Soft Epidermal Paperfluidics for Sweat Analysis by Ratiometric Raman Spectroscopy
Abstract
:1. Introduction
2. Methodology
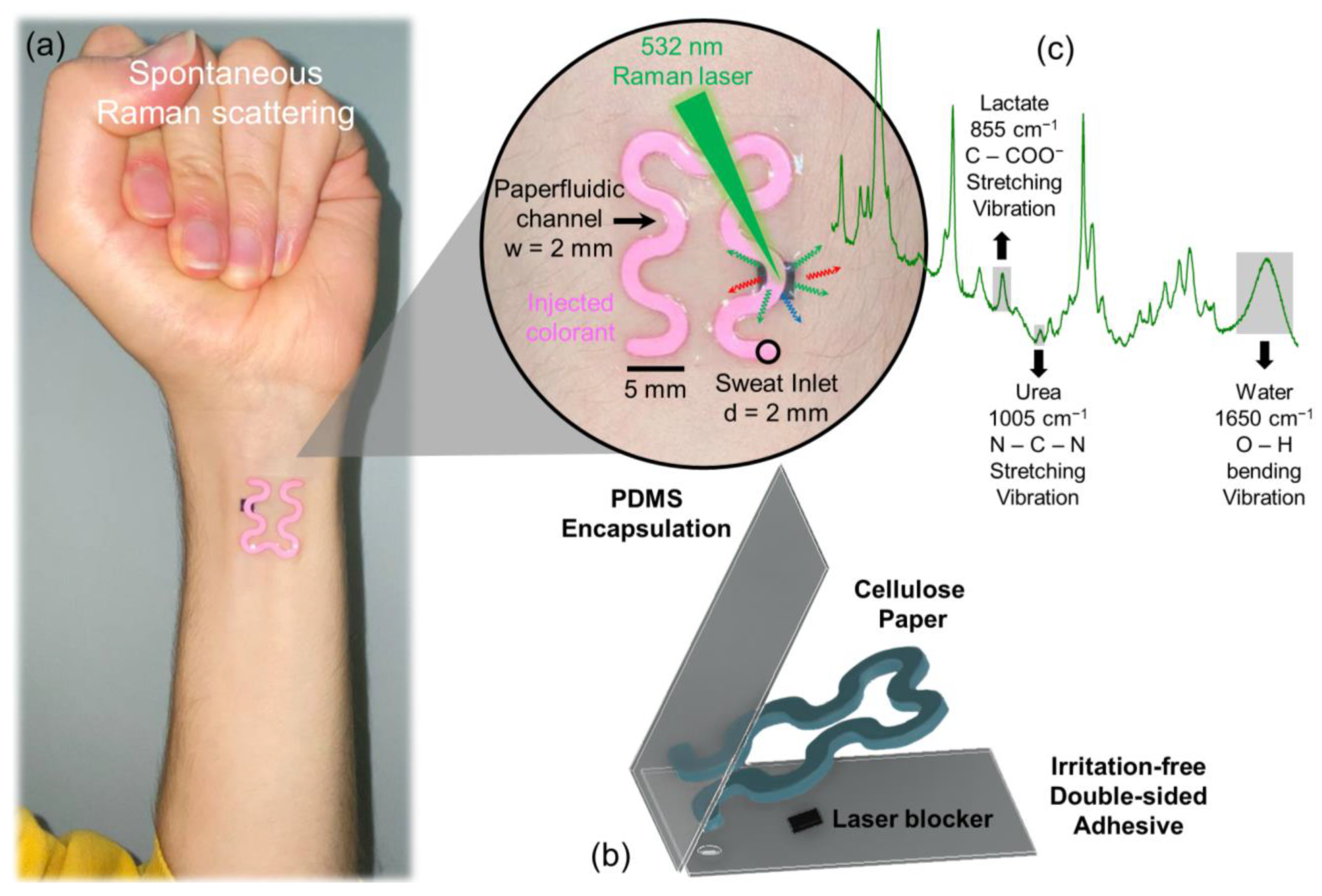
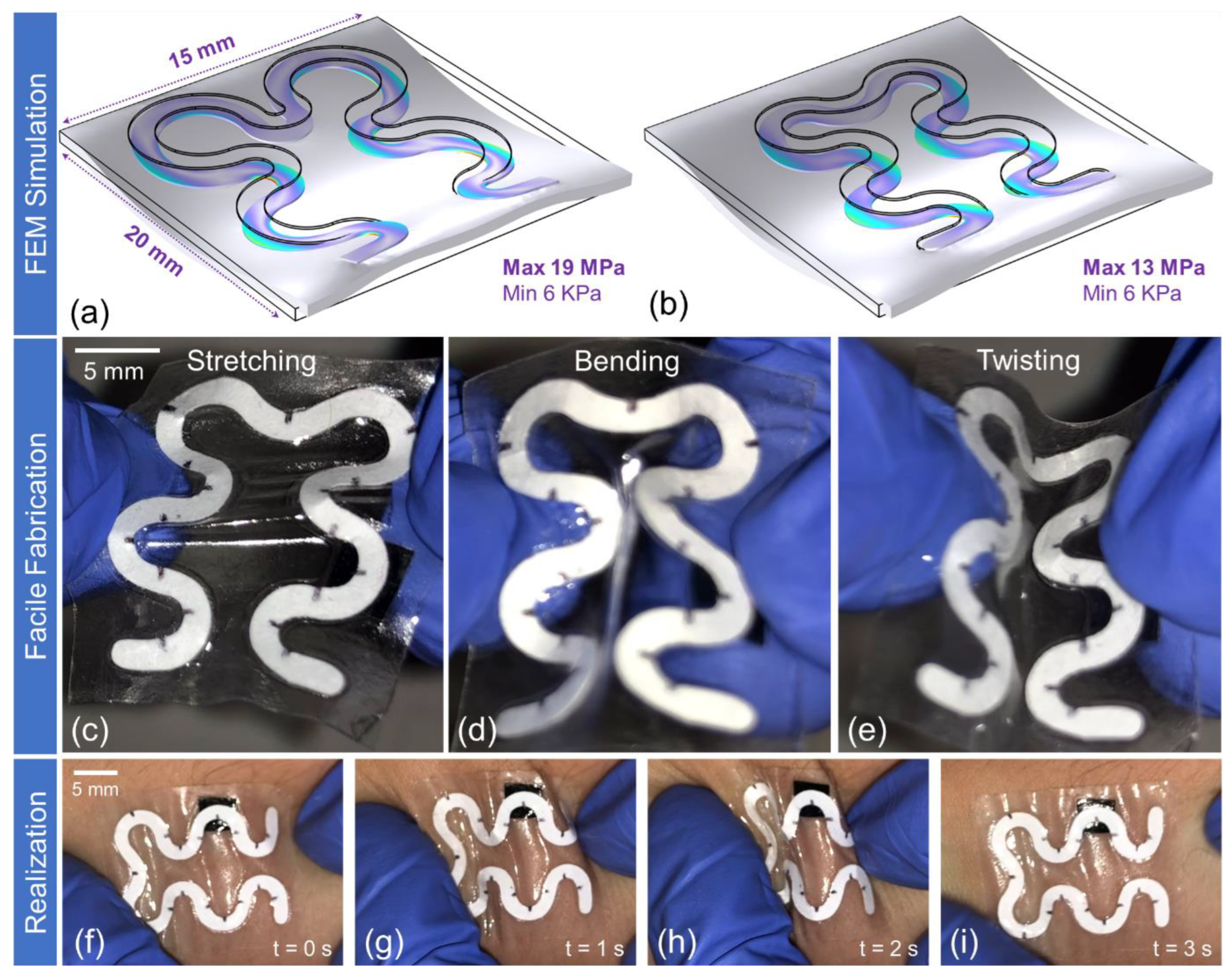
Soft Paper-Based Optofluidic Device: Design, FEM Simulation, and Fabrication
3. Results
3.1. Wearable Epidermal Paper-Based Optofluidics
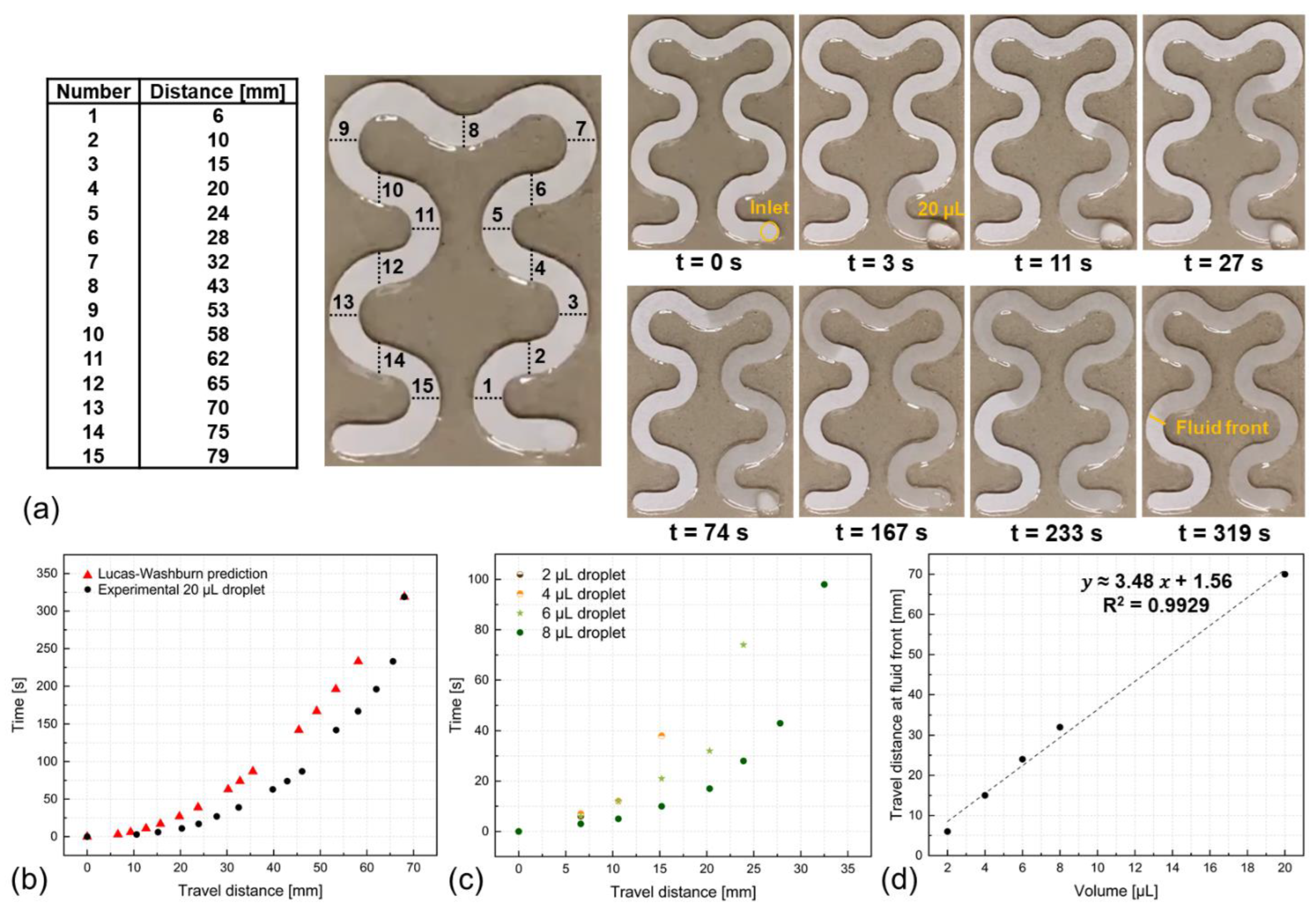
3.2. Impact of the Sweat Rate on the Accuracy of Sweat Components Analysis
3.3. Chip-Free and Imaging-Less Visual Sweat Rate Estimation
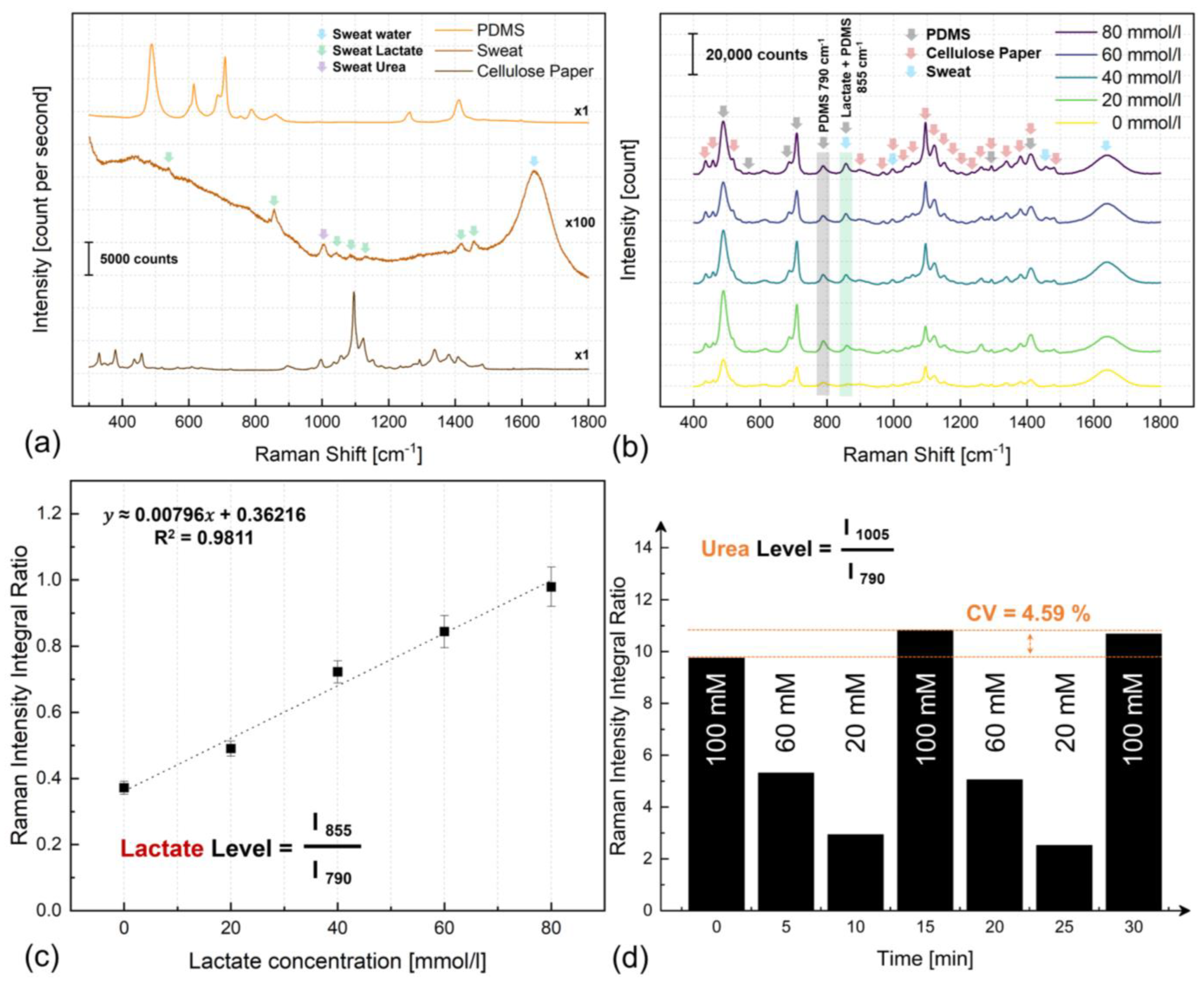
3.4. Optical Sweat Biochemistry Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozturk, O.; Golparvar, A.; Acar, G.; Guler, S.; Yapici, M.K. Single-arm diagnostic electrocardiography with printed graphene on wearable textiles. Sens. Actuators A Phys. 2023, 349, 114058. [Google Scholar] [CrossRef]
- Mirbakht, S.; Golparvar, A.; Umar, M.; Yapici, M.K. Flexible silk-based graphene bioelectronics for wearable multimodal physiological monitoring. In Proceedings of the IEEE 36th International Conference on Micro Electro Mechanical Systems (MEMS), Munich, Germany, 15–19 January 2023; pp. 335–338. [Google Scholar] [CrossRef]
- Golparvar, A.; Ozturk, O.; Yapici, M.K. Gel-free wearable electroencephalography (EEG) with soft graphene textiles. In Proceedings of the IEEE Sensors Conference, Sydney, Australia, 31 October–3 November 2021. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab A Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.C.; Lim, C.M. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narvaez, E.; Guder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Golparvar, A.; Kim, J.; Boukhayma, A.; Briand, D.; Carrara, S. Highly accurate multimodal monitoring of lactate and urea in sweat by soft epidermal optofluidics with single-band Raman scattering. Sens. Actuators B Chem. 2023, 387, 133814. [Google Scholar] [CrossRef]
- Economou, A.; Kokkinos, C.; Prodromidis, M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab A Chip 2018, 18, 1812–1830. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, X.; Fang, X.; Kong, J. Wearable chem-biosensing devices: From basic research to commercial market. Lab A Chip 2021, 21, 4285–4310. [Google Scholar] [CrossRef]
- Niederberger, C.; Vermeersch, A.; Davidhi, F.; Ewald, C.Y.; Havenith, G.; Goldhahn, J.; Dincer, C.; Brasier, N. Wearable sweat analysis to determine biological age. Trends Biotechnol. 2023, 41, 1113–1116. [Google Scholar] [CrossRef]
