Advancement in Paper-Based Electrochemical Biosensing and Emerging Diagnostic Methods
Abstract
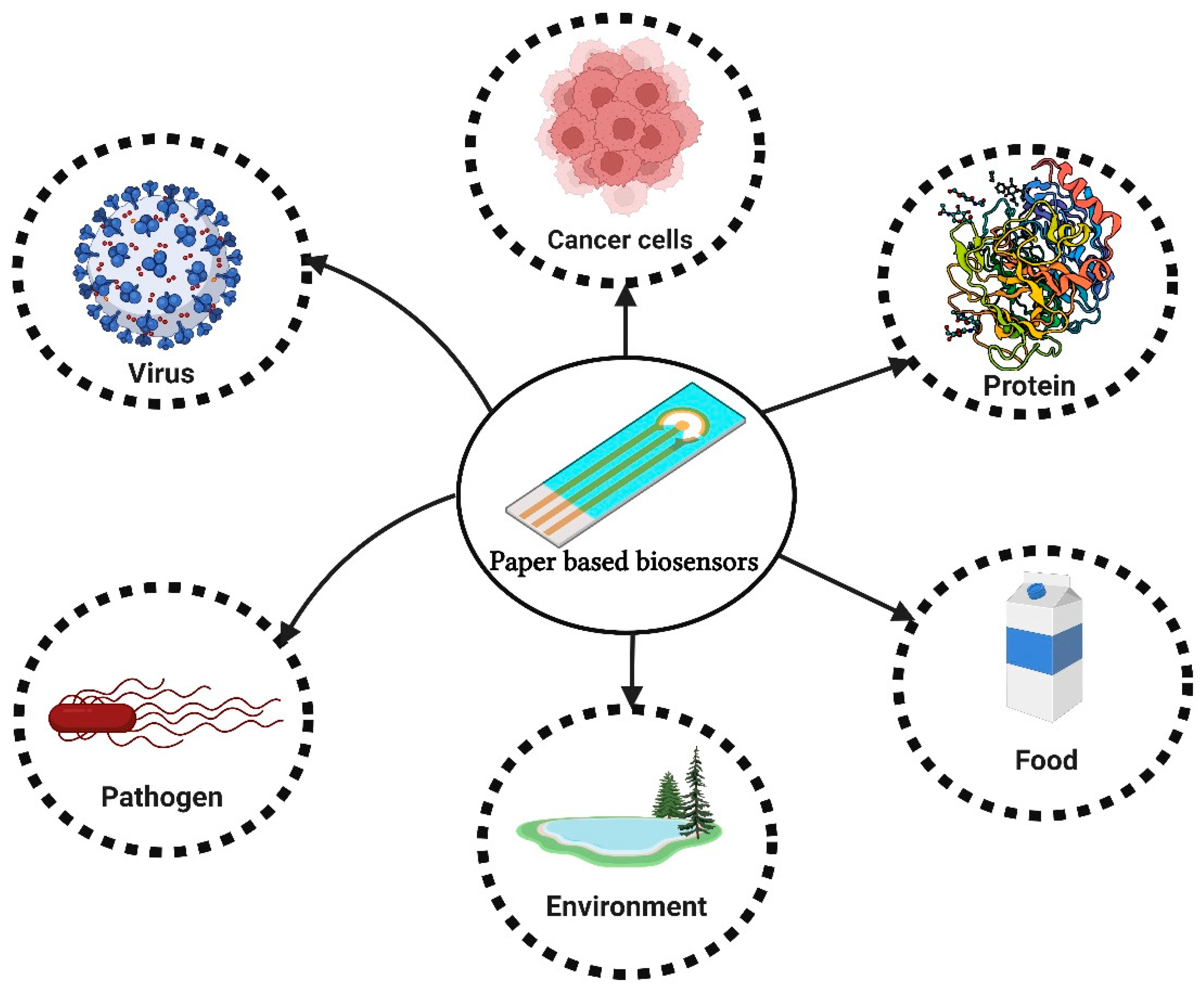
:1. Introduction
Paper-Based Biosensors
2. Design and Fabrication of Paper
2.1. Paper Types Used in Electroanalytical Devices
2.2. Fabrication of Paper-Based Electrodes
2.3. Electrochemical Biosensors
2.4. Improvement of ePAD Detection Performance by Nanomaterials
2.5. Designing and Fabricating 3D Paper Devices
2.6. Wearable Sensors
3. Applications
3.1. Clinical Analysis
Detection of Nucleic Acid
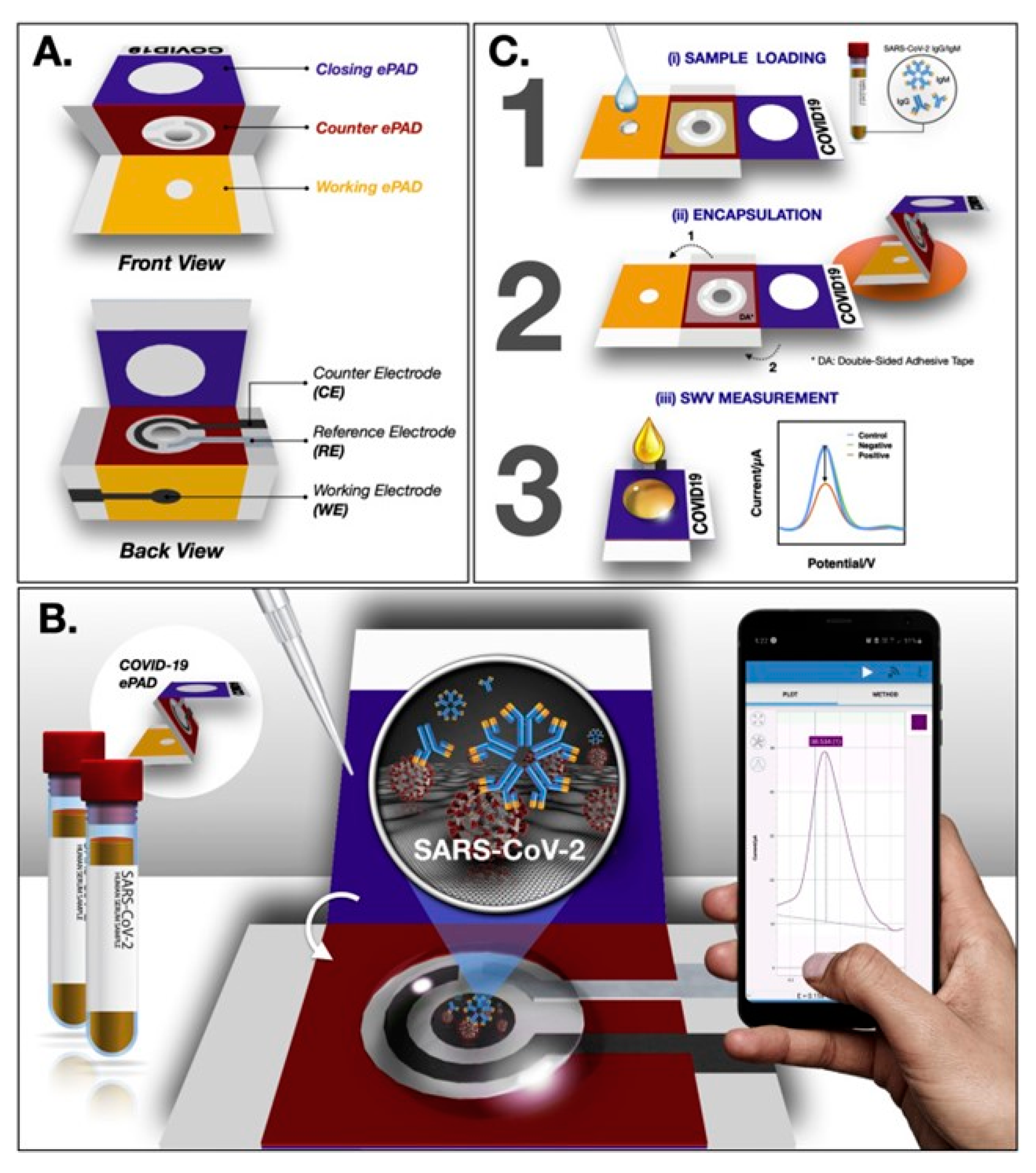
3.2. Detection of Viruses
COVID-19 Detection Using Paper-Based Biosensors
3.3. Biomarkers for Disease Detection
3.3.1. Detection of Cardiovascular Biomarkers
3.3.2. Detection of Cancer Biomarkers
3.3.3. Detection of Other Molecules/Macromolecules
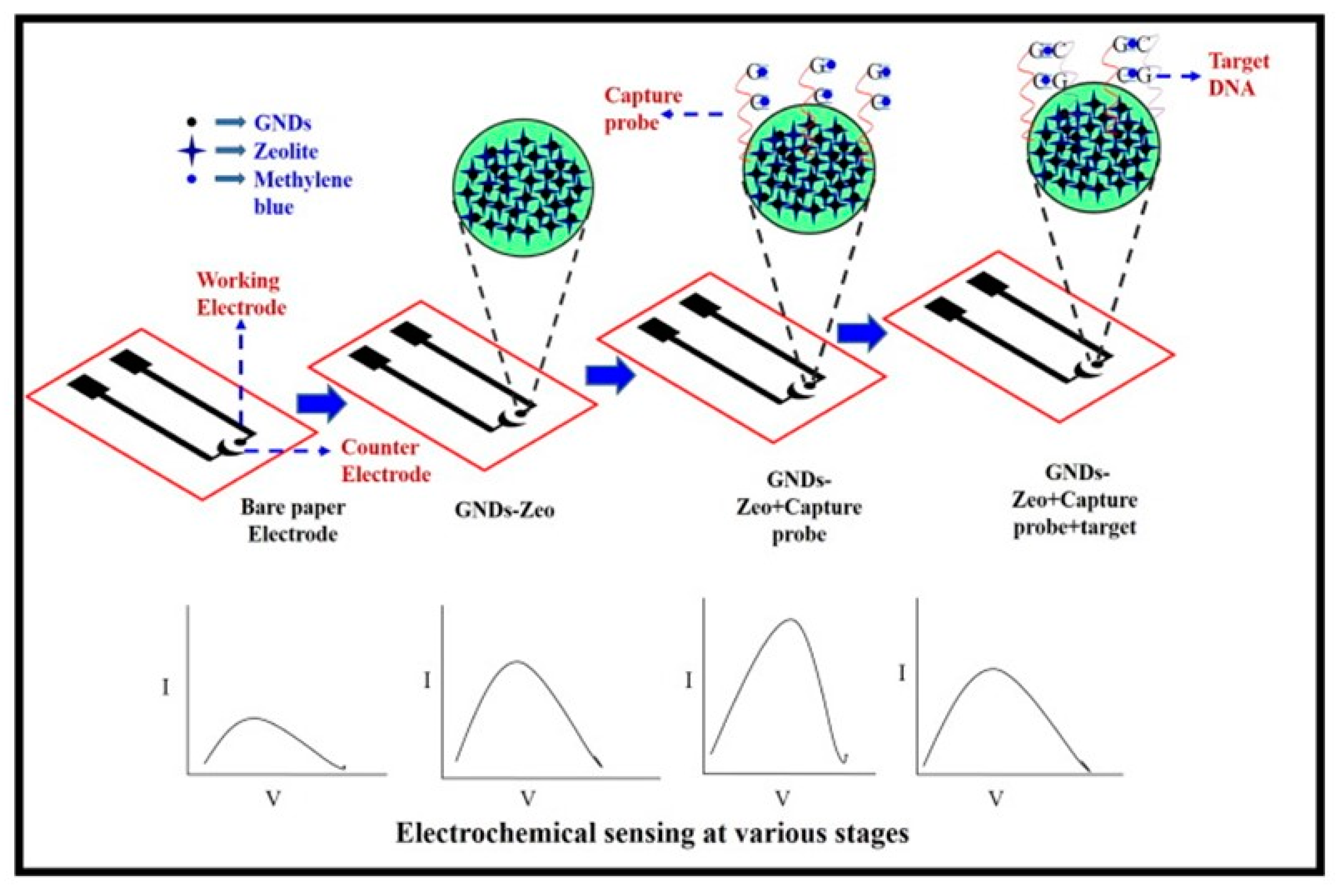
3.4. Detection of Genes
Viral RNA Testing
3.5. Point-of-Care ePADs for Neurotransmitter Monitoring
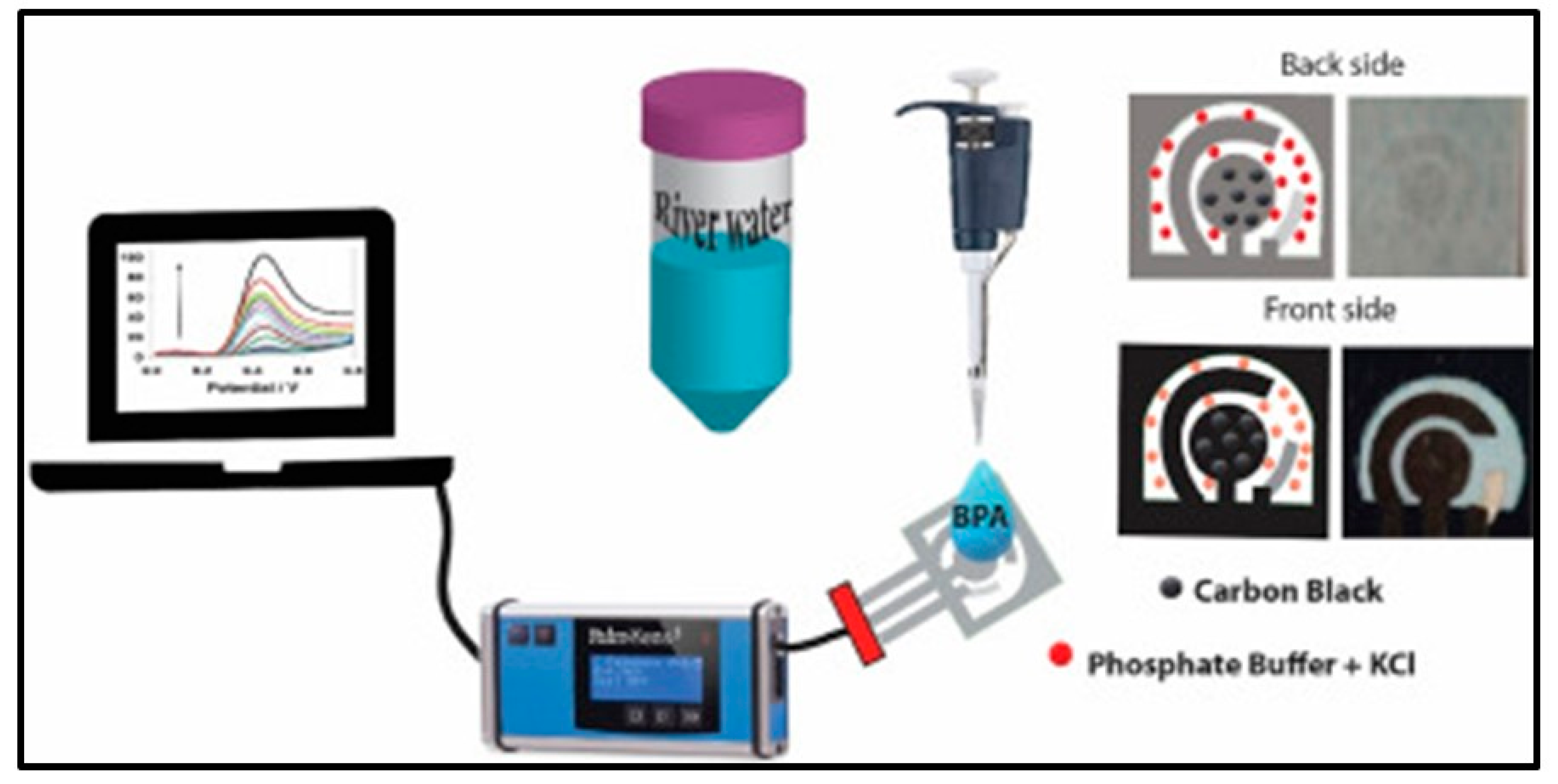
3.6. Environmental and Food Analysis
Humidity Sensors
3.7. Foodborne Pathogens
3.8. Paper-Based Electrochemical Microfluidic Devices
Integration of Electrochemical Cells in Paper-Based Analytical Devices
4. Carbon Paper
Limitations
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baharfar, M.; Rahbar, M.; Tajik, M.; Liu, G. Engineering Strategies for Enhancing the Performance of Electrochemical Paper-Based Analytical Devices. Biosens. Bioelectron. 2020, 167, 112506. [Google Scholar]
- Free, T.J.; Tucker, R.W.; Simonson, K.M.; Smith, S.A.; Lindgren, C.M.; Pitt, W.G.; Bundy, B.C. Engineering At-Home Dilution and Filtration Methods to Enable Paper-Based Colorimetric Biosensing in Human Blood with Cell-Free Protein Synthesis. Biosensors 2023, 13, 104. [Google Scholar] [CrossRef]
- Sher, M.; Zhuang, R.; Demirci, U.; Asghar, W. Paper-Based Analytical Devices for Clinical Diagnosis: Recent Advances in the Fabrication Techniques and Sensing Mechanisms. Expert Rev. Mol. Diagn. 2017, 17, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Calvo, A.; Fernández-Abedul, M.T.; Blanco-López, M.C.; Costa-García, A. Paper-Based Electrochemical Transducer Modified with Nanomaterials for Mercury Determination in Environmental Waters. Sens. Actuators B Chem. 2019, 290, 87–92. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-Based Electrochemical Biosensors for Food Safety Analysis. Biosensors 2022, 12, 1088. [Google Scholar] [PubMed]
- Solhi, E.; Hasanzadeh, M.; Babaie, P. Electrochemical Paper-Based Analytical Devices (EPADs) toward Biosensing: Recent Advances and Challenges in Bioanalysis. Anal. Methods 2020, 12, 1398–1414. [Google Scholar] [CrossRef]
- Antonacci, A.; Scognamiglio, V.; Mazzaracchio, V.; Caratelli, V.; Fiore, L.; Moscone, D.; Arduini, F. Paper-Based Electrochemical Devices for the Pharmaceutical Field: State of the Art and Perspectives. Front. Bioeng. Biotechnol. 2020, 8, 339. [Google Scholar]
- Valentine, C.J.; Takagishi, K.; Umezu, S.; Daly, R.; De Volder, M. Paper-Based Electrochemical Sensors Using Paper as a Scaffold to Create Porous Carbon Nanotube Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 30680–30685. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.-P.; Ku, C.-H.; Yang, R.-J. Porosity Estimation Using Electric Current Measurements for Paper-Based Microfluidics. Microfluid. Nanofluid. 2019, 23, 59. [Google Scholar] [CrossRef]
- Amor-Gutiérrez, O.; Costa-Rama, E.; Fernández-Abedul, M.T. Paper-Based Enzymatic Electrochemical Sensors for Glucose Determination. Sensors 2022, 22, 6232. [Google Scholar] [CrossRef]
- Shen, Y.; Tran, T.T.; Modha, S.; Tsutsui, H.; Mulchandani, A. A Paper-Based Chemiresistive Biosensor Employing Single-Walled Carbon Nanotubes for Low-Cost, Point-of-Care Detection. Biosens. Bioelectron. 2019, 130, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Sharma, A.; Jang, J. Vertical Flow-Based Paper Immunosensor for Rapid Electrochemical and Colorimetric Detection of Influenza Virus Using a Different Pore Size Sample Pad. Biosens. Bioelectron. 2019, 126, 36–43. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Dinh, T.; Foisal, A.R.M.; Nguyen, T.; Phan, H.P.; Dao, D.V.; Nguyen, N.T. Paper-Based Electronics Using Graphite and Silver Nanoparticles for Respiration Monitoring. IEEE Sens. J. 2019, 19, 11784–11790. [Google Scholar] [CrossRef]
- Guan, X.; Hou, Z.; Wu, K.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. Flexible Humidity Sensor Based on Modified Cellulose Paper. Sens. Actuators B Chem. 2021, 339, 129879. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Ziaie, B. Comparison of Direct and Indirect Laser Ablation of Metallized Paper for Inexpensive Paper-Based Sensors. ACS Appl. Mater. Interfaces 2018, 10, 36332–36341. [Google Scholar] [CrossRef]
- Thiyagarajan, K.; Rajini, G.K.; Maji, D. Flexible, Highly Sensitive Paper-Based Screen Printed MWCNT/PDMS Composite Breath Sensor for Human Respiration Monitoring. IEEE Sens. J. 2021, 21, 13985–13995. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Goh, C.-H.; Yap, K.Z.; Ramakrishnan, N. One-Step Fabrication of Paper-Based Inkjet-Printed Graphene for Breath Monitor Sensors. Biosensors 2023, 13, 209. [Google Scholar] [CrossRef]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of Paper-Based Devices for Reagentless Electrochemical (Bio)Sensor Strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Han, G.R.; Koo, H.J.; Ki, H.; Kim, M.G. Paper/Soluble Polymer Hybrid-Based Lateral Flow Biosensing Platform for High-Performance Point-of-Care Testing. ACS Appl. Mater. Interfaces 2020, 12, 34564–34575. [Google Scholar] [CrossRef]
- Ray, A.; Mohan, J.M.; Amreen, K.; Dubey, S.K.; Javed, A.; Ponnalagu, R.N.; Goel, S. Ink-Jet-Printed CuO Nanoparticle-Enhanced Miniaturized Paper-Based Electrochemical Platform for Hypochlorite Sensing. Appl. Nanosci. 2023, 13, 1855–1861. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Kefeni, K.K.; Mishra, S.B.; Nxumalo, E.N.; Ntsendwana, B. Recent Developments in Nanotechnology-Based Printing Electrode Systems for Electrochemical Sensors. Talanta 2021, 225, 121951. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Siangproh, W.; Khongchareonporn, N.; Ngamrojanavanich, N.; Chailapakul, O. Development of an Automated Wax-Printed Paper-Based Lateral Flow Device for Alpha-Fetoprotein Enzyme-Linked Immunosorbent Assay. Biosens. Bioelectron. 2018, 102, 27–32. [Google Scholar] [CrossRef]
- Ullah, Z.; Kainat, F.; Manzoor, S.; Liaquat, H.; Waheed, A.; Akhtar, S.; Rafiq, I.; Jafri, S.H.M.; Li, H.; Razaq, A. Natural Fibers and Zinc Hydroxystannate 3D Microspheres Based Composite Paper Sheets for Modern Bendable Energy Storage Application. J. Appl. Polym. Sci. 2023, 140, e53275. [Google Scholar] [CrossRef]
- Bhattacharya, G.; Fishlock, S.J.; Hussain, S.; Choudhury, S.; Xiang, A.; Kandola, B.; Pritam, A.; Soin, N.; Roy, S.S.; McLaughlin, J.A. Disposable Paper-Based Biosensors: Optimizing the Electrochemical Properties of Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2022, 14, 31109–31120. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Shamkhalichenar, H.; Choi, J.W. Inkjet-Printed and Paper-Based Electrochemical Sensors. Appl. Sci. 2018, 8, 288. [Google Scholar]
- Torrinha, Á.; Morais, S. Electrochemical (Bio)Sensors Based on Carbon Cloth and Carbon Paper: An Overview. TrAC Trends Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.Q.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in Paper-Based Point-of-Care Diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar]
- Fu, L.M.; Wang, Y.N. Detection Methods and Applications of Microfluidic Paper-Based Analytical Devices. TrAC—Trends Anal. Chem. 2018, 107, 196–211. [Google Scholar]
- Benjamin, S.R.S.R.; de Oliveira Neto, J.R.; de Macedo, I.Y.L.I.Y.L.; Bara, M.T.F.M.T.F.; da Cunha, L.C.L.C.; de Faria Carvalho, L.A.L.A.; de Souza Gil, E. Electroanalysis for Quality Control of Acerola (Malpighia Emarginata) Fruits and Their Commercial Products. Food Anal. Methods 2015, 8, 86–92. [Google Scholar] [CrossRef]
- Oliveira-Neto, J.R.; Rezende, S.G.; de Fátima Reis, C.; Benjamin, S.R.; Rocha, M.L.; de Souza Gil, E. Electrochemical Behavior and Determination of Major Phenolic Antioxidants in Selected Coffee Samples. Food Chem. 2016, 190, 506–512. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, S.R.; Ribeiro Júnior, E.J.M.; de Andrade, G.M.; Oriá, R.B. Zero-Dimensional Carbon Nanomaterials for Cancer Diagnosis. In Zero-Dimensional Carbon Nanomaterials; IOP Publishing: Bristol, UK, 2022; pp. 7–14. [Google Scholar]
- de Souza Nascimento, T.; Benjamin, S.R.; Costa de Assis, A.L.; Bezerra, J.R.; Gomes, J.M.P.; de Andrade, G.M.; Oriá, R.B. Zero-Dimensional Carbon Nanomaterials for Central Nervous System Diseases. In Zero-Dimensional Carbon Nanomaterials; IOP Publishing: Bristol, UK, 2022; pp. 9–21. [Google Scholar]
- Benjamin, S.R.; Miranda Ribeiro Júnior, E.J. Graphene Based Electrochemical Sensors for Detection of Environmental Pollutants. Curr. Opin. Environ. Sci. Health 2022, 29, 100381. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A Paper-Based Electrochemical Immunosensor with Reduced Graphene Oxide/Thionine/Gold Nanoparticles Nanocomposites Modification for the Detection of Cancer Antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhu, Q.; Wang, Z. The Recent Development of Nanomaterials Enhanced Paper-Based Electrochemical Analytical Devices. J. Electroanal. Chem. 2022, 909, 116140. [Google Scholar] [CrossRef]
- Eksin, E.; Torul, H.; Yarali, E.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-Based Electrode Assemble for Impedimetric Detection of MiRNA. Talanta 2021, 225, 122043. [Google Scholar] [CrossRef]
- Cinti, S.; Proietti, E.; Casotto, F.; Moscone, D.; Arduini, F. Paper-Based Strips for the Electrochemical Detection of Single and Double Stranded DNA. Anal. Chem. 2018, 90, 13680–13686. [Google Scholar] [CrossRef]
- Tran, V.K.; Ko, E.; Geng, Y.; Kim, M.K.; Jin, G.H.; Son, S.E.; Hur, W.; Seong, G.H. Micro-Patterning of Single-Walled Carbon Nanotubes and Its Surface Modification with Gold Nanoparticles for Electrochemical Paper-Based Non-Enzymatic Glucose Sensor. J. Electroanal. Chem. 2018, 826, 29–37. [Google Scholar] [CrossRef]
- Suntornsuk, W.; Suntornsuk, L. Recent Applications of Paper-based Point-of-care Devices for Biomarker Detection. Electrophoresis 2020, 41, 287–305. [Google Scholar] [CrossRef]
- de Oliveira, R.A.G.; Camargo, F.; Pesquero, N.C.; Faria, R.C. A Simple Method to Produce 2D and 3D Microfluidic Paper-Based Analytical Devices for Clinical Analysis. Anal. Chim. Acta 2017, 957, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-A.; Yuan, H.; Chen, C.-W.; Chien, Y.-S.; Sheng, W.-H.; Chen, C.-F. An Electricity- and Instrument-Free Infectious Disease Sensor Based on a 3D Origami Paper-Based Analytical Device. Lab Chip 2021, 21, 1908–1915. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, K.; Xu, F.; Guo, R.; Fang, A.; Sun, B.; Meng, X.; Liu, Y.; Bai, M. An Integrated Platform for Fibrinogen Quantification on a Microfluidic Paper-Based Analytical Device. Lab Chip 2020, 20, 2724–2734. [Google Scholar] [CrossRef]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Electrochemical Impedance-Based DNA Sensor Using Pyrrolidinyl Peptide Nucleic Acids for Tuberculosis Detection. Anal. Chim. Acta 2018, 1044, 102–109. [Google Scholar] [CrossRef]
- Deroco, P.B.; Wachholz Junior, D.; Kubota, L.T. Paper-based Wearable Electrochemical Sensors: A New Generation of Analytical Devices. Electroanalysis 2023, 35, e202200177. [Google Scholar] [CrossRef]
- Lin, P.-H.; Nien, H.-H.; Li, B.-R. Wearable Microfluidics for Continuous Assay. Annu. Rev. Anal. Chem. 2023, 16, 181–203. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, G.; Zhu, L.; Fei, Q.; Zhang, Z.; Chen, Z.; An, F.; Chen, Y.; Ling, Y.; Guo, P.; et al. Pencil-Paper on-Skin Electronics. Proc. Natl. Acad. Sci. USA 2020, 117, 18292–18301. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, C.; Li, L.; Zhang, C.; Liu, J.; Yu, H.D.; Huang, W. All Paper-Based Flexible and Wearable Piezoresistive Pressure Sensor. ACS Appl. Mater. Interfaces 2019, 11, 25034–25042. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Sumesh, C.K. WS 2 Nanosheet/Graphene Heterostructures for Paper-Based Flexible Photodetectors. ACS Appl. Nano Mater. 2020, 3, 6935–6944. [Google Scholar] [CrossRef]
- Hassanpour, S.; Hasanzadeh, M.; Saadati, A.; Shadjou, N.; Soleymani, J.; Jouyban, A. A Novel Paper Based Immunoassay of Breast Cancer Specific Carbohydrate (CA 15.3) Using Silver Nanoparticles-Reduced Graphene Oxide Nano-Ink Technology: A New Platform to Construction of Microfluidic Paper-Based Analytical Devices (ΜPADs) towards Biomedica. Microchem. J. 2019, 146, 345–358. [Google Scholar] [CrossRef]
- Jaewjaroenwattana, J.; Phoolcharoen, W.; Pasomsub, E.; Teengam, P.; Chailapakul, O. Electrochemical Paper-Based Antigen Sensing Platform Using Plant-Derived Monoclonal Antibody for Detecting SARS-CoV-2. Talanta 2023, 251, 123783. [Google Scholar] [CrossRef]
- Mao, X.; Li, Y.; Han, P.; Wang, X.; Yang, S.; Zhang, F.; Gong, X.; Cao, Y. One-Pot and One-Step Colorimetric Detection of Aminopeptidase N Activity Based on Gold Nanoparticles-Based Supramolecular Structure. Sens. Actuators B Chem. 2018, 267, 336–341. [Google Scholar] [CrossRef]
- Sun, X.; Jian, Y.; Wang, H.; Ge, S.; Yan, M.; Yu, J. Ultrasensitive Microfluidic Paper-Based Electrochemical Biosensor Based on Molecularly Imprinted Film and Boronate Affinity Sandwich Assay for Glycoprotein Detection. ACS Appl. Mater. Interfaces 2019, 11, 16198–16206. [Google Scholar] [CrossRef]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Feng, J.; Jin, D.; Zhu, A.; Xu, Q.; Hu, X. Paper-Based Electrochemical Immunosensor Device via Ni-Co MOF Nanosheet as a Peroxidase Mimic for the Label-Free Detection of Alpha-Fetoprotein. Sens. Actuators B Chem. 2022, 373, 132736. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Wang, H.; Han, Y.; Liu, J.; Lu, G.; Yu, H. A MXene-Functionalized Paper-Based Electrochemical Immunosensor for Label-Free Detection of Cardiac Troponin I. J. Semicond. 2021, 42, 092601. [Google Scholar] [CrossRef]
- Canhete de Moraes, N.; Marques Petroni, J.; de Lima, F.; Souza Ferreira, V.; Gabriel Lucca, B. Paper-Based Electrochemical Platform Modified with Graphene Nanoribbons: A New and Affordable Approach for Analysis of 5-Hydroxy-l-Tryptophan. Microchem. J. 2022, 183, 108030. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-Free Microfluidic Paper-Based Electrochemical Aptasensor for Ultrasensitive and Simultaneous Multiplexed Detection of Cancer Biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Ming, T.; Cheng, Y.; Xing, Y.; Luo, J.; Mao, G.; Liu, J.; Sun, S.; Kong, F.; Jin, H.; Cai, X. Electrochemical Microfluidic Paper-Based Aptasensor Platform Based on a Biotin-Streptavidin System for Label-Free Detection of Biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 46317–46324. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, C.; Ping, J. Flexible Freestanding Graphene Paper-Based Potentiometric Enzymatic Aptasensor for Ultrasensitive Wireless Detection of Kanamycin. Biosens. Bioelectron. 2019, 123, 178–184. [Google Scholar] [CrossRef]
- Khoshroo, A.; Fattahi, A.; Hosseinzadeh, L. Development of Paper-Based Aptasensor for Circulating Tumor Cells Detection in the Breast Cancer. J. Electroanal. Chem. 2022, 910, 116182. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, J.; Sun, S.; Ming, T.; Wang, Y.; Luo, J.; Xiao, G.; Li, X.; Xie, J.; Cai, X. New Electrochemical Method for Programmed Death-Ligand 1 Detection Based on a Paper-Based Microfluidic Aptasensor. Bioelectrochemistry 2021, 140, 107789. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Khajehsharifi, H.; Hajihosseini, S. Detection of Oxytetracycline Using an Electrochemical Label-Free Aptamer-Based Biosensor. Biosensors 2022, 12, 468. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Acedo, P. An Electrochemical Impedance Spectroscopy-Based Aptasensor for the Determination of SARS-CoV-2-RBD Using a Carbon Nanofiber–Gold Nanocomposite Modified Screen-Printed Electrode. Biosensors 2022, 12, 142. [Google Scholar] [CrossRef]
- Maier, D.; Laubender, E.; Basavanna, A.; Schumann, S.; Güder, F.; Urban, G.A.; Dincer, C. Toward Continuous Monitoring of Breath Biochemistry: A Paper-Based Wearable Sensor for Real-Time Hydrogen Peroxide Measurement in Simulated Breath. ACS Sens. 2019, 4, 2945–2951. [Google Scholar] [CrossRef]
- Nontawong, N.; Amatatongchai, M.; Wuepchaiyaphum, W.; Chairam, S.; Pimmongkol, S.; Panich, S.; Tamuang, S.; Jarujamrus, P. Fabrication of a Three-Dimensional Electrochemical Paper-Based Device (3D-EPAD) for Individual and Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Int. J. Electrochem. Sci. 2018, 13, 6940–6957. [Google Scholar] [CrossRef]
- Kammarchedu, V.; Butler, D.; Ebrahimi, A. A Machine Learning-Based Multimodal Electrochemical Analytical Device Based on EMoSx-LIG for Multiplexed Detection of Tyrosine and Uric Acid in Sweat and Saliva. Anal. Chim. Acta 2022, 1232, 340447. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Bagheri, N.; Chiara, F.; Fiore, L.; Moscone, D.; Roggero, S.; Arduini, F. A Smart Paper-Based Electrochemical Sensor for Reliable Detection of Iron Ions in Serum. Anal. Bioanal. Chem. 2023, 415, 1149–1157. [Google Scholar]
- Yamaoka, K.; Eguchi, J.; Uno, S. Potentiometric Glucose Detection by Paper-Based Electrochemical Sensor on CMOS Chip. TELKOMNIKA (Telecommun. Comput. Electron. Control) 2017, 15, 836. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Tahernia, M.; Choi, S. An Equipment-Free, Paper-Based Electrochemical Sensor for Visual Monitoring of Glucose Levels in Urine. SLAS Technol. 2019, 24, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Razavi, F.; Khajehsharifi, H. Paper-Based Electrochemical Sensor with a Commercial Glucose Meter to Determine L-Cysteine by Graphene Electrode and Ag-Doped Silica Nanoporous SBA-16. J. Iran. Chem. Soc. 2022, 19, 4349–4357. [Google Scholar] [CrossRef]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Henry, C.S.; Vilaivan, T.; Chailapakul, O. Electrochemical Paper-Based Peptide Nucleic Acid Biosensor for Detecting Human Papillomavirus. Anal. Chim. Acta 2017, 952, 32–40. [Google Scholar] [CrossRef]
- Pinheiro, T.; Cardoso, A.R.; Sousa, C.E.A.; Marques, A.C.; Tavares, A.P.M.; Matos, A.M.; Cruz, M.T.; Moreira, F.T.C.; Martins, R.; Fortunato, E.; et al. Paper-Based Biosensors for COVID-19: A Review of Innovative Tools for Controlling the Pandemic. ACS Omega 2021, 6, 29268–29290. [Google Scholar] [CrossRef]
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-Based Electrochemical Biosensor for Diagnosing COVID-19: Detection of SARS-CoV-2 Antibodies and Antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khan, S.A.; Rehman, A. Screen-Printed Graphene/Carbon Electrodes on Paper Substrates as Impedance Sensors for Detection of Coronavirus in Nasopharyngeal Fluid Samples. Diagnostics 2021, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.R.; RB Singh, K.; de Souza Nascimento, T.; Roque, C.R.; de Andrade, G.M.; Oriá, R.B. Nanobiosensors Potentialities for Monitoring SARS-CoV-2 in the Environment. In Nanobiosensors for Environmental Monitoring; Springer International Publishing: Cham, Switzerland, 2022; pp. 363–391. [Google Scholar]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.U.; Seo, S.B.; Jang, S.; Choi, J.; Lim, J.; Lee, D.K.; Kim, H.; Seo, S.; Kang, T.; Jung, J.; et al. Naked-Eye Detection of Pandemic Influenza a (PH1N1) Virus by Polydiacetylene (PDA)-Based Paper Sensor as a Point-of-Care Diagnostic Platform. Sens. Actuators B Chem. 2019, 291, 257–265. [Google Scholar] [CrossRef]
- Jin, Z.; Mantri, Y.; Retout, M.; Cheng, Y.; Zhou, J.; Jorns, A.; Fajtova, P.; Yim, W.; Moore, C.; Xu, M.; et al. A Charge-Switchable Zwitterionic Peptide for Rapid Detection of SARS-CoV-2 Main Protease. Angew. Chem. Int. Ed. 2022, 61, e202112995. [Google Scholar] [CrossRef]
- Vasantham, S.; Alhans, R.; Singhal, C.; Nagabooshanam, S.; Nissar, S.; Basu, T.; Ray, S.C.; Wadhwa, S.; Narang, J.; Mathur, A. Paper Based Point of Care Immunosensor for the Impedimetric Detection of Cardiac Troponin I Biomarker. Biomed. Microdevices 2020, 22, 6. [Google Scholar] [CrossRef]
- Boonkaew, S.; Jang, I.; Noviana, E.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Electrochemical Paper-Based Analytical Device for Multiplexed, Point-of-Care Detection of Cardiovascular Disease Biomarkers. Sens. Actuators B Chem. 2021, 330, 129336. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, K.; Kong, Q.; Zhang, L.; Ge, S.; Yu, J. A Self-Powered Origami Paper Analytical Device with a Pop-up Structure for Dual-Mode Electrochemical Sensing of ATP Assisted by Glucose Oxidase-Triggered Reaction. Biosens. Bioelectron. 2020, 148, 111839. [Google Scholar] [CrossRef]
- Lee, D.; Bhardwaj, J.; Jang, J. Paper-Based Electrochemical Immunosensor for Label-Free Detection of Multiple Avian Influenza Virus Antigens Using Flexible Screen-Printed Carbon Nanotube-Polydimethylsiloxane Electrodes. Sci. Rep. 2022, 12, 2311. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Yarali, E.; Eksin, E.; Ganguly, A.; Benson, J.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-Based Electrochemical Biosensors for Voltammetric Detection of MiRNA Biomarkers Using Reduced Graphene Oxide or MoS2 Nanosheets Decorated with Gold Nanoparticle Electrodes. Biosensors 2021, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gulati, S.; Ansari, A.H.; Phutela, R.; Acharya, S.; Azhar, M.; Murthy, J.; Kathpalia, P.; Kanakan, A.; Maurya, R.; et al. FnCas9-Based CRISPR Diagnostic for Rapid and Accurate Detection of Major SARS-CoV-2 Variants on a Paper Strip. Elife 2021, 10, e67130. [Google Scholar] [CrossRef]
- He, C.; Lin, C.; Mo, G.; Xi, B.; Li, A.; Huang, D.; Wan, Y.; Chen, F.; Liang, Y.; Zuo, Q.; et al. Rapid and Accurate Detection of SARS-CoV-2 Mutations Using a Cas12a-Based Sensing Platform. Biosens. Bioelectron. 2022, 198, 113857. [Google Scholar] [CrossRef] [PubMed]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, Ultrasensitive, and Quantitative Detection of SARS-CoV-2 Using Antisense Oligonucleotides Directed Electrochemical Biosensor Chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef]
- Shen, H.; Song, E.; Wang, Y.; Meng, L.; Dong, J.; Lin, B.; Huang, D.; Guan, Z.; Yang, C.; Zhu, Z. In Situ Raman Enhancement Strategy for Highly Sensitive and Quantitative Lateral Flow Assay. Anal. Bioanal. Chem. 2022, 414, 507–513. [Google Scholar] [CrossRef]
- Tegegne, W.A.; Su, W.N.; Beyene, A.B.; Huang, W.H.; Tsai, M.C.; Hwang, B.J. Flexible Hydrophobic Filter Paper-Based SERS Substrate Using Silver Nanocubes for Sensitive and Rapid Detection of Adenine. Microchem. J. 2021, 168, 106349. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-Based CRISPR/Cas Assay on Microfluidic Paper Analytical Devices for Supersensitive Detection of Pathogenic Bacteria in Foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar] [CrossRef]
- Hariharan, A.; Chelli, S.M.; Muthukumar, V.S.; Belliraj, S.K.; Ferrari, M.; Krishna Narasimha, N.; Vishnubhatla, K.C. Paper-Microfluidics Based SERS Substrate for PPB Level Detection of Catechol. Opt. Mater. 2019, 94, 305–310. [Google Scholar] [CrossRef]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-Sampling Paper-Based Analytical Device for Colorimetric/Surface-Enhanced Raman Scattering Dual Sensing of Sulfur Dioxide in Wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef]
- Ying, B.; Park, S.; Chen, L.; Dong, X.; Young, E.W.K.; Liu, X. NanoPADs and NanoFACEs: An Optically Transparent Nanopaper-Based Device for Biomedical Applications. Lab Chip 2020, 20, 3322–3333. [Google Scholar] [CrossRef] [PubMed]
- Siebe, H.S.; Chen, Q.; Li, X.; Xu, Y.; Browne, W.R.; Bell, S.E.J. Filter Paper Based SERS Substrate for the Direct Detection of Analytes in Complex Matrices. Analyst 2021, 146, 1281–1288. [Google Scholar] [CrossRef]
- Settu, K.; Huang, Y.-M.; Zhou, S.-X. A Facile Approach for the Electrochemical Sensing of Dopamine Using Paper-Based PEDOT:PSS/RGO Graphene Biosensor. ECS J. Solid State Sci. Technol. 2020, 9, 121002. [Google Scholar] [CrossRef]
- Nantaphol, S.; Kava, A.A.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Janus Electrochemistry: Simultaneous Electrochemical Detection at Multiple Working Conditions in a Paper-Based Analytical Device. Anal. Chim. Acta 2019, 1056, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ji, Y.; Li, H.; Wang, Z.; Shi, L.; Zhang, S.; Wang, T.; Gong, Z. Recent Advances of Environmental Pollutants Detection via Paper-based Sensing Strategy. Luminescence 2021, 36, 1818–1836. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Nguyen, T.-A.; Tran, A.Q.; Bagheri, H. Optoelectronic Nose Based on an Origami Paper Sensor for Selective Detection of Pesticide Aerosols. Sci. Rep. 2020, 10, 17302. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami Multiple Paper-Based Electrochemical Biosensors for Pesticide Detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef]
- Colozza, N.; Kehe, K.; Dionisi, G.; Popp, T.; Tsoutsoulopoulos, A.; Steinritz, D.; Moscone, D.; Arduini, F. A Wearable Origami-like Paper-Based Electrochemical Biosensor for Sulfur Mustard Detection. Biosens. Bioelectron. 2019, 129, 15–23. [Google Scholar] [CrossRef]
- Wang, C.; Wu, R.; Ling, H.; Zhao, Z.; Han, W.; Shi, X.; Payne, G.F.; Wang, X. Toward Scalable Fabrication of Electrochemical Paper Sensor without Surface Functionalization. npj Flex. Electron. 2022, 6, 12. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a Paper-Based, Inexpensive, and Disposable Electrochemical Sensing Platform for Nitrite Detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- An, Y.; Wang, W.; Lv, Q.; Zhang, Q.; Wang, X. A Dual-Readout Paper-Based Analytical Device for the Simultaneous Determination of Hexavalent Cr and Total Cr. Microchim. Acta 2022, 189, 445. [Google Scholar] [CrossRef]
- Jemmeli, D.; Marcoccio, E.; Moscone, D.; Dridi, C.; Arduini, F. Highly Sensitive Paper-Based Electrochemical Sensor for Reagent Free Detection of Bisphenol A. Talanta 2020, 216, 120924. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.L.; dos Santos, D.M.; Soares, A.C.; Mattoso, L.H.C.; Oliveira, O.N.; Correa, D.S. Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1. Chemosensors 2020, 8, 87. [Google Scholar] [CrossRef]
- Fu, E.; Downs, C. Progress in the Development and Integration of Fluid Flow Control Tools in Paper Microfluidics. Lab Chip 2017, 17, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a Paper-Based Electrochemical Immunosensor Using an Antibody-Single Walled Carbon Nanotubes Bio-Conjugate Modified Electrode for Label-Free Detection of Foodborne Pathogens. Sens. Actuators B Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Mathur, A.; Gupta, R.; Kondal, S.; Wadhwa, S.; Pudake, R.N.; Shivani; Kansal, R.; Pundir, C.S.; Narang, J. A New Tactics for the Detection of S. aureus via Paper Based Geno-Interface Incorporated with Graphene Nano Dots and Zeolites. Int. J. Biol. Macromol. 2018, 112, 364–370. [Google Scholar] [CrossRef]
- Yin, M.; Liu, C.; Ge, R.; Fang, Y.; Wei, J.; Chen, X.; Chen, Q.; Chen, X. Paper-Supported near-Infrared-Light-Triggered Photoelectrochemical Platform for Monitoring Escherichia coli O157:H7 Based on Silver Nanoparticles-Sensitized-Upconversion Nanophosphors. Biosens. Bioelectron. 2022, 203, 114022. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Almeida, C.M.R.; Magalhães, J.M.C.S.; Gonçalves, M.P.; Freire, C.; Delerue-Matos, C. Development of a Disposable Paper-Based Potentiometric Immunosensor for Real-Time Detection of a Foodborne Pathogen. Biosens. Bioelectron. 2019, 141, 111317. [Google Scholar] [CrossRef]
- Bagheri Pebdeni, A.; Hosseini, M. Fast and Selective Whole Cell Detection of Staphylococcus aureus Bacteria in Food Samples by Paper Based Colorimetric Nanobiosensor Using Peroxidase-like Catalytic Activity of DNA-Au/Pt Bimetallic Nanoclusters. Microchem. J. 2020, 159, 105475. [Google Scholar] [CrossRef]
- Anushka; Bandopadhyay, A.; Das, P.K. Paper Based Microfluidic Devices: A Review of Fabrication Techniques and Applications. Eur. Phys. J. Spec. Top. 2022, 232, 781–815. [Google Scholar] [CrossRef]
- Cao, L.; Han, G.-C.; Xiao, H.; Chen, Z.; Fang, C. A Novel 3D Paper-Based Microfluidic Electrochemical Glucose Biosensor Based on RGO-TEPA/PB Sensitive Film. Anal. Chim. Acta 2020, 1096, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Liu, X. A Paper-Based Microfluidic Biosensor Integrating Zinc Oxide Nanowires for Electrochemical Glucose Detection. Microsyst. Nanoeng. 2015, 1, 15014. [Google Scholar] [CrossRef] [Green Version]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; PN, A.K.; Pundir, C.S. Detection of Alprazolam with a Lab on Paper Economical Device Integrated with Urchin like Ag@ Pd Shell Nano-Hybrids. Mater. Sci. Eng. C 2017, 80, 728–735. [Google Scholar] [CrossRef]
- Ozer, T.; Henry, C.S. Paper-Based Analytical Devices for Virus Detection: Recent Strategies for Current and Future Pandemics. TrAC Trends Anal. Chem. 2021, 144, 116424. [Google Scholar] [CrossRef]
- Sun, S.; Luo, J.; Zhu, Y.; Kong, F.; Mao, G.; Ming, T.; Xing, Y.; Liu, J.; Dai, Y.; Yan, S.; et al. Multifunctional Self-Driven Origami Paper-Based Integrated Microfluidic Chip to Detect CRP and PAB in Whole Blood. Biosens. Bioelectron. 2022, 208, 114225. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon Black as an Outstanding and Affordable Nanomaterial for Electrochemical (Bio)Sensor Design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef]
- Lettieri, S.; Battaglino, B.; Sacco, A.; Saracco, G.; Pagliano, C. A Green and Easy-to-Assemble Electrochemical Biosensor Based on Thylakoid Membranes for Photosynthetic Herbicides Detection. Biosens. Bioelectron. 2022, 198, 113838. [Google Scholar] [CrossRef]
| Type of Paper Substrate | Sensing Material | Fabrication Method | RH Range (%) | Sensitivity | Reference |
|---|---|---|---|---|---|
| Filter paper (Whatman 4) | Carbon black (CB) and Reduced graphene oxide (rGO) | Coating and drying | 33–95% | 0.7 (33−75%) 1.5 (75−95%) | [13] |
| Printing paper | Polyimide | Laser writing | 0–90% | - | [14] |
| Cellulose filter paper | Cobalt chloride | Soaking and drying | 11–98% | - | [15] |
| A4 printing paper | A4 printing paper | Facile pasting | 7.2–91% | - | [16] |
| A4 porous paper (metallic pearl) | Graphite and silver nanoparticles | Screen printing and pencil drawing technique | 70–95% | 0.0564% | [17] |
| Printing paper | Glycidyl trimethyl Ammonium chloride (EPTAC) | Screen printing | 11–95% | 1.59 (54 RH%) 63.7 (95 RH%) | [15] |
| Metalized paper (aluminum-coated paper) | Polymeric layer | Laser ablation | 2–85% | 18.9 fF/%RH | [16] |
| Cellulose paper | Carbon nanotube and Polydimethy siloxane composite | Screen printing | 30–95% | 0.375 pF/RH% (30–70%) 8.24 pF/RH% (70–95%) | [17] |
| Glossy paper | Graphene printing ink | Inkjet printing | 40–70% | 0.03 pF/RH% | [18] |
| S.NO | Target | Material | Detection Methods | Linear Range | LOD | References |
|---|---|---|---|---|---|---|
| Electrochemical immunosensor | ||||||
| 1. | CA 15.3 | Ag-RGO/CysA-Au NPs | ChA | 15–125 U/mL | 15 U/mL | [51] |
| 2. | CA 125 | rGO/Thi/AuNPs | CV, DPV | 0.1~200 U/mL | 0.01 U/mL | [36] |
| 3. | Spike protein of SARS-CoV-2 | CNC-SPGE | DPV | 0.1 pg/mL to 500 ng/mL | 2.0 fg/mL | [52] |
| 4. | Human IFN-γ | PANI/G | EIS | 5–1000 pg/mL | 3.4 pg/mL | [53] |
| 5. | Glycoprotein | SiO2@Au/dsDNA/CeO2 | DPV | 1 pg/mL–1000 ng/mL | 0.87 pg/mL | [54] |
| 6. | Alpha-fetoprotein | Ni-Co MOF | DPV | 1–200 ng/mL | 0.3 ng/mL | [55] |
| 7. | Cardiac troponin I | MXene | EIS | 5–100 ng/mL | 0.58 ng/mL | [56] |
| 8. | 5-hydroxy-l-tryptophan | Graphene nanoribbons | SWV | 25–1000 μmol/L | 7.6 μmol/L | [57] |
| Aptasensor | ||||||
| 9. | Carcinoembryonic antigen | NG-THI-AuNPs-PB-PEDOT | DPV | 0.01–500 ng/mL | 2 pg/mL | [58] |
| 10. | 17β-Estradiol | Amino redox graphene/thionine/streptavidin-modified gold nanoparticles/chitosan | DPV | 10 pg/mL to 100 ng/mL | 10 pg/mL | [59] |
| 11. | Kanamycin | Graphene | Potentiometry | 0.05–30 pM | 0.05 pM | [60] |
| 12. | Breast cancer cells (MCF-7) | Paper-based aptasensor and gold nanoparticles | CV, DPV and EIS | 20 to 1 × 106 cells mL−1 | 7 cells mL−1 | [61] |
| 13. | Programmed death-ligand 1 | Amino carbon nanotubes/methylene blue | CV, DPV | 10 pg/mL–2.5 ng/mL | 10 pg/mL | [62] |
| 14. | Oxytetracycline | Multiwall carbon nanotubes, gold nanoparticles, reduced graphene oxide and chitosan | CV, DPV and EIS | 1.00–540 nM | 10 nM | [63] |
| 15. | SARS-CoV-2 | Carbon nanofibers and gold nanoparticles | EIS | 0.01–64 nM | 7.0 pM | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamin, S.R.; de Lima, F.; Nascimento, V.A.d.; de Andrade, G.M.; Oriá, R.B. Advancement in Paper-Based Electrochemical Biosensing and Emerging Diagnostic Methods. Biosensors 2023, 13, 689. https://doi.org/10.3390/bios13070689
Benjamin SR, de Lima F, Nascimento VAd, de Andrade GM, Oriá RB. Advancement in Paper-Based Electrochemical Biosensing and Emerging Diagnostic Methods. Biosensors. 2023; 13(7):689. https://doi.org/10.3390/bios13070689
Chicago/Turabian StyleBenjamin, Stephen Rathinaraj, Fábio de Lima, Valter Aragão do Nascimento, Geanne Matos de Andrade, and Reinaldo Barreto Oriá. 2023. "Advancement in Paper-Based Electrochemical Biosensing and Emerging Diagnostic Methods" Biosensors 13, no. 7: 689. https://doi.org/10.3390/bios13070689





