Shielded Cone Coil Array for Non-Invasive Deep Brain Magnetic Stimulation
Abstract
:1. Introduction
2. System Conceptual and Design Principle
2.1. Modeling and Simulation
2.2. The Structural Design of the Cone Coil
3. Design of the Coil Array
3.1. Number, Orientation, and Current Direction of the Coil
3.2. Coil Array System Optimization
4. System Validation
4.1. Coil Array Fabrication
4.2. Experimental Setup
4.3. In Vitro Validation
4.4. Validation on Postmortem Tissue
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rakic, P. Progress: Neurogenesis in adult primate neocortex: An evaluation of the evidence. Nat. Rev. Neurosci. 2002, 3, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.L.; Diamond, M.C.; Krech, D.; Rosenzweig, M.R. Chemical and Anatomical Plasticity of Brain. Science 1964, 146, 610–619. Available online: http://www.jstor.org/stable/1714515 (accessed on 1 March 2022). [CrossRef] [PubMed]
- Poorganji, M.; Zomorrodi, R.; Zrenner, C.; Bansal, A.; Hawco, C.; Hill, A.T.; Hadas, I.; Rajji, T.K.; Chen, R.; Zrenner, B.; et al. Pre-Stimulus Power but Not Phase Predicts Prefrontal Cortical Excitability in TMS-EEG. Biosensors 2023, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Islam, M.T.; Rahman, T.; Chowdhury, M.E.H.; Tahir, A.; Kiranyaz, S.; Mat, K.; Beng, G.K.; Soliman, M.S. Brain Tumor Segmentation and Classification from Sensor-Based Portable Microwave Brain Imaging System Using Lightweight Deep Learning Models. Biosensors 2023, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet 1985, 325, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Wada, M.; Zomorrodi, R.; Taniguchi, K.; Li, X.; Honda, S.; Tobari, Y.; Mimura, Y.; Nakajima, S.; Kitahata, R.; et al. Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition. Biosensors 2022, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Abu Sufian, S.M.; Mehdi, H.; Siddique-E-Rabbani, K. Designing a transcranial magnetic stimulator coil for deep brain stimulation. In Proceedings of the 9th International Conference on Electrical and Computer Engineering, ICECE 2016, Dhaka, Bangladesh, 20–22 December 2016; pp. 303–306. [Google Scholar] [CrossRef]
- Lu, M.; Ueno, S.; Dong, X.W. Deep transcranial magnetic stimulation using the semi-Halo coil. In Proceedings of the 2016 Progress in Electromagnetics Research Symposium, PIERS 2016, Shanghai, China, 8–11 August 2016; pp. 4644–4647. [Google Scholar] [CrossRef]
- Roth, Y.; Zangen, A.; Hallett, M. A Coil Design for Transcranial Magnetic Stimulation of Deep Brain Regions. J. Clin. Neurophysiol. 2002, 19, 361–370. [Google Scholar] [CrossRef]
- Sorkhabi, M.M.; Frounchi, J.; Shahabi, P.; Veladi, H. Deep-brain transcranial stimulation: A novel approach for high 3-D resolution. IEEE Access 2017, 5, 3157–3171. [Google Scholar] [CrossRef]
- Williams, P.I.; Marketos, P.; Crowther, L.J.; Jiles, D.C. New designs for deep brain transcranial magnetic stimulation. IEEE Trans. Magn. 2012, 48, 1171–1178. [Google Scholar] [CrossRef]
- Chernov, M.M.; Swan, C.B.; Leiter, J.C. In Search of a Feedback Signal for Closed-Loop Deep Brain Stimulation: Stimulation of the Subthalamic Nucleus Reveals Altered Glutamate Dynamics in the Globus Pallidus in Anesthetized, 6-Hydroxydopamine-Treated Rats. Biosensors 2023, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Di Barba, P.; Mognaschi, M.E.; Savini, A. Synthesizing a field source for magnetic stimulation of peripheral nerves. IEEE Trans. Magn. 2007, 43, 4023–4029. (In English) [Google Scholar] [CrossRef]
