalk and interference: Dry electrodes may be susceptible to cross-talk and interference from other sources, such as electrical noise from nearby devices or from other electrodes on the same individual;
- Biodegradability: Some of the primary environmental concerns, such as biodegradability, are being addressed by growing trends towards the manufacturing of green textile electrodes. Cotton is one of the more popular substrates for making textile electrodes. They are biodegradable, soft, and flexible [99]. Polyester-based textile electrodes are not biodegradable and can pose a risk to the environment [151,152];
- Addressing these challenges and limitations is critical for the widespread adoption of dry textile electrodes in ECG and EMG monitoring applications. Ongoing research is focused on developing new materials, designs, and manufacturing processes to overcome these limitations and improve the performance of dry textile electrodes for biopotential monitoring.
8. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mshali, H.; Lemlouma, T.; Moloney, M.; Magoni, D. A Survey on Health Monitoring Systems for Health Smart Homes. Int. J. Ind. Ergon. 2018, 66, 26–56. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Lee, W.; Kim, Y.J. A Real-time Wearable Physiological Monitoring System for Home-based Healthcare Applications. Sensors 2022, 22, 104. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Martin, S.S.; Steinhubl, S.R.; Muse, E.D. Connected Health Technology for Cardiovascular Disease Prevention and Management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 29. [Google Scholar] [CrossRef]
- Düking, P.; Hotho, A.; Holmberg, H.C.; Fuss, F.K.; Sperlich, B. Comparison of Non-Invasive Individual Monitoring of the Training and Health of Athletes with Commercially Available Wearable Technologies. Front. Physiol. 2016, 7, 71. [Google Scholar] [CrossRef]
- Byrom, B.; McCarthy, M.; Schueler, P.; Muehlhausen, W. Brain Monitoring Devices in Neuroscience Clinical Research: The Potential of Remote Monitoring Using Sensors, Wearables, and Mobile Devices. Clin. Pharmacol. Ther. 2018, 104, 59–71. [Google Scholar] [CrossRef] [Green Version]
- IHME. New Report Tracks Latest Trends in Global Cardiovascular Health; IHME: Seattle, WA, USA, 2022. [Google Scholar]
- Robinson, T.; Condell, J.; Ramsey, E.; Leavey, G. Self-Management of Subclinical Common Mental Health Disorders (Anxiety, Depression and Sleep Disorders) Using Wearable Devices. Int. J. Environ. Res. Public Health 2023, 20, 2636. [Google Scholar] [CrossRef]
- Munch Nielsen, J.; Zibrandtsen, I.C.; Masulli, P.; Lykke Sørensen, T.; Andersen, T.S.; Wesenberg Kjær, T. Towards a Wearable Multi-Modal Seizure Detection System in Epilepsy: A Pilot Study. Clin. Neurophysiol. 2022, 136, 40–48. [Google Scholar] [CrossRef]
- Channa, A.; Popescu, N.; Ciobanu, V. Wearable Solutions for Patients with Parkinson’s Disease and Neurocognitive Disorder: A Systematic Review. Sensors 2020, 20, 2713. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Wang, Y.; Zhao, Y.; Qin, Y.; Zhu, Z.; Yu, Z.; Wang, J. An Ultralight Self-Powered Fire Alarm e-Textile Based on Conductive Aerogel Fiber with Repeatable Temperature Monitoring Performance Used in Firefighting Clothing. ACS Nano 2022, 16, 2953–2967. [Google Scholar] [CrossRef]
- Gumus, C.; Ozlem, K.; Khalilbayli, F.; Erzurumluoglu, O.F.; Ince, G.; Atalay, O.; Atalay, A.T. Textile-Based Pressure Sensor Arrays: A Novel Scalable Manufacturing Technique. Micro. Nano Eng. 2022, 15, 100140. [Google Scholar] [CrossRef]
- Alam, T.; Saidane, F.; Al Faisal, A.; Khan, A.; Hossain, G. Smart-Textile Strain Sensor for Human Joint Monitoring. Sens. Actuators A Phys. 2022, 341, 113587. [Google Scholar] [CrossRef]
- Zhou, Y.; Myant, C.; Stewart, R. Multifunctional and Stretchable Graphene/Textile Composite Sensor for Human Motion Monitoring. J. Appl. Polym. Sci. 2022, 139, e52755. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hung, Y.W.; Su, P.H.; Lee, I.P.; Chen, J.Y. Biosignal Monitoring Clothing System for the Acquisition of ECG and Respiratory Signals. IEEE Access 2022, 10, 66083–66097. [Google Scholar] [CrossRef]
- Heo, J.S.; Eom, J.; Kim, Y.H.; Park, S.K. Recent Progress of Textile-Based Wearable Electronics: A Comprehensive Review of Materials, Devices, and Applications. Small 2018, 14, 1703034. [Google Scholar] [CrossRef]
- Ismar, E.; Kurşun Bahadir, S.; Kalaoglu, F.; Koncar, V. Futuristic Clothes: Electronic Textiles and Wearable Technologies. Glob. Chall. 2020, 4, 1900092. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Pirbhulal, S.; Dong, K.; Wu, W.; Mei, X. Performance Evaluation of Plain Weave and Honeycomb Weave Electrodes for Human ECG Monitoring. J. Sens. 2017, 2017, 7539840. [Google Scholar] [CrossRef] [Green Version]
- Puurtinen, M.M.; Komulainen, S.M.; Kauppinen, P.K.; Malmivuo, J.A.V.; Hyttinen, J.A.K. Measurement of Noise and Impedance of Dry and Wet Textile Electrodes, and Textile Electrodes with Hydrogel. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; IEEE: New York, NY, USA, 2006. [Google Scholar]
- Cömert, A.; Honkala, M.; Hyttinen, J. Effect of Pressure and Padding on Motion Artifact of Textile Electrodes. BioMed. Eng. Online 2013, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Uz Zaman, S.; Tao, X.; Cochrane, C.; Koncar, V. Understanding the Washing Damage to Textile ECG Dry Skin Electrodes, Embroidered and Fabric-Based; Set up of Equivalent Laboratory Tests. Sensors 2020, 20, 1272. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, H.; Ozhan, O.; Karadana, Y.; Gulcu, M.; Macit, S.; Husain, F. A Portable Wearable Tele-ECG Monitoring System. IEEE Trans. Instrum. Meas. 2020, 69, 173–182. [Google Scholar] [CrossRef]
- Park, Y.G.; Lee, S.; Park, J.U. Recent Progress in Wireless Sensors for Wearable Electronics. Sensors 2019, 19, 4353. [Google Scholar] [CrossRef] [Green Version]
- Sverre, G.; Martinsen, Ø.G. Bioimpedance and Bioelectricity Basics, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Webster, J.G. (Ed.) Measurement, Instrumentation, and Sensors Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Bandara, V.; Nanayakkara, A. A Low-Cost, Portable Biopotential Monitoring System to Study Electrical Activities of the Human Brain and Body. Eur. J. Phys. 2020, 41, 065801. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Maithani, Y.; Choudhuri, B.; Mehta, B.R.; Singh, J.P. A Comprehensive Review of the Fabrication and Performance Evaluation of Dry Electrodes for Long-Term ECG Monitoring. In Modelling and Analysis of Active Biopotential Signals in Healthcare; IOP Publishing: Bristol, UK, 2020; Volume 2, pp. 8-1–8-32. [Google Scholar]
- Santana, L.F.; Cheng, E.P.; Lederer, W.J. How Does the Shape of the Cardiac Action Potential Control Calcium Signaling and Contraction in the Heart? J. Mol. Cell. Cardiol. 2010, 49, 901–903. [Google Scholar] [CrossRef] [Green Version]
- Hurst, J.W. Naming of the Waves in the ECG, with a Brief Account of Their Genesis. Circulation 1998, 98, 1937–1942. [Google Scholar] [CrossRef] [Green Version]
- Konrad, P. The ABC of EMG—A Practical Introduction to Kinesiological Electromyography; Noraxon USA, Inc.: Scottsdale, AZ, USA, 2006. [Google Scholar]
- Yousif, H.A.; Zakaria, A.; Rahim, N.A.; Salleh, A.F.B.; Mahmood, M.; Alfarhan, K.A.; Kamarudin, L.M.; Mamduh, S.M.; Hasan, A.M.; Hussain, M.K. Assessment of Muscles Fatigue Based on Surface EMG Signals Using Machine Learning and Statistical Approaches: A Review. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 705. [Google Scholar]
- Ozturk, O.; Golparvar, A.; Yapici, M.K. Smart Armband with Graphene Textile Electrodes for EMG-Based Muscle Fatigue Monitoring. In Proceedings of the IEEE Sensors, Sydney, Australia, 17 December 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021. [Google Scholar]
- Sumner, B.; Mancuso, C.; Paradiso, R. Performances Evaluation of Textile Electrodes for EMG Remote Measurements. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Osaka, Japan, 3–7 July 2013; EMBS: Piscataway, NJ, USA, 2013; pp. 6510–6513. [Google Scholar]
- Can, Y.; Coimbra, M.T.; Vijaya Kumar, B.V.K. Arrhythmia Detection and Classification Using Morphological and Dynamic Features of ECG Signals. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; IEEE: Buenos Aires, Argentina, 2010; pp. 1918–1921. [Google Scholar]
- Reddy, K. Recent Advances in the Diagnosis and Treatment of Acute Myocardial Infarction. World J. Cardiol. 2015, 7, 243. [Google Scholar] [CrossRef]
- Kehri, V.; Awale, R.N. EMG Signal Analysis for Diagnosis of Muscular Dystrophy Using Wavelet Transform, SVM and ANN. Biomed. Pharm. J. 2018, 11, 1583–1591. [Google Scholar] [CrossRef]
- Goen, A. Classification of EMG Signals for Assessment of Neuromuscular Disorders. Int. J. Electron. Electr. Eng. 2014, 2, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Neuman, M.R. Biopotential Electrodes. In The Biomedical Engineering Handbook, 2nd ed.; Bronzino, J.D., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kim, M.; Kim, T.; Kim, D.S.; Chung, W.K. Curved Microneedle Array-Based SEMG Electrode for Robust Long-Term Measurements and High Selectivity. Sensors 2015, 15, 16265–16280. [Google Scholar] [CrossRef] [Green Version]
- Polachan, K.; Chatterjee, B.; Weigand, S.; Sen, S. Human Body–Electrode Interfaces for Wide-Frequency Sensing and Communication: A Review. Nanomaterials 2021, 11, 2152. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Reyes, B.A.; Burnham, K.; Pennace, J.; Chon, K.H. Low Impedance Carbon Adhesive Electrodes with Long Shelf Life. Ann. Biomed. Eng. 2015, 43, 2374–2382. [Google Scholar] [CrossRef]
- Chi, M.; Zhao, J.; Dong, Y.; Wang, X. Flexible Carbon Nanotube-Based Polymer Electrode for Long-Term Electrocardiographic Recording. Materials 2019, 12, 971. [Google Scholar] [CrossRef] [Green Version]
- Gruetzmann, A.; Hansen, S.; Müller, J. Novel Dry Electrodes for ECG Monitoring. Physiol. Meas. 2007, 28, 1375–1390. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Song, Y.; Gou, L.; Zou, Y. A Novel Wearable Flexible Dry Electrode Based on Cowhide for ECG Measurement. Biosensors 2021, 11, 101. [Google Scholar] [CrossRef]
- Nunes, T.; da Silva, H.P. Characterization and Validation of Flexible Dry Electrodes for Wearable Integration. Sensors 2023, 23, 1468. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry Electrodes for Electrocardiography. Physiol. Meas. 2013, 34, R47. [Google Scholar] [CrossRef]
- Niu, X.; Gao, X.; Liu, Y.; Liu, H. Surface Bioelectric Dry Electrodes: A Review. Measurement 2021, 183, 109774. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, Q.; Chen, K.; Chen, Z.; Pan, C.; Jiang, L. Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring. Sensors 2016, 16, 908. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Zhang, S.; Hjort, K.; Hilborn, J.; Wu, Z.G. PDMS-Based Elastomer Tuned Soft, Stretchable, and Sticky for Epidermal Electronics. Adv. Mater. 2016, 28, 5830–5836. [Google Scholar] [CrossRef]
- Lee, E.; Cho, G. PU Nanoweb-Based Textile Electrode Treated with Single-Walled Carbon Nanotube/Silver Nanowire and Its Application to ECG Monitoring. Smart Mater. Struct. 2019, 28, 045004. [Google Scholar] [CrossRef]
- Arquilla, K.; Webb, A.K.; Anderson, A.P. Textile Electrocardiogram (ECG) Electrodes for Wearable Health Monitoring. Sensors 2020, 20, 1013. [Google Scholar] [CrossRef] [Green Version]
- Gorgutsa, S.; Bélanger-Garnier, V.; Ung, B.; Viens, J.; Gosselin, B.; LaRochelle, S.; Messaddeq, Y. Novel Wireless-Communicating Textiles Made from Multi-Material and Minimally-Invasive Fibers. Sensors 2014, 14, 19260–19274. [Google Scholar] [CrossRef] [Green Version]
- Linz, T.; Gourmelon, L.; Langereis, G. Contactless EMG Sensors Embroidered onto Textile. In Proceedings of the 4th International Workshop on Wearable and Implantable Body Sensor Networks (BSN 2007), Aachen, Germany, 26–28 March 2007; Springer: New York, NY, USA, 2007. [Google Scholar]
- Gao, Y.; Soman, V.V.; Lombardi, J.P.; Rajbhandari, P.P.; Dhakal, T.P.; Wilson, D.G.; Poliks, M.D.; Ghose, K.; Turner, J.N.; Jin, Z. Heart Monitor Using Flexible Capacitive ECG Electrodes. IEEE Trans. Instrum. Meas. 2020, 69, 4314–4323. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. J. Manag. 2016, 68, 1145–1155. [Google Scholar] [CrossRef]
- Lim, Y.G.; Lee, J.S.; Lee, S.M.; Lee, H.J.; Park, K.S. Capacitive Measurement of ECG for Ubiquitous Healthcare. Ann. Biomed. Eng. 2014, 42, 2218–2227. [Google Scholar] [CrossRef]
- Su, P.C.; Hsueh, Y.H.; Ke, M.T.; Chen, J.J.; Lai, P.C. Noncontact ECG Monitoring by Capacitive Coupling of Textiles in a Chair. J. Healthc. Eng. 2021, 2021, 6698567. [Google Scholar] [CrossRef]
- Feng, B.; Wei, H.; Shi, B.; Zhao, D.; Ye, S.; Wu, G.; Wang, R.; Zuo, G.; Wu, Z.; Chen, J.; et al. Sleeping Heart Monitoring Using Hydrogel-Textile Capacitive ECG Electrodes. IEEE Sens. J. 2022, 22, 9255–9267. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Liu, M.; Li, L.; Zhao, Y.; Wang, Y.; Bai, Y.; Lu, Q.; Xiong, Z.; Feng, S.; et al. All-Nanofiber-Based Janus Epidermal Electrode with Directional Sweat Permeability for Artifact-Free Biopotential Monitoring. Small 2022, 18, 2106477. [Google Scholar] [CrossRef]
- Luo, D.; Sun, H.; Li, Q.; Niu, X.; He, Y.; Liu, H. Flexible Sweat Sensors: From Films to Textiles. ACS Sens. 2023, 8, 465–481. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, M.; Li, H.; Dou, J.; Jian, M.; Xia, K.; Li, S.; Zhang, Y. Hydrophilic, Breathable, and Washable Graphene Decorated Textile Assisted by Silk Sericin for Integrated Multimodal Smart Wearables. Adv. Funct. Mater. 2022, 32, 2200162. [Google Scholar] [CrossRef]
- Soroudi, A.; Hernández, N.; Wipenmyr, J.; Nierstrasz, V. Surface Modification of Textile Electrodes to Improve Electrocardiography Signals in Wearable Smart Garment. J. Mater. Sci. Mater. Electron. 2019, 30, 16666–16675. [Google Scholar] [CrossRef] [Green Version]
- Lyons, G.; Nixon, R. Allergic Contact Dermatitis to Methacrylates in ECG Electrode Dots. Australas. J. Dermatol. 2013, 54, 39–40. [Google Scholar] [CrossRef]
- Alshabouna, F.; Lee, H.S.; Barandun, G.; Tan, E.; Cotur, Y.; Asfour, T.; Gonzalez-Macia, L.; Coatsworth, P.; Núnez-Bajo, E.; Kim, J.S.; et al. PEDOT:PSS-Modified Cotton Conductive Thread for Mass Manufacturing of Textile-Based Electrical Wearable Sensors by Computerized Embroidery. Mater. Today 2022, 59, 56–67. [Google Scholar] [CrossRef]
- Pitou, S.; Michael, B.; Thompson, K.; Howard, M. Hand-Made Embroidered Electromyography: Towards a Solution for Low-Income Countries. Sensors 2020, 20, 3347. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Lim, D.; Jeong, W. Development and Characterization of Embroidery-Based Textile Electrodes for Surface EMG Detection. Sensors 2022, 22, 2726. [Google Scholar] [CrossRef]
- Murciego, L.P.; Komolafe, A.; Peřinka, N.; Nunes-Matos, H.; Junker, K.; Díez, A.G.; Lanceros-Méndez, S.; Torah, R.; Spaich, E.G.; Dosen, S. A Novel Screen-Printed Textile Interface for High-Density Electromyography Recording. Sensors 2023, 23, 1113. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Malengier, B.; Mengistie, D.A.; Tseghai, G.B.; Van Langenhove, L. Development of Washable Silver Printed Textile Electrodes for Long-Term ECG Monitoring. Sensors 2020, 20, 6233. [Google Scholar] [CrossRef]
- Rayhan, G.S.; Khan, K.H.; Shoily, M.T.; Rahman, H.; Rahman, R.; Akon, T.; Hoque, M.; Khan, R.; Rifat, T.R.; Tisha, F.A.; et al. Conductive Textiles for Signal Sensing and Technical Applications. Signals 2022, 4, 1–39. [Google Scholar] [CrossRef]
- Liu, W.; Shangguan, D.; Lee, J.C.B. Evaluation of Launderability of Electrically Conductive Fabrics for E-Textile Applications. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 763–769. [Google Scholar] [CrossRef]
- Pawlak, R.; Korzeniewska, E.; Koneczny, C.; Hałgas, B. Properties of Thin Metal Layers Deposited on Textile Composites by Using the PVD Method for Textronic Applications. Autex Res. J. 2017, 17, 229–237. [Google Scholar] [CrossRef]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.F.; Abächerli, R.; Meyer, V.R.; Rossi, R.M. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Cho, J.; Jeong, K.; Cho, G. Exploring Possibilities of ECG Electrodes for Bio-Monitoring Smartwear with Cu Sputtered Fabrics. In Lecture Notes in Computer Science; Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics; Springer: New York, NY, USA, 2007; Volume 4551, pp. 1130–1137. [Google Scholar]
- Wang, L.; Pan, Y.; He, D.; Qian, L.; Cao, X.; He, B.; Li, J. Conductive Polyester Fabrics with High Washability as Electrocardiogram Textile Electrodes. ACS Appl. Polym. Mater. 2022, 4, 1440–1447. [Google Scholar] [CrossRef]
- He, D.; Qin, H.; Qian, L.; Cao, X.; Huang, J.; Li, J. Wearable Cellulosic Textile Electrodes with High Washability Based on Silver Nanowires to Capture Electrocardiogram. Fibers Polym. 2023, 24, 1963–1973. [Google Scholar] [CrossRef]
- Kaynak, A. Conductive Polymer Coatings. In Active Coatings for Smart Textiles; Woodhead Publishing: Shaxton, UK, 2016. [Google Scholar]
- Jin Young, O.; Kim, S.; Baik, H.K.; Jeong, U. Conducting Polymer Dough for Deformable Electronics. Adv. Mater. 2016, 28, 4455–4461. [Google Scholar] [CrossRef]
- Pradhan, S.; Yadavalli, V.K. Photolithographically Printed Flexible Silk/PEDOT:PSS Temperature Sensors. ACS Appl. Electron. Mater. 2021, 3, 21–29. [Google Scholar] [CrossRef]
- Spanu, A.; Botter, A.; Zedda, A.; Cerone, G.L.; Bonfiglio, A.; Pani, D. Dynamic Surface Electromyography Using Stretchable Screen-Printed Textile Electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1661–1668. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Nigusse, A.B.; Van Langenhove, L. Development of a Flex and Stretchy Conductive Cotton Fabric via Flat Screen Printing of PEDOT:PSS/PDMS Conductive Polymer Composite. Sensors 2020, 20, 1742. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Zakirov, A.S.; Kumar, G.M.; Yuldashev, S.U.; Cho, H.D.; Kang, T.W.; Mamadalimov, A.T. Highly Efficient CNT Functionalized Cotton Fabrics for Flexible/Wearable Heating Applications. RSC Adv. 2015, 5, 10697–10702. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for Application of Electrospinning and Electrospun Nanofibers Technology in Textile Industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Ibanez Labiano, I.; Arslan, D.; Ozden Yenigun, E.; Asadi, A.; Cebeci, H.; Alomainy, A. Screen Printing Carbon Nanotubes Textiles Antennas for Smart Wearables. Sensors 2021, 21, 4934. [Google Scholar] [CrossRef]
- Sadi, M.S.; Pan, J.; Xu, A.; Cheng, D.; Cai, G.; Wang, X. Direct Dip-Coating of Carbon Nanotubes onto Polydopamine-Templated Cotton Fabrics for Wearable Applications. Cellulose 2019, 26, 7569–7579. [Google Scholar] [CrossRef]
- Ozturk, O.; Yapici, M.K. Surface Electromyography with Wearable Graphene Textiles. IEEE Sens. J. 2021, 21, 14397–14406. [Google Scholar] [CrossRef]
- Soltani, T.; Kyu Lee, B. A Benign Ultrasonic Route to Reduced Graphene Oxide from Pristine Graphite. J. Colloid Interface Sci. 2017, 486, 337–343. [Google Scholar] [CrossRef]
- Shathi, M.A.; Chen, M.; Khoso, N.A.; Rahman, M.T.; Bhattacharjee, B. Graphene Coated Textile Based Highly Flexible and Washable Sports Bra for Human Health Monitoring. Mater. Des. 2020, 193, 108792. [Google Scholar] [CrossRef]
- Masihi, S.; Panahi, M.; Maddipatla, D.; Hanson, A.J.; Fenech, S.; Bonek, L.; Sapoznik, N.; Fleming, P.D.; Bazuin, B.J.; Atashbar, M.Z. Development of a Flexible Wireless ECG Monitoring Device with Dry Fabric Electrodes for Wearable Applications. IEEE Sens. J. 2022, 22, 11223–11232. [Google Scholar] [CrossRef]
- Boncel, S.; Jȩdrysiak, R.G.; Czerw, M.; Kolanowska, A.; Blacha, A.W.; Imielski, M.; Jóźwiak, B.; Dzida, M.H.; Greer, H.F.; Sobotnicki, A. Paintable Carbon Nanotube Coating-Based Textronics for Sustained Holter-Type Electrocardiography. ACS Appl. Nano Mater. 2022, 5, 15762–15774. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; McCay, R.N.; Goswami, S.; Xu, Y.; Zhang, C.; Ling, Y.; Lin, J.; Yan, Z. Gas-Permeable, Multifunctional On-Skin Electronics Based on Laser-Induced Porous Graphene and Sugar-Templated Elastomer Sponges. Adv. Mater. 2018, 30, 1804327. [Google Scholar] [CrossRef]
- Maithani, Y.; Mehta, B.R.; Singh, J.P. PEDOT:PSS-Treated Laser-Induced Graphene-Based Smart Textile Dry Electrodes for Long-Term ECG Monitoring. New J. Chem. 2022, 47, 1832–1841. [Google Scholar] [CrossRef]
- An, X.; Stylios, G.K. A Hybrid Textile Electrode for Electrocardiogram (ECG) Measurement and Motion Tracking. Materials 2018, 11, 1887. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Guo, N.; Gao, Q.; Li, H.; Wang, Z. Design, Characterization, and Performance of Woven Fabric Electrodes for Electrocardiogram Signal Monitoring. Sensors 2022, 22, 5472. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.; Liang, W.; Zhao, L.; Dong, Y.; Wang, X.; Lin, L. Water-Retentive, 3D Knitted Textile Electrode for Long-Term and Motion State Bioelectrical Signal Acquisition. Compos. Sci. Technol. 2022, 227, 109606. [Google Scholar] [CrossRef]
- Hossain, M.M.; Li, B.M.; Sennik, B.; Jur, J.S.; Bradford, P.D. Adhesive Free, Conformable and Washable Carbon Nanotube Fabric Electrodes for Biosensing. NPJ Flex. Electron. 2022, 6, 97. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Luo, G.; Xie, J.; Liu, J.; Zhang, Q.; Luo, Y.; Li, M.; Zhou, W.; Chen, K.; Li, Z.; Yang, P.; et al. Highly Conductive, Stretchable, Durable, Breathable Electrodes Based on Electrospun Polyurethane Mats Superficially Decorated with Carbon Nanotubes for Multifunctional Wearable Electronics. Chem. Eng. J. 2023, 451, 138549. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, C.Y.; Li, J.W.; Li, C.L. Flexible Hybrid Electronics Nanofiber Electrodes with Excellent Stretchability and Highly Stable Electrical Conductivity for Smart Clothing. ACS Appl. Mater. Interfaces 2022, 14, 42441–42453. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chiu, C.W. Facile Fabrication of a Stretchable and Flexible Nanofiber Carbon Film-Sensing Electrode by Electrospinning and Its Application in Smart Clothing for ECG and EMG Monitoring. ACS Appl. Electron. Mater. 2021, 3, 676–686. [Google Scholar] [CrossRef]
- Yan, X.; Chen, S.; Zhang, G.; Shi, W.; Peng, Z.; Liu, Z.; Chen, Y.; Huang, Y.; Liu, L. Highly Breathable, Surface-Hydrophobic and Wet-Adhesive Silk Based Epidermal Electrode for Long-Term Electrophysiological Monitoring. Compos. Sci. Technol. 2022, 230, 109751. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; Novoselov, K.S.; Yeates, S.G. All Inkjet-Printed Graphene-Silver Composite Ink on Textiles for Highly Conductive Wearable Electronics Applications. Sci. Rep. 2019, 9, 8035. [Google Scholar] [CrossRef] [Green Version]
- Marra, F.; Minutillo, S.; Tamburrano, A.; Sarto, M.S. Production and Characterization of Graphene Nanoplatelet-Based Ink for Smart Textile Strain Sensors via Screen Printing Technique. Mater. Des. 2021, 198, 109306. [Google Scholar] [CrossRef]
- Court, D.; Torah, R. Development of a Printed E-Textile for the Measurement of Muscle Activation via EMG for the Purpose of Gesture Control. Proceedings 2021, 68, 8. [Google Scholar]
- Xu, X.; Luo, M.; He, P.; Yang, J. Washable and Flexible Screen Printed Graphene Electrode on Textiles for Wearable Healthcare Monitoring. J. Phys. D Appl. Phys. 2020, 53, 125402. [Google Scholar] [CrossRef]
- Stempien, Z.; Rybicki, E.; Rybicki, T.; Lesnikowski, J. Inkjet-Printing Deposition of Silver Electro-Conductive Layers on Textile Substrates at Low Sintering Temperature by Using an Aqueous Silver Ions-Containing Ink for Textronic Applications. Sens. Actuators B Chem. 2015, 224, 714–725. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Q.; Zhang, C.; Zhang, W.; Yun, W.; Yang, L. Fabrication of Silver Electrical Circuits on Textile Substrates by Reactive Inkjet Printing. IEEE Sens. J. 2022, 22, 11056–11064. [Google Scholar] [CrossRef]
- Ojstršek, A.; Jug, L.; Plohl, O. A Review of Electro Conductive Textiles Utilizing the Dip-Coating Technique: Their Functionality, Durability and Sustainability. Polymers 2022, 14, 4713. [Google Scholar] [CrossRef]
- Maithani, Y.; Singh, A.; Mehta, B.R.; Singh, J.P. PEDOT: PSS Treated Cotton-Based Textile Dry Electrode for ECG Sensing. Mater. Today Proc. 2022, 62, 4052–4057. [Google Scholar] [CrossRef]
- Akter Shathi, M.; Minzhi, C.; Khoso, N.A.; Deb, H.; Ahmed, A.; Sai Sai, W. All Organic Graphene Oxide and Poly (3,4-Ethylene Dioxythiophene)—Poly (Styrene Sulfonate) Coated Knitted Textile Fabrics for Wearable Electrocardiography (ECG) Monitoring. Synth. Met. 2020, 263, 116329. [Google Scholar] [CrossRef]
- Yang, J.; Nithyanandam, P.; Kanetkar, S.; Kwon, K.Y.; Ma, J.; Im, S.; Oh, J.H.; Shamsi, M.; Wilkins, M.; Daniele, M.; et al. Liquid Metal Coated Textiles with Autonomous Electrical Healing and Antibacterial Properties. Adv. Mater. Technol. 2023, 2023, 2202183. [Google Scholar] [CrossRef]
- Lim, T.; Kim, H.J.; Won, S.; Kim, C.H.; Yoo, J.; Lee, J.H.; Son, K.S.; Nam, I.W.; Kim, K.; Yeo, S.Y.; et al. Liquid Metal-Based Electronic Textiles Coated with Au Nanoparticles as Stretchable Electrode Materials for Healthcare Monitoring. ACS Appl. Nano Mater. 2023, 6, 8482–8494. [Google Scholar] [CrossRef]
- Qian, L.; He, D.; Cao, X.; Huang, J.; Li, J. Robust Conductive Polyester Fabric with Enhanced Multi-Layer Silver Deposition for Textile Electrodes. Coll. Surf. A Physicochem. Eng. Asp. 2022, 644, 128857. [Google Scholar] [CrossRef]
- Jain, K.; Wang, Z.; Garma, L.D.; Engel, E.; Ciftci, G.C.; Fager, C.; Larsson, P.A.; Wågberg, L. 3D Printable Composites of Modified Cellulose Fibers and Conductive Polymers and Their Use in Wearable Electronics. Appl. Mater. Today 2023, 30, 101703. [Google Scholar] [CrossRef]
- Ohiri, K.A.; Pyles, C.O.; Hamilton, L.H.; Baker, M.M.; McGuire, M.T.; Nguyen, E.Q.; Osborn, L.E.; Rossick, K.M.; McDowell, E.G.; Strohsnitter, L.M.; et al. E-Textile Based Modular SEMG Suit for Large Area Level of Effort Analysis. Sci. Rep. 2022, 12, 9650. [Google Scholar] [CrossRef]
- Dong, J.; Wang, D.; Peng, Y.; Zhang, C.; Lai, F.; He, G.; Ma, P.; Dong, W.; Huang, Y.; Parkin, I.P.; et al. Ultra-Stretchable and Superhydrophobic Textile-Based Bioelectrodes for Robust Self-Cleaning and Personal Health Monitoring. Nano Energy 2022, 97, 107160. [Google Scholar] [CrossRef]
- Ali, B.; Cheraghi Bidsorkhi, H.; D’Aloia, A.G.; Laracca, M.; Sarto, M.S. Wearable Graphene-Based Fabric Electrodes for Enhanced and Long-Term Biosignal Detection. Sens. Actuators Rep. 2023, 5, 100161. [Google Scholar] [CrossRef]
- Guler, S.; Golparvar, A.; Ozturk, O.; Yapici, M.K. Ear Electrocardiography with Soft Graphene Textiles for Hearable Applications. IEEE Sens. Lett. 2022, 6, 6003004. [Google Scholar] [CrossRef]
- Etana, B.B.; Malengier, B.; Kwa, T.; Krishnamoorthy, J.; Langenhove, L. Van Evaluation of Novel Embroidered Textile-Electrodes Made from Hybrid Polyamide Conductive Threads for Surface EMG Sensing. Sensors 2023, 23, 4397. [Google Scholar] [CrossRef]
- Alizadeh-Meghrazi, M.; Ying, B.; Schlums, A.; Lam, E.; Eskandarian, L.; Abbas, F.; Sidhu, G.; Mahnam, A.; Moineau, B.; Popovic, M.R. Evaluation of Dry Textile Electrodes for Long-Term Electrocardiographic Monitoring. BioMed. Eng. Online 2021, 20, 68. [Google Scholar] [CrossRef]
- Chun, S.; Kim, S.; Kim, J. Human Arm Workout Classification by Arm Sleeve Device Based on Machine Learning Algorithms. Sensors 2023, 23, 3106. [Google Scholar] [CrossRef]
- Hakansson, E.; Kaynak, A.; Lin, T.; Nahavandi, S.; Jones, T.; Hu, E. Characterization of Conducting Polymer Coated Synthetic Fabrics for Heat Generation. Synth. Met. 2004, 144, 21–28. [Google Scholar] [CrossRef]
- Kolanowska, A.; Kuziel, A.W.; Herman, A.P.; Jędrysiak, R.G.; Giżewski, T.; Boncel, S. Electroconductive Textile Coatings from Pastes Based on Individualized Multi-Wall Carbon Nanotubes—Synergy of Surfactant and Nanotube Aspect Ratio. Prog. Org. Coat. 2019, 130, 260–269. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Fully Printed Electrodes on Stretchable Textiles for Long-Term Electrophysiology. Adv. Mater. Technol. 2017, 2, 1600251. [Google Scholar] [CrossRef]
- Le, K.; Servati, A.; Soltanian, S.; Servati, P.; Ko, F. Performance and Signal Quality Analysis of Electrocardiogram Textile Electrodes for Smart Apparel Applications. Front. Electron. 2021, 2, 685264. [Google Scholar] [CrossRef]
- Goncu-Berk, G.; Tuna, B.G. The Effect of Sleeve Pattern and Fit on E-Textile Electromyography (EMG) Electrode Performance in Smart Clothing Design. Sensors 2021, 21, 5621. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Kaya Yapici, M. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A Review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xiong, W.; Li, Y. Wearable Measurement of ECG Signals Based on Smart Clothing. Int. J. Telemed. Appl. 2020, 2020, 6329360. [Google Scholar] [CrossRef] [Green Version]
- Brehm, P.J.; Anderson, A.P. Modeling the Design Characteristics of Woven Textile Electrodes for Long−Term ECG Monitoring. Sensors 2023, 23, 598. [Google Scholar] [CrossRef]
- Kubicek, J.; Fiedorova, K.; Vilimek, D.; Cerny, M.; Penhaker, M.; Janura, M.; Rosicky, J. Recent Trends, Construction, and Applications of Smart Textiles and Clothing for Monitoring of Health Activity: A Comprehensive Multidisciplinary Review. IEEE Rev. Biomed. Eng. 2022, 15, 36–60. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Jeong, W. Emg Measurement with Textile-Based Electrodes in Different Electrode Sizes and Clothing Pressures for Smart Clothing Design Optimization. Polymers 2020, 12, 2406. [Google Scholar] [CrossRef]
- Belbasis, A.; Fuss, F.K. Muscle Performance Investigated with a Novel Smart Compression Garment Based on Pressure Sensor Force Myography and Its Validation against EMG. Front. Physiol. 2018, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of Textile Electrodes and Conductors Using Standardized Measurement Setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef]
- Takeshita, T.; Yoshida, M.; Takei, Y.; Ouchi, A.; Hinoki, A.; Uchida, H.; Kobayashi, T. Relationship between Contact Pressure and Motion Artifacts in ECG Measurement with Electrostatic Flocked Electrodes Fabricated on Textile. Sci. Rep. 2019, 9, 5897. [Google Scholar] [CrossRef] [Green Version]
- Fouassier, D.; Roy, X.; Blanchard, A.; Hulot, J.S. Assessment of Signal Quality Measured with a Smart 12-Lead ECG Acquisition T-Shirt. Ann. Noninvasive Electrocardiol. 2020, 25, e12682. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; Daminello-Raimundo, R.; de Abreu, L.C. Main Artifacts in Electrocardiography. Ann. Noninvasive Electrocardiol. 2018, 23, e12494. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.J.; Donald Gilmore, L.; Kuznetsov, M.; Roy, S.H. Filtering the Surface EMG Signal: Movement Artifact and Baseline Noise Contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef]
- Hamilton, P.S.; Curley, M.G.; Aimi, R.M.; Sae-Hau, C.; Limited, E.P. Comparison of Methods for Adaptive Removal of Motion Artifact. In Proceedings of the Computers in Cardiology, Cambridge, MA, USA, 24–27 September 2000; IEEE: New York, NY, USA, 2000. [Google Scholar]
- Xie, X.; Liu, H.; Shu, M.; Zhu, Q.; Huang, A.; Kong, X.; Wang, Y. A Multi-Stage Denoising Framework for Ambulatory ECG Signal Based on Domain Knowledge and Motion Artifact Detection. Future Gener. Comput. Syst. 2021, 116, 103–116. [Google Scholar] [CrossRef]
- Lee, J.; McManus, D.D.; Merchant, S.; Chon, K.H. Automatic Motion and Noise Artifact Detection in Holter ECG Data Using Empirical Mode Decomposition and Statistical Approaches. IEEE Trans. Biomed. Eng. 2012, 59, 1499–1506. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, S.-Y.; Van Helleputte, N.; Berset, T.; Geng, D.; Romero, I.; Penders, J.; Van Hoof, C. Refet Firat Yazicioglu Motion Artifact Removal Using Cascade Adaptive Filtering for Ambulatory ECG Monitoring System. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Hsinchu, Taiwan, 28–30 November 2012; IEEE: Hsinchu, Taiwan, 2012; pp. 160–163. [Google Scholar]
- Ricardo, L.; Luiz, J.; Bigliassi, M.; Dias Kanthack, T.F.; de Moraes, A.C.; Abrao, T. Influence of Different Strategies of Treatment Muscle Contraction and Relaxation Phases on EMG Signal Processing and Analysis During Cyclic Exercise. In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; InTech: Jakarta, Indonesia, 2012. [Google Scholar]
- Zubair, M.; Chandra Mouli, G.N.V.S.; Shaik, R.A. Removal of Motion Artifacts from ECG Signals by Combination of Recurrent Neural Networks and Deep Neural Networks. In Proceedings of the 2020 2nd International Conference on Electrical, Control and Instrumentation Engineering (ICECIE), Kuala Lumpur, Malaysia, 28–28 November 2020; IEEE: Kuala Lumpur, Malaysia, 2020; pp. 1–7. [Google Scholar]
- Zivanovic, M.; González-Izal, M. Simultaneous Powerline Interference and Baseline Wander Removal from ECG and EMG Signals by Sinusoidal Modeling. Med. Eng. Phys. 2013, 35, 1431–1441. [Google Scholar] [CrossRef]
- Lee, J.C.B.; Liu, W.; Lo, C.H.; Chen, C.C. Laundering Reliability of Electrically Conductive Fabrics for E-Textile Applications. In Proceedings of the Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2019; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2019; pp. 1826–1832. [Google Scholar]
- Ding, Y.; Invernale, M.A.; Sotzing, G.A. Conductivity Trends of Pedot-Pss Impregnated Fabric and the Effect of Conductivity on Electrochromic Textile. ACS Appl. Mater. Interfaces 2010, 2, 1588–1593. [Google Scholar] [CrossRef]
- Isaia, C.; McNally, D.S.; McMaster, S.A.; Branson, D.T. Effect of Mechanical Preconditioning on the Electrical Properties of Knitted Conductive Textiles during Cyclic Loading. Text. Res. J. 2019, 89, 445–460. [Google Scholar] [CrossRef]
- Egan, J.; Salmon, S. Strategies and Progress in Synthetic Textile Fiber Biodegradability. SN Appl. Sci. 2022, 4, 22. [Google Scholar] [CrossRef]
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective Conversion of Waste Polyester-Cotton Textile to Ethanol and Recovery of Polyester by Alkaline Pretreatment. Chem. Eng. J. 2014, 253, 40–45. [Google Scholar] [CrossRef]













| References | Textile Electrode Type | Fabrication Method | Benefits | Drawbacks |
|---|---|---|---|---|
| [114] Yang et al. (2023) | Metal based electrodes | Dip coating fabric into Ga- liquid metal particles solution | Conductive patterns heals automatically when cut, permeability, conductivity, easy to fabricate, antibacterial property | Coating of textile as a whole, selective region coatings can be explored |
| [115] Lim et al. (2023) | Metal based electrodes | Ga-liquid metal particles spray coated on nylon/spandex fabric and was immersed in aqueous Au solution | Good electrical and mechanical stability under stretching, good adhesion between conductive layers | Using gold can increase the cost of fabrication |
| [116] Qian et al. (2022) | Metal based electrodes | Ag particles deposited on polyester fabric by electroless plating | High washing stability, electrical stability even after 500 washing cycles | Biodegradability can be a concern for the polyester fabric |
| [112] Maithani et al. (2022) | Conducting polymer | Coating of PEDOT:PSS on the fabric | Good electrical conductivity, easy to fabricate | Loose their mechanical property during wet conditions |
| [117] Jain et al. (2023) | Conducting polymer | Bio-conductive of modified cellulose fibers and PEDOT: PSS prepared for 3D printing | Printed patterns have good conductivity at low PEDOT: PSS concentration, high tensile strain | Washing stability and reusability has to be explored if to be used in wearables |
| [118] Ohiri et al. (2022) | Conducting polymer | Dip coating polyester fabrics into PEDOT: PSS solution | Conductive textiles incorporated into compressive garments can be machine laundered, resistance to high strain, rapid prototyping possible | Use proprietary materials for conductive surface (Dupont CCSM) |
| [119] Dong et al. (2022) | Carbon-based electrodes | Dip coating of electrospun woven fabric into CB/CNT mixture | Ultra stretchable, self healing non woven fabric, long term use stability under harsh environment | Reusability and skin contact impedance can be studied |
| [120] Ali et al. (2023) | Carbon-based electrodes | Graphene NP/PVDF mixture cast and cured on polyester | Reusable, biocompatible, non irritant to skin, flexible | High temperature involved in the synthesis procedure |
| [121] Guler et al. (2022) | Carbon-based electrodes | Three step print-dry-reduce spray painting process to coat graphene on textile | ECG acquisition from region behind the ear using this soft graphene coated textile, High SNR 29.87 dB | Prone to motion artefacts when the electrodes move, need to explore this by postprocessing of the recorded ECG |
| [122] Etana et al. (2023) | Embroidered conductive threads electrodes | Computerized embroidery machine used to make conductive fabric with polyester multifilament with conductive hybrid thread | Comparison of optimum pressure and the signal quality | EMG signals were affected by motion artefacts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidhya, C.M.; Maithani, Y.; Singh, J.P. Recent Advances and Challenges in Textile Electrodes for Wearable Biopotential Signal Monitoring: A Comprehensive Review. Biosensors 2023, 13, 679. https://doi.org/10.3390/bios13070679
Vidhya CM, Maithani Y, Singh JP. Recent Advances and Challenges in Textile Electrodes for Wearable Biopotential Signal Monitoring: A Comprehensive Review. Biosensors. 2023; 13(7):679. https://doi.org/10.3390/bios13070679
Chicago/Turabian StyleVidhya, C. M., Yogita Maithani, and Jitendra P. Singh. 2023. "Recent Advances and Challenges in Textile Electrodes for Wearable Biopotential Signal Monitoring: A Comprehensive Review" Biosensors 13, no. 7: 679. https://doi.org/10.3390/bios13070679