- Parolo, C.; Idili, A.; Heikenfeld, J.; Plaxco, K.W. Conformational-switch biosensors as novel tools to support continuous, real-time molecular monitoring in lab-on-a-chip devices. Lab A Chip 2023, 23, 1339–1348. [Google Scholar] [CrossRef]
- Kim, J.; Wu, Y.; Luan, H.; Yang, D.S.; Cho, D.; Kwak, S.S.; Liu, S.; Ryu, H.; Ghaffari, R.; Rogers, A. A skin-interfaced, miniaturized microfluidic analysis and delivery system for colorimetric measurements of nutrients in sweat and supply of vitamins through the skin. Adv. Sci. 2022, 9, 2103331. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Chen, L.; Zhao, Y.; Gong, J.; Li, Z.; Zhang, J. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring. Lab A Chip 2021, 21, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.R.; Zhou, Y.; Dey, A.A.; Arellano, D.L.G.; Okoroanyanwu, U.; Secor, E.B.; Hersam, M.C.; Morse, J.; Rothstein, J.P.; Carter, K.R.; et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab A Chip 2022, 22, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef] [PubMed]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Bradbury, A.R.M.; Knappik, A.; Pluckthun, A.; Borrebaeck, C.A.K.; Dubel, S. Animal-free alternatives and the antibody iceberg. Nat. Biotechnol. 2020, 38, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Romanholo, P.V.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Machado, S.A.S.; Sgobbi, L.F. Biomimetic electrochemical sensors: New horizons and challenges in biosensing applications. Biosens. Bioelectron. 2021, 185, 113242. [Google Scholar] [CrossRef]
- Uhlen, M.; Svahn, C. Lab on a chip technologies for bioenergy and biosustainability research. Lab A Chip 2011, 11, 3389–3393. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab A Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Patari, S.; Mahapatra, P.S. Liquid wicking in a paper strip: An experimental and numerical study. ACS Omega 2020, 5, 22931–22939. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.M.; Wee, E.J.; Wang, Y.; Trau, M. Enabling miniaturised personalised diagnostics: From lab-on-a-chip to lab-in-a-drop. Lab A Chip 2017, 17, 3200–3220. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pancorbo, P.M.; Xiao, T.-H.; Noguchi, S.; Marumi, M.; Segawa, H.; Karhadkar, S.; de Pablo, J.G.; Hiramatsu, K.; Kitahama, Y.; et al. Highly scalable, wearable surface-enhanced Raman spectroscopy. Adv. Opt. Mater. 2022, 10, 2200054. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Nguyen, N. Microfluidic solutions for biofluids handling in on-skin wearable systems. Lab A Chip 2023, 23, 913–937. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Park, Y.S.; Chang, H.; Lee, W.; Singh, S.P.; Choi, W.; Galindo, L.H.; Dasari, R.R.; Nam, S.H.; Park, J.; et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 2020, 6, eaay5206. [Google Scholar] [CrossRef]
- Golparvar, A.; Boukhayma, A.; Loayza, T.; Caizzone, A.; Enz, C.; Carrara, S. Very selective detection of low physiopathological glucose levels by spontaneous Raman spectroscopy with univariate data analysis. BioNanoScience 2021, 11, 871–877. [Google Scholar] [CrossRef]
- Park, Y.; Kim, U.J.; Lee, S.; Kim, H.; Kim, J.; Ma, H.; Son, H.; Yoon, Y.Z.; Lee, J.-S.; Park, M.; et al. On-chip Raman spectrometers using narrow band filter array combined with CMOS image sensors. Sens. Actuators B Chem. 2023, 381, 133442. [Google Scholar] [CrossRef]
- Yang, Z.; Albrow-Owen, T.; Cai, W.; Hasan, T. Miniaturization of optical spectrometers. Science 2021, 371, eabe0722. [Google Scholar] [CrossRef]
- Golparvar, A.; Boukhayma, A.; Carrara, S. Single-band Raman Shift Detection for Spectroscopy-less Optical Biosensors. IEEE Sens. Lett. 2023, 7, 6004904. [Google Scholar] [CrossRef]
- Golparvar, A.; Boukhayma, A.; Enz, C.; Carrara, S. Rapid, sensitive and selective optical glucose sensing with stimulated Raman scattering (SRS). In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Messina, Italy, 22–24 June 2022. [Google Scholar] [CrossRef]
- Walter, A.; Marz, A.; Schumacher, W.; Rosch, P.; Popp, J. Towards a fast, high specific and reliable discrimination of bacteria on strain level by means of SERS in a microfluidic device. Lab A Chip 2011, 11, 1013–1021. [Google Scholar] [CrossRef]
- He, X.; Fan, C.; Luo, Y.; Xu, T.; Zhang, X. Flexible microfluidic nanoplasmonic sensors for refreshable and portable recognition of sweat biochemical fingerprint. NPJ Flex. Electron. 2022, 6, 60. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Wang, J.; Luo, X.; Xie, L.; Zhan, S.; Kim, J.; Wang, X.; Liu, X.; Ying, Y. Wearable plasmonic-metasurface sensor for non-invasive and universal molecular fingerprint detection on biointerfaces. Sci. Adv. 2021, 7, eabe4553. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Lee, W.-C.; Choi, Y.-J.; Moon, J.-I.; Jang, J.; Park, S.-G.; Choo, J.; Kim, D.-H.; Jung, H.S. A wearable surface-enhanced Raman scattering sensor for label-free molecular detection. ACS Appl. Mater. Interfaces 2021, 13, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Caldarola, M.; Albella, P.; Cortés, E.; Rahmani, M.; Roschuk, T.; Grinblat, G.; Oulton, R.F.; Bragas, A.V.; Maier, S.A. Non-plasmonic nanoantennas for surface enhanced spectroscopies with ultra-low heat conversion. Nat. Commun. 2015, 6, 7915. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la Paz, E.; Pei, K.; Sonsa-ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J. Stiff as a board: Perspectives on the crystalline modulus of cellulose. ACS Macro Lett. 2012, 1, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Boppart, S.A. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans. Biomed. Eng. 2009, 57, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Gerhardt, L.-C.; Lee, Z.S.; Byers, R.A.; Woods, D.; Sanz-Herrera, J.A.; Franklin, S.E.; Lewis, R.; Matcher, S.J.; Carré, M.J. In vivo measurement of skin surface strain and sub-surface layer deformation induced by natural tissue stretching. J. Mech. Behav. Biomed. Mater. 2016, 62, 556–569. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B: Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Fu, J.; Wu, W. Fabrication of paper-based microfluidic analysis devices: A review. RSC Adv. 2015, 5, 78109–78127. [Google Scholar] [CrossRef]
- Golparvar, A.; Boukhayma, A.; Carrara, S. Flexible Microfluidics for Raman Measurements on Skin. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Yang, G.; Wu, J. Flow physics of wicking into woven screens with hybrid micro-/nanoporous structures. Langmuir 2021, 37, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Han, G.; Yang, Q.; Liu, D. Confinement–unconfinement transformation of ILs in IL@ MOF composite with multiple adsorption sites for efficient water capture and release. Adv. Mater. Interfaces 2022, 9, 2102354. [Google Scholar] [CrossRef]