- Hsiao, I.N.; Lin, V.W.-H. Improved coil design for functional magnetic stimulation of expiratory muscles. IEEE Trans. Biomed. Eng. 2001, 48, 684–694. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Tashiro, T.; Harada, K. Localized Stimulation of Neural Tissues in the Brain by Means of a Paired Configuration of Time-Varying Magnetic-Fields. J. Appl. Phys. 1988, 64, 5862–5864. (In English) [Google Scholar] [CrossRef]
- Hsu, K.H.; Durand, D.M. A 3-D differential coil design for localized magnetic stimulation. IEEE Trans. Biomed. Eng. 2001, 48, 1162–1168. (In English) [Google Scholar] [PubMed]
- Kim, D.-H.; Georghiou, G.; Won, C. Improved field localization in transcranial magnetic stimulation of the brain with the utilization of a conductive shield plate in the stimulator. IEEE Trans. Biomed. Eng. 2006, 53, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Tang, Y.; Zhang, B.; Lee, E.G.; Hadimani, R.L.; Jiles, D.C. Quadruple Butterfly Coil with Passive Magnetic Shielding for Focused Transcranial Magnetic Stimulation. IEEE Trans. Magn. 2017, 53, 2016. [Google Scholar] [CrossRef]
- Lontis, E.R.; Voigt, M.; Struijk, J.J. Focality assessment in transcranial magnetic stimulation with double and cone coils. J. Clin. Neurophysiol. 2006, 23, 462–471. [Google Scholar] [CrossRef]
- Salvador, R.; Miranda, P.C.; Roth, Y.; Zangen, A. High permeability cores to optimize the stimulation of deeply located brain regions using transcranial magnetic stimulation. Phys. Med. Biol. 2009, 54, 3113–3128. [Google Scholar] [CrossRef]
- Lu, M.; Ueno, S. Deep Transcranial Magnetic Stimulation Using Figure-of-Eight and Halo Coils. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Han, B.H.; Chun, I.K.; Lee, S.C.; Lee, S.Y. Multichannel magnetic stimulation system design considering mutual couplings among the stimulation coils. IEEE Trans. Biomed. Eng. 2004, 51, 812–817. [Google Scholar] [CrossRef]
- Lu, M.; Ueno, S.; Thorlin, T.; Persson, M. Calculating the Current Density and Electric Field in Human Head by Multichannel Transcranial Magnetic Stimulation. IEEE Trans. Magn. 2009, 45, 1662–1665. [Google Scholar] [CrossRef]
- Epstein, C.M.; Davey, K.R. Iron-core coils for transcranial magnetic stimulation. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2002, 19, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Lee, S.Y.; Kim, J.H.; Yi, J.H. Some technical aspects of magnetic stimulation coil design with the ferromagnetic effect. Med. Biol. Eng. Comput. 2003, 41, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Miranda, P.C.; Roth, Y.; Zangen, A. High-permeability core coils for transcranial magnetic stimulation of deep brain regions. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology—Proceedings 2007, Lyon, France, 22–26 August 2007; pp. 6652–6655. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyawaki, Y.; Saitoh, Y.; Sekino, M. Improvement in Efficiency of Transcranial Magnetic Stimulator Coil by Combination of Iron Core Plates Laminated in Different Directions. IEEE Trans. Magn. 2016, 52, 22–25. [Google Scholar] [CrossRef]
- Bottauscio, O.; Zucca, M.; Chiampi, M.; Zilberti, L. Evaluation of the Electric Field Induced in Transcranial Magnetic Stimulation Operators. IEEE Trans. Magn. 2016, 52, 2–5. [Google Scholar] [CrossRef]
- Zucca, M.; Bottauscio, O.; Chiampi, M.; Zilberti, L. Operator safety and field focality in shielded transcranial magnetic stimulation. In Proceedings of the 2017 IEEE International Magnetics Conference, INTERMAG 2017, Dublin, Ireland, 24–28 April 2017; p. 10. [Google Scholar] [CrossRef]
