A Facile Method for the Fabrication of the Microneedle Electrode and Its Application in the Enzymatic Determination of Glutamate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus
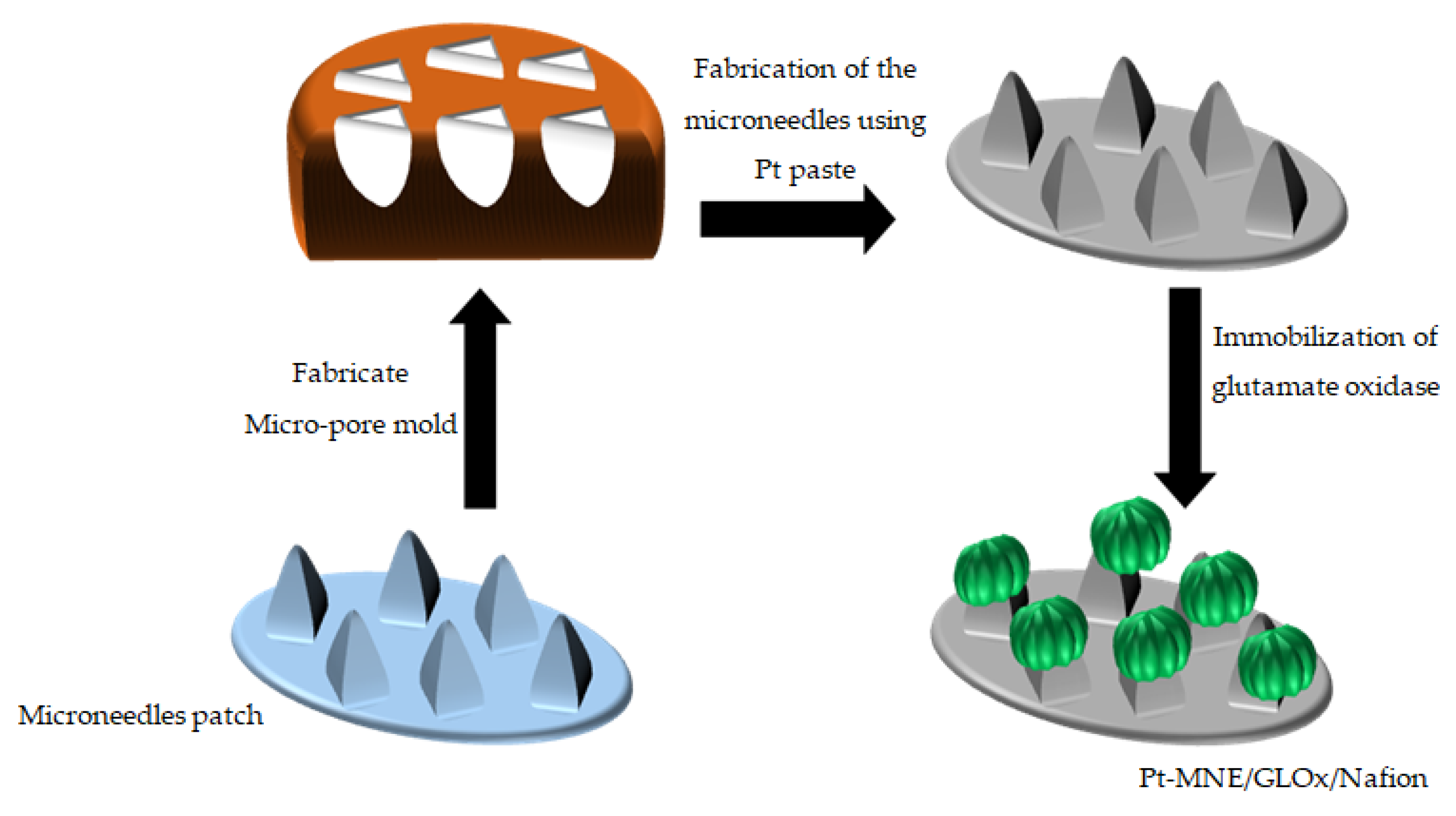
2.3. Fabrication of the Micro-Pore Mold (MPM)
2.4. Fabrication of the Pt-MNE
2.5. Fabrication of the Biosensor
3. Results and Discussion
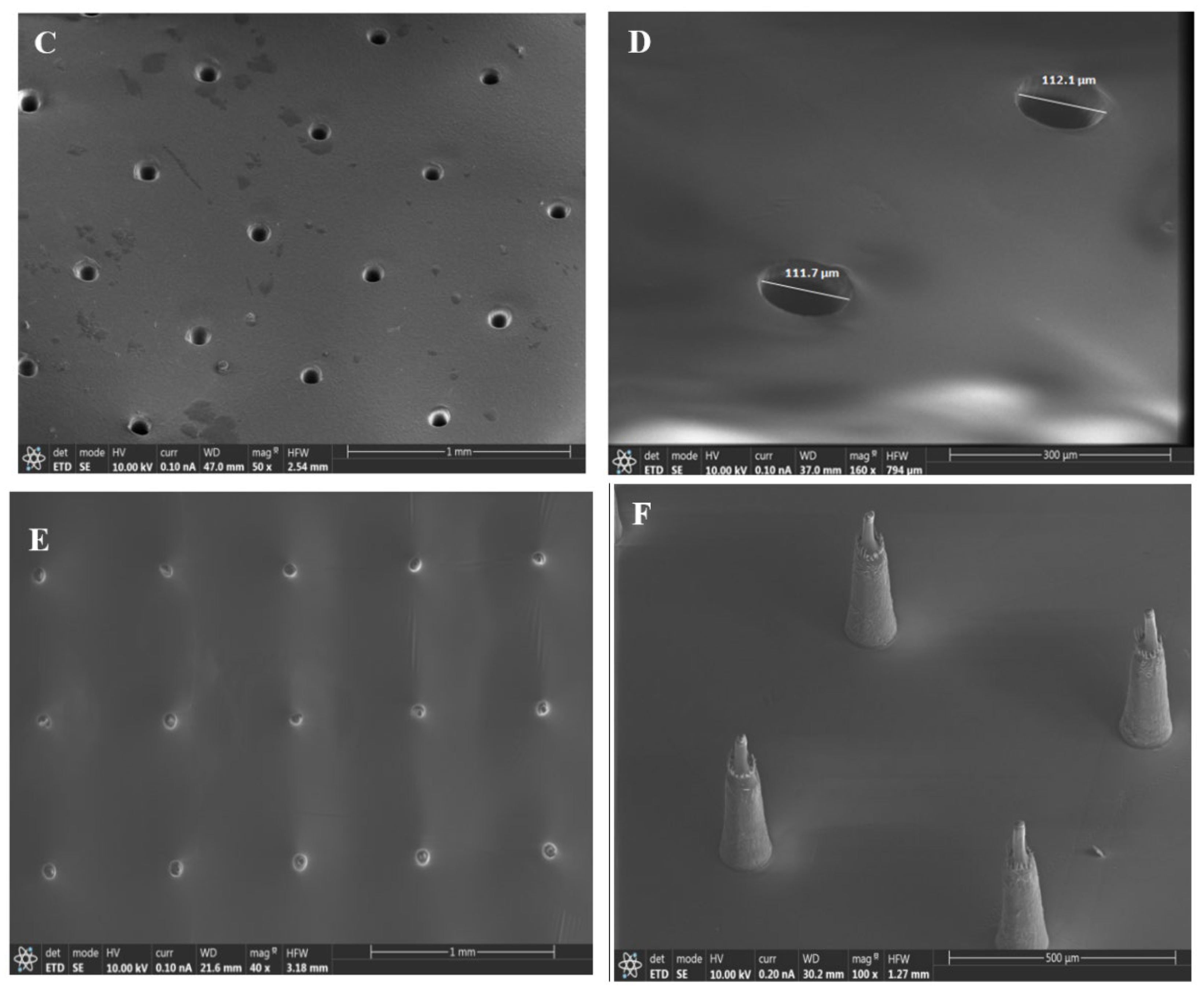
3.1. Surface Characterization of the Samples
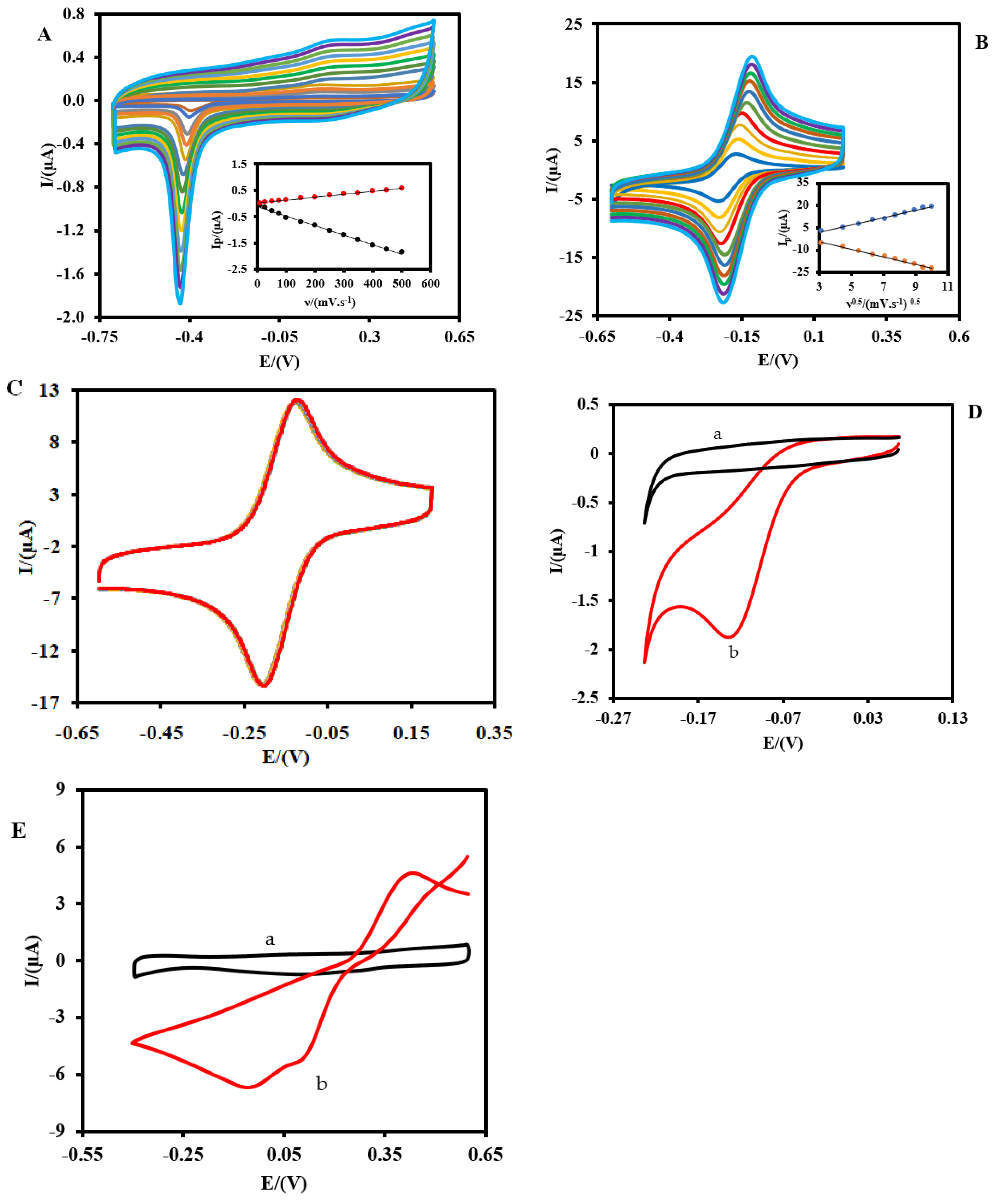
3.2. Electrochemical Behavior of Pt-MNE
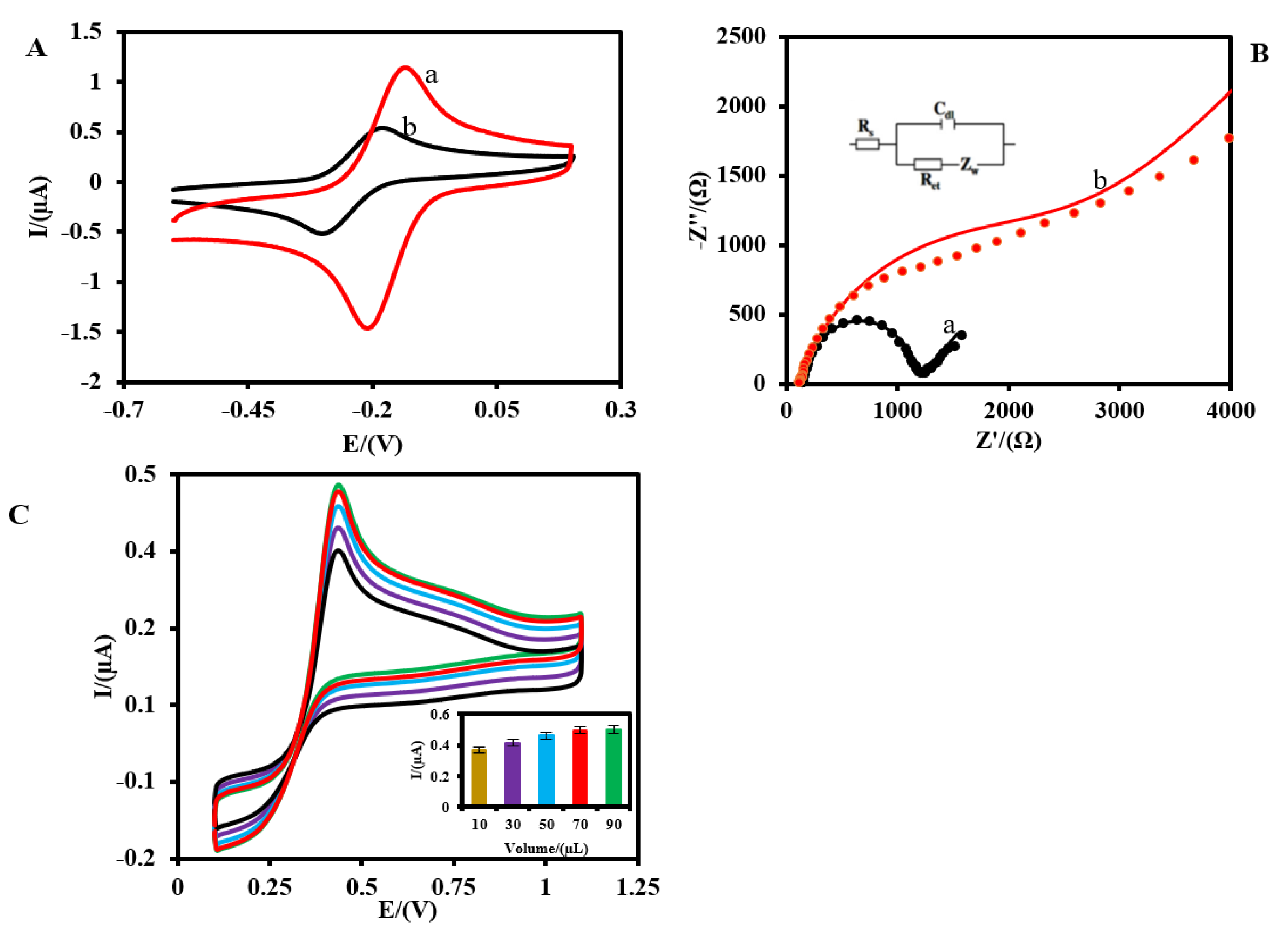
3.3. The Electrochemical Performance of Pt-MNE/GLOx/Nafion
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [PubMed]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Dardano, P.; Rea, I.; De Stefano, L. Microneedles-based electrochemical sensors: New tools for advanced biosensing. Curr. Opin. Electrochem. 2019, 17, 121–127. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Cai, R.; Zheng, H.; Yu, J.; Zhang, Y.; Gu, Z. Microneedle-based transdermal detection and sensing devices. Lab Chip 2023, 23, 869–887. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-based wearable electrochemical devices: A review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Miller, P.R.; Narayan, R.J.; Polsky, R. Microneedle-based sensors for medical diagnosis. J. Mater. Chem. B 2016, 4, 1379–1383. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Weng, Z.; Sun, H.; Nistala, R.; Zhang, Y. Microneedle-based potentiometric sensing system for continuous monitoring of multiple electrolytes in skin interstitial fluids. ACS Sens. 2021, 6, 2181–2190. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Hung, V.W.S.; Jia, W.; Valdés-Ramírez, G.; Windmiller, J.R.; Martinez, A.G.; Ramírez, J.; Chan, G.; Kerman, K.; Wang, J. Tattoo-based potentiometric ion-selective sensors for epidermal ph monitoring. Analyst 2013, 138, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. TrAC Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 2022, 249, 123695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Yang, Y.; Zhao, Z.Q.; Guo, X.D. A gold nanoparticles deposited polymer microneedle enzymatic biosensor for glucose sensing. Electrochim. Acta 2020, 358, 136917. [Google Scholar] [CrossRef]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef]
- Mohan, A.M.V.; Windmiller, J.R.; Mishra, R.K.; Wang, J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens. Bioelectron. 2017, 91, 574–579. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, M.; Voelcker, N.H. Gold microneedles fabricated by casting of gold ink used for urea sensing. Mater. Lett. 2019, 243, 50–53. [Google Scholar] [CrossRef]
- Wu, Y.; Tehrani, F.; Teymourian, H.; Mack, J.; Shaver, A.; Reynoso, M.; Kavner, J.; Huang, N.; Furmidge, A.; Duvvuri, A.; et al. Microneedle aptamer-based sensors for continuous, real-time therapeutic drug monitoring. Anal. Chem. 2022, 94, 8335–8345. [Google Scholar] [CrossRef]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: Toward parkinson management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Mishra, R.K.; Vinu Mohan, A.M.; Soto, F.; Chrostowski, R.; Wang, J. A microneedle biosensor for minimally-invasive transdermal detection of nerve agents. Analyst 2017, 142, 918–924. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 2020, 92, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Lai, L.; Li, Y. Preparation of microneedle array mold based on mems lithography technology. Micromachines 2021, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, T.; Chujo, K.; Miyamoto, K.; Okida, M.; Nakamura, K.; Aoki, N.; Morita, R. Metal microneedle fabrication using twisted light with spin. Opt. Express 2010, 18, 17967–17973. [Google Scholar] [CrossRef]
- Mansoor, I.; Liu, Y.; Häfeli, U.O.; Stoeber, B. Arrays of hollow out-of-plane microneedles made by metal electrodeposition onto solvent cast conductive polymer structures. J. Micromech. Microeng. 2013, 23, 085011. [Google Scholar] [CrossRef]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef]
- Silvestre, S.L.; Araújo, D.; Marques, A.C.; Pires, C.; Matos, M.; Alves, V.; Martins, R.; Freitas, F.; Reis, M.A.M.; Fortunato, E. Microneedle arrays of polyhydroxyalkanoate by laser-based micromolding technique. ACS Appl. Bio. Mater. 2020, 3, 5856–5864. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, D.D.; Chen, Y.; Guo, X.D. A fabrication method of microneedle molds with controlled microstructures. Mater. Sci. Eng. C 2016, 65, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3d printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Sharma, S. Chapter fifteen—Microneedle enzyme sensor arrays for continuous in vivo monitoring. In Methods in Enzymology; Thompson, R.B., Fierke, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 589, pp. 413–427. [Google Scholar]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [PubMed]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in parkinson’s disease: The role of glial cells. J. Pharm. Sci. 2020, 144, 151–164. [Google Scholar]
- Wang, R.; Reddy, P.H. Role of glutamate and nmda receptors in alzheimer’s disease. J.Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar]
- Wu, C.; Barkova, D.; Komarova, N.; Offenhäusser, A.; Andrianova, M.; Hu, Z.; Kuznetsov, A.; Mayer, D. Highly selective and sensitive detection of glutamate by an electrochemical aptasensor. Anal. Bioanal. Chem. 2022, 414, 1609–1622. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Gao, Y.; Gao, H.; Deng, L.; Gui, Q.; Cao, Z.; Yin, Y.; Feng, Z. A peptide aptamer based electrochemical amperometric sensor for sensitive l-glutamate detection. Bioelectrochemistry 2022, 146, 108165. [Google Scholar] [CrossRef]
- Wang, Y.; Mishra, D.; Bergman, J.; Keighron, J.D.; Skibicka, K.P.; Cans, A.-S. Ultrafast glutamate biosensor recordings in brain slices reveal complex single exocytosis transients. ACS Chem. Neurosci. 2019, 10, 1744–1752. [Google Scholar] [CrossRef]
- Özel, R.E.; Ispas, C.; Ganesana, M.; Leiter, J.C.; Andreescu, S. Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments. Biosens. Bioelectron. 2014, 52, 397–402. [Google Scholar] [CrossRef]
- Yang, X.-K.; Zhang, F.-L.; Wu, W.-T.; Tang, Y.; Yan, J.; Liu, Y.-L.; Amatore, C.; Huang, W.-H. Quantitative nano-amperometric measurement of intravesicular glutamate content and its sub-quantal release by living neurons. Angew. Chem. Int. Ed. 2021, 60, 15803–15808. [Google Scholar] [CrossRef]
- Bermingham, K.P.; Doran, M.M.; Bolger, F.B.; Lowry, J.P. Design optimisation and characterisation of an amperometric glutamate oxidase-based composite biosensor for neurotransmitter l-glutamic acid. Anal. Chim. Acta 2022, 1224, 340205. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Valdés-Ramírez, G.; Zhou, N.; Zhou, M.; Miller, P.R.; Jin, C.; Brozik, S.M.; Polsky, R.; Katz, E.; Narayan, R.; et al. Bicomponent microneedle array biosensor for minimally-invasive glutamate monitoring. Electroanalysis 2011, 23, 2302–2309. [Google Scholar] [CrossRef]
- Benveniste, H.; Drejer, J.; Schousboe, A.; Diemer, N.H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 1984, 43, 1369–1374. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, Y.G. Homeostasis of the intraparenchymal-blood glutamate concentration gradient: Maintenance, imbalance, and regulation. Front. Mol. Neurosci. 2017, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, H.; Kim, I.J.; Kim, J.R.; Lee, J.I.; Ree, M. Bacterial adhesion, cell adhesion and biocompatibility of nafion films. J. Biomater. Sci. Polym. Ed. 2009, 20, 1687–1707. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.F.B.; Harrison, D.J.; Rojotte, R.V. Preliminary in vivo biocompatibility studies on perfluorosulphonic acid polymer membranes for biosensor applications. Biomaterials 1991, 12, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.M.; Belzer, T. An amperometric glucose biosensor composed of prussian blue, nafion, and glucose oxidase studied by flow injection analysis. J. Chem. Educ. 2023, 100, 760–766. [Google Scholar] [CrossRef]
- Haghighi, B.; Tabrizi, M.A. Direct electron transfer from glucose oxidase immobilized on a nano-porous glassy carbon electrode. Electrochim. Acta 2011, 56, 10101–10106. [Google Scholar] [CrossRef]
- Lee, W.; Jeong, S.-H.; Lim, Y.-W.; Lee, H.; Kang, J.; Lee, H.; Lee, I.; Han, H.-S.; Kobayashi, S.; Tanaka, M.; et al. Conformable microneedle ph sensors via the integration of two different siloxane polymers for mapping peripheral artery disease. Sci. Adv. 2021, 7, eabi6290. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, Z.; Jiang, F.; Zheng, Z.; Lu, S. Silk/polyols/god microneedle based electrochemical biosensor for continuous glucose monitoring. RSC Adv. 2020, 10, 6163–6171. [Google Scholar] [CrossRef]
- Prass, S.; St-Pierre, J.; Klingele, M.; Friedrich, K.A.; Zamel, N. Hydrogen oxidation artifact during platinum oxide reduction in cyclic voltammetry analysis of low-loaded pemfc electrodes. Electrocatalysis 2021, 12, 45–55. [Google Scholar] [CrossRef]
- Wu, J.; Fan, X.; Liu, J.; Luo, Q.; Xu, J.; Chen, X. Promoter engineering of cascade biocatalysis for α-ketoglutaric acid production by coexpressing l-glutamate oxidase and catalase. Appl. Microbiol. Biotechnol. 2018, 102, 4755–4764. [Google Scholar] [CrossRef]
- Maity, D.; Kumar, R.T.R. Highly sensitive amperometric detection of glutamate by glutamic oxidase immobilized pt nanoparticle decorated multiwalled carbon nanotubes(mwcnts)/polypyrrole composite. Biosens. Bioelectron. 2019, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Kumari, S.; Pundir, C.S. Construction of glutamate biosensor based on covalent immobilization of glutmate oxidase on polypyrrole nanoparticles/polyaniline modified gold electrode. Enzym. Microb. Technol. 2014, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and nad+, integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sens. Actuators B Chem. 2015, 216, 614–621. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Soldatkin, O.O.; Kasap, B.O.; Kucherenko, D.Y.; Kucherenko, I.S.; Kurc, B.A.; Dzyadevych, S.V. A novel amperometric glutamate biosensor based on glutamate oxidase adsorbed on silicalite. Nanoscale Res Lett. 2017, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bai, R.; Xie, B.; Zhuang, N.; Lv, Z.; Chen, M.; Dong, W.; Zhou, J.; Jiang, M. A biosensor based on oriented immobilization of an engineered l-glutamate oxidase on a screen-printed microchip for detection of l-glutamate in fermentation processes. Food Chem. 2023, 405, 134792. [Google Scholar] [CrossRef]
- Zeynaloo, E.; Yang, Y.-P.; Dikici, E.; Landgraf, R.; Bachas, L.G.; Daunert, S. Design of a mediator-free, non-enzymatic electrochemical biosensor for glutamate detection. Nanomed. NBM 2021, 31, 102305. [Google Scholar] [CrossRef]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, S.; Lang, Q.; Song, J.; Han, L.; Liu, A. Amperometric l-glutamate biosensor based on bacterial cell-surface displayed glutamate dehydrogenase. Anal. Chim. Acta 2015, 884, 83–89. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Domínguez-Saldaña, A.; Villa-Manso, A.M.; Gutiérrez-Sánchez, C.; Revenga-Parra, M.; Mateo-Martí, E.; Pariente, F.; Lorenzo, E. Azure a embedded in carbon dots as nadh electrocatalyst: Development of a glutamate electrochemical biosensor. Sens. Actuators B Chem. 2023, 374, 132761. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Chen, G.; Liu, Y. Highly sensitive glutamate biosensor based on platinum nanoparticles decorated mxene-ti3c2tx for l-glutamate determination in foodstuffs. LWT 2021, 148, 111748. [Google Scholar] [CrossRef]
- Wang, X.; Duan, J.; Cai, Y.; Liu, D.; Li, X.; Dong, Y.; Hu, F. A modified nanocomposite biosensor for quantitative l-glutamate detection in beef. Meat Sci. 2020, 168, 108185. [Google Scholar] [CrossRef] [PubMed]
- Mentana, A.; Nardiello, D.; Palermo, C.; Centonze, D. Accurate glutamate monitoring in foodstuffs by a sensitive and interference-free glutamate oxidase based disposable amperometric biosensor. Anal. Chim. Acta 2020, 1115, 16–22. [Google Scholar] [CrossRef] [PubMed]



| Sample | Obtained Value Using Pt-MNE/GLOx/Nafion/ (µM) | Mean/(µM) | Standard Deviation | Count | Standard Error of Mean | Degree of Freedom | Hypothesized Mean/(µM) | T-Value † | p-Value | Obtained Value Using Glutamate Assay Kit/ (µM) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45; 45.5, 45.2; 45.4 | 45.27 | 0.22 | 4.0 | 0.11 | 3.0 | 45.0 | 2.48 | 0.089 | 45 |
| 2 | 50.1; 50.5; 50.2; 50.4 | 50.3 | 0.18 | 4.0 | 0.09 | 3.0 | 50.0 | 3.28 | 0.046 | 50.0 |
| Biosensor | Method | Linear Range | Limit of Detection | References |
|---|---|---|---|---|
| Pt/P(o-PD)/GLOx | CA | 20–140 µM | 10 µM | [43] |
| Pt wire/P(o-PD)/GLOx/Chit/ASOx | CA | 5–35 µM | 0.59 µM | [40] |
| Gold Electrode/PNAI/PPyNps | CA | 0.02–400 µM | 0.1 nM | [55] |
| MWCNT-Chit-MB/GLDH-NAD-Chit-MB/MWCNT-Chit -MB | CA | 7.5–105 μM | 3.0 μM | [56] |
| Pt wire/GLOx | CA | 2.5–450 μM | 1.0 μM | [57] |
| SPC/PB/GLOx/Chit | CA | 25–300 μM | 9.0μM | [58] |
| SPCE/AuNP/GluBP | CV | 0.1–0.8 mM | 0.15 mM | [59] |
| Gold electrode/Chit/AuNP/cMWCNT/GluOx | CA | 5–500 μM | 1.6 μM | [60] |
| GCE/PEI-MWNTs/bacteria-Gldh/Nafion | CA | 10 μM–1 mM and 2–10 mM | 2.0 μM | [61] |
| SPCE/Chit-AA-CDs/GLDH | CA | 11–125 μM | 3.0 μM | [62] |
| GCE/PtNP@MXene-Ti3C2Tx/Chi | CA | 10–110 | 0.45 | [63] |
| Gold electrode/Chi/GO/Au NPs/GluOx | DPV | 0.2–1.4 mM | 0.023 mM | [64] |
| SPPtE/PPYox/GLOx | CA | 0.005–1.0 mM | 1.8 μM | [65] |
| Pt-MNE/GLOx/Nafion | CV CA | 1–150 μM 1–150 μM | 0.25 μM 0.41 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amouzadeh Tabrizi, M. A Facile Method for the Fabrication of the Microneedle Electrode and Its Application in the Enzymatic Determination of Glutamate. Biosensors 2023, 13, 828. https://doi.org/10.3390/bios13080828
Amouzadeh Tabrizi M. A Facile Method for the Fabrication of the Microneedle Electrode and Its Application in the Enzymatic Determination of Glutamate. Biosensors. 2023; 13(8):828. https://doi.org/10.3390/bios13080828
Chicago/Turabian StyleAmouzadeh Tabrizi, Mahmoud. 2023. "A Facile Method for the Fabrication of the Microneedle Electrode and Its Application in the Enzymatic Determination of Glutamate" Biosensors 13, no. 8: 828. https://doi.org/10.3390/bios13080828