- Elizalde, E.; Urteaga, R.; Berli, C. Rational design of capillary-driven flows for paper-based microfluidics. Lab A Chip 2015, 15, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pamula, V.K.; Fair, R. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab A Chip 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Huang, J.; Liu, Y.; Peng, J.; Chen, S.; Song, K.; Ouyang, X.; Cheng, H.; Wang, X. Skin-interfaced microfluidic devices with one-opening chambers and hydrophobic valves for sweat collection and analysis. Lab A Chip 2020, 20, 2635–2645. [Google Scholar] [CrossRef]
- Wang, S.; Rovira, M.; Demuru, S.; Kim, J.; Kunnel, B.P.; Besson, C.; Fernandez-Sanchez, C. Multisensing wearables for real-time monitoring of sweat electrolyte biomarkers during exercise and analysis on their correlation with core body temperature. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 808–817. [Google Scholar] [CrossRef]
- Choi, J.; Ghaffari, R.; Baker, L.B.; Rogers, J.A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 2018, 4, eaar3921. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T. Recent progress challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef]
- Ohashi, T.; Gerrett, N.; Shinkawa, S.; Sato, T.; Miyake, R.; Kondo, N.; Mitsuzawa, S. Fluidic Patch device to sample sweat for accurate measurement of sweat rate and chemical composition: A proof-of-concept study. Anal. Chem. 2020, 92, 15534–15541. [Google Scholar] [CrossRef]
- Tonello, S.; Golparvar, A.; Meimandi, A.; Carrara, S. Multimodal Sweat Ion and Sweat Rate Sensing with Inkjet-printed Flexible Bracelet and Paperfluidics. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023. [Google Scholar] [CrossRef]
- Yang, Q.; Rosati, G.; Abarintos, V.; Aroca, M.A.; Osma, J.F.; Merkoci, A. Wearable and fully printed microfluidic nanosensor for sweat rate, conductivity, and copper detection with healthcare applications. Biosens. Bioelectron. 2022, 202, 114005. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Demchyshyn, S.; Sempionatto, J.R.; Song, Y.; Hailegnaw, B.; Xu, C.; Yang, Y.; Solomon, S. An autonomous wearable biosensor powered by a perovskite solar cell. Nat. Electron. 2023, 6, 630–641. [Google Scholar] [CrossRef]
- Baker, L.B.; Model, J.B.; Barnes, K.A.; Anderson, M.L.; Lee, S.P.; Lee, K.A.; Brown, S.D.; Reimel, A.J.; Roberts, T.J.; Nuccio, R.P.; et al. Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv. 2020, 6, eabe3929. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, W.; Suarez, J.E.; Athavan, H.; Wang, Y.; Yeung, C.; Lin, S.; Sankararaman, S.; Milla, C.; Emaminejad, S. Autonomous wearable sweat rate monitoring based on digitized microbubble detection. Lab A Chip 2022, 22, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Aranyosi, A.; Lee, S.; Model, J.; Baker, L. The Gx Sweat Patch for personalized hydration management. Nat. Rev. Bioeng. 2023, 1, 5–7. [Google Scholar] [CrossRef]
- Persichetti, G.; Grimaldi, I.A.; Testa, G.; Bernini, R. Multifunctional optofluidic lab-on-chip platform for Raman and fluorescence spectroscopic microfluidic analysis. Lab A Chip 2017, 17, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Soum, V.; Park, S.; Brilian, A.I.; Kwon, O.; Shin, K. Programmable paper-based microfluidic devices for biomarker detections. Micromachines 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Cassanas, G.; Morssli, M.; Fabregue, E.; Bardet, L. Vibrational spectra of lactic acid and lactates. J. Raman Spectrosc. 1991, 22, 409–413. [Google Scholar] [CrossRef]
- Keuleers, R.; Desseyn, H.; Rousseau, B.; Van Alsenoy, C. Vibrational analysis of urea. J. Phys. Chem. A 1991, 103, 4621–4630. [Google Scholar] [CrossRef]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, mid-infrared, near-infrared and ultraviolet–visible spectroscopy of PDMS silicone rubber for characterization of polymer optical waveguide materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Choi, D.; Gonzales, M.; Kitchen, G.B.; Phan, D.; Searson, P.C. A capacitive sweat rate sensor for continuous and real-time monitoring of sweat loss. ACS Sens. 2020, 5, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research). Nephrol. Dial. Transplant. 2018, 1, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.W.; Bailey, J.L.; Wang, Y.; Klein, J.D.; Sands, J.M. Urea transporters and sweat response to uremia. Physiol. Rep. 2016, 4, e12825. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, N.; Lodebo, B.T.; Shah, A.; Kopple, J.D. Is there a role for diaphoresis therapy for advanced chronic kidney disease patients? J. Ren. Nutr. 2017, 27, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Alvear-Ordenes, I.; García-López, D.; De Paz, J.; González-Gallego, J. Sweat lactate, ammonia, and urea in rugby players. Int. J. Sports Med. 2005, 26, 632–637. [Google Scholar] [CrossRef]
- Bonini, A.; Vivaldi, F.M.; Herrera, E.; Melai, B.; Kirchhain, A.; Sajama, N.V.P.; Mat, M.; Caprioli, R.; Lomonaco, T.; Di Francesco, F.; et al. A graphenic biosensor for real-time monitoring of urea during dialysis. IEEE Sens. J. 2020, 20, 4571–4578. [Google Scholar] [CrossRef]
- Bigot, A.; Tchan, M.C.; Thoreau, B.; Blasco, H.; Maillot, F. Liver involvement in urea cycle disorders: A review of the literature. J. Inherit. Metab. Dis. 2017, 40, 757–769. [Google Scholar] [CrossRef]
- Watabe, A.; Sugawara, T.; Kikuchi, K.; Yamasaki, K.; Sakai, S.; Aiba, S. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J. Dermatol. Sci. 2013, 72, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Van Hoovels, K.; Xuan, X.; Cuartero, M.; Gijssel, M.; Swarén, M.; Crespo, G.A. Can wearable sweat lactate sensors contribute to sports physiology? ACS Sens. 2021, 6, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhong, T.; He, H.; Zhao, T.; Xing, L.; Zhang, Y.; Xue, X. A self-powered wearable sweat-evaporation-biosensing analyzer for building sports big data. Nano Energy 2019, 59, 754–761. [Google Scholar] [CrossRef]
- Klous, L.; De Ruiter, C.; Scherrer, S.; Gerrett, N.; Daanen, H.A.M. The (in) dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 2021, 121, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Havenith, G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur. J. Appl. Physiol. 2011, 111, 1391–1404. [Google Scholar] [CrossRef]
- Golparvar, A.; Boukhayma, A.; Enz, C.; Carrara, S. Optimized Detection of Hypoglycemic Glucose Ranges in Human Serum by Raman Spectroscopy with 532 nm Laser Excitation. Photoptics 2022, 1, 158–165. [Google Scholar] [CrossRef]
- Tfaili, S.; Gobinet, C.; Josse, G.; Angiboust, J.; Manfait, M.; Piot, O. Confocal Raman microspectroscopy for skin characterization: A comparative study between human skin and pig skin. Analyst 2012, 137, 3673–3682. [Google Scholar] [CrossRef]
- Tonello, S.; Fapanni, T.; Bonaldo, S.; Giorgi, G.; Narduzzi, C.; Paccagnella, A.; Serpellon, M. Amperometric measurements by a novel aerosol jet printed flexible sensor for wearable applications. IEEE Trans. Instrum. Meas. 2022, 72, 7500512. [Google Scholar] [CrossRef]
- Coull, N.A.; West, A.M.; Hodder, S.G.; Wheeler, P.; Havenith, G. Body mapping of regional sweat distribution in young and older males. Eur. J. Appl. Physiol. 2021, 121, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Ceseracciu, L.; Heredia-Guerrero, J.A.; Dante, S.; Athanassiou, A.; Bayer, I. Robust and biodegradable elastomers based on corn starch and polydimethylsiloxane (PDMS). ACS Appl. Mater. Interfaces 2015, 7, 3742–3753. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, J.; Luo, Y.; Xu, T.; Zhang, X. Wearable Plasmonic Sweat Biosensor for Acetaminophen Drug Monitoring. ACS Sens. 2023, 8, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, H.; Lee, J.; Lee, T.; Yun, J.; Lee, G.; Hong, Y. Hand-held Raman spectrometer-based dual detection of creatinine and cortisol in human sweat using silver nanoflakes. Anal. Chem. 2021, 93, 14996–15004. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.L.; Jahani, Y.; Leitis, A.; Altug, H. Dielectric metasurfaces enabling advanced optical biosensors. ACS Photonics 2020, 8, 47–60. [Google Scholar] [CrossRef]
- Eskandari, V.; Sahbafar, H.; Zeinalizad, L.; Marashipour, R.; Hadi, A. A review of paper-based substrates as surface-enhanced Raman spectroscopy (SERS) biosensors and microfluidic paper-based SERS platforms. J. Comput. Appl. Mech. 2022, 53, 142–156. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Han, S.; Liu, Y.; Hasi, W.; Wang, L. Flexible fabrication of a paper-fluidic SERS sensor coated with a monolayer of core–shell nanospheres for reliable quantitative SERS measurements. Anal. Chim. Acta 2020, 1108, 167–176. [Google Scholar] [CrossRef]
- Hoppmann, E.P.; Wei, W.Y.; White, I.M. Inkjet-printed fluidic paper devices for chemical and biological analytics using surface-enhanced Raman spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2013, 20, 195–204. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golparvar, A.; Thenot, L.; Boukhayma, A.; Carrara, S. Soft Epidermal Paperfluidics for Sweat Analysis by Ratiometric Raman Spectroscopy. Biosensors 2024, 14, 12. https://doi.org/10.3390/bios14010012
Golparvar A, Thenot L, Boukhayma A, Carrara S. Soft Epidermal Paperfluidics for Sweat Analysis by Ratiometric Raman Spectroscopy. Biosensors. 2024; 14(1):12. https://doi.org/10.3390/bios14010012
Chicago/Turabian StyleGolparvar, Ata, Lucie Thenot, Assim Boukhayma, and Sandro Carrara. 2024. "Soft Epidermal Paperfluidics for Sweat Analysis by Ratiometric Raman Spectroscopy" Biosensors 14, no. 1: 12. https://doi.org/10.3390/bios14010012
APA StyleGolparvar, A., Thenot, L., Boukhayma, A., & Carrara, S. (2024). Soft Epidermal Paperfluidics for Sweat Analysis by Ratiometric Raman Spectroscopy. Biosensors, 14(1), 12. https://doi.org/10.3390/bios14010012