- Li, Y.; Cosoroaba, E.; Maharjan, L.; Fahimi, B. Comparative Study of a New Coil Design with Traditional Shielded Figure-of-Eight Coil for Transcranial Magnetic Stimulation. IEEE Trans. Magn. 2017, 54, 1–4. [Google Scholar] [CrossRef]
- Lu, M.; Ueno, S. Calculating the electric field in real human head by transcranial magnetic stimulation with shield plate. J. Appl. Phys. 2009, 105, 07B322. [Google Scholar] [CrossRef]
- Yosef, R.A.; Toaha Mobashsher, A. The Effects of Using Ferromagnetic Core and Shield on Deep TMS Systems. In Proceedings of the 2021 IEEE Asia-Pacific Microwave Conference (APMC), Brisbane, Australia, 28 November–1 December 2021. [Google Scholar]
- Liang, Y.-W.; Lai, M.-L.; Chiu, F.-M.; Tseng, H.-Y.; Lo, Y.-C.; Li, S.-J.; Chang, C.-W.; Chen, P.-C.; Chen, Y.-Y. Experimental Verification for Numerical Simulation of Thalamic Stimulation-Evoked Calcium-Sensitive Fluorescence and Electrophysiology with Self-Assembled Multifunctional Optrode. Biosensors 2023, 13, 265. [Google Scholar] [CrossRef]
- Thackston, K.A.; Sievenpiper, D.F. Limits of Near-Field Directivity for a Wire Array. IEEE Magn. Lett. 2018, 9, 1306604. (In English) [Google Scholar] [CrossRef]
- Gao, F.; Zhang, F.; Wakatsuchi, H.; Sievenpiper, D.F. Synthesis and Design of Programmable Subwavelength Coil Array for Near-Field Manipulation. IEEE Trans. Microw. Theory 2015, 63, 2971–2982. [Google Scholar] [CrossRef]
- Li, J.T.; Jiang, W.H.; Liang, Z.; Zhao, Z.J.; Li, J.H. Flexible Multichannel Transcranial Magnetic Stimulation Coil Array. In Proceedings of the 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), Brooklyn, NY, USA, 16–19 April 2015; pp. 1490–1493. (In English). [Google Scholar]
- De Lara, L.I.N.; Daneshzand, M.; Mascarenas, A.; Paulson, D.; Pratt, K.; Okada, Y.; Raij, T.; Makarov, S.N.; Nummenmaa, A. A 3-axis coil design for multichannel TMS arrays. Neuroimage 2021, 224, 117355. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Xu, G.; Fu, W.N.; Yang, Q.; Hou, H.; Yan, W. Optimization of Array Magnetic Coil Design for Functional Magnetic Stimulation Based on Improved Genetic Algorithm. IEEE Trans. Magn. 2009, 45, 4849–4852. [Google Scholar] [CrossRef]
- Lee, S.W. Selective Activation of Cortical Columns Using Multichannel Magnetic Stimulation with a Bent Flat Microwire Array. IEEE Trans. Biomed. Eng. 2021, 68, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Ruohonen, J.; Ilmoniemi, R.J. Focusing and targeting of magnetic brain stimulation using multiple coils. Med. Biol. Eng. Comput. 1998, 36, 297–301. (In English) [Google Scholar] [CrossRef]
- Ruohonen, J.; Ravazzani, P.; Grandori, F.; Ilmoniemi, R. Theory of multichannel magnetic stimulation: Toward functional neuromuscular rehabilitation. IEEE Trans. Biomed. Eng. 1999, 46, 646–651. [Google Scholar] [CrossRef]
- Jiang, R.; Jansen, B.H.; Sheth, B.R.; Chen, J. Dynamic multi-channel TMS with reconfigurable coil. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 370–375. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Zhao, Z.; Zheng, M.; Liu, Y.; He, J.; Sun, Y.; Ren, Z. A Study on the Multi-Channel TMS Device. IEEE Trans. Magn. 2017, 53, 5000805. [Google Scholar] [CrossRef]
- ZMT Zurich MedTech AG. Sim4Life. Available online: https://www.zurichmedtech.com/sim4life/ (accessed on 1 April 2023).
- Iacono, M.I.; Neufeld, E.; Akinnagbe, E.; Bower, K.; Wolf, J.; Oikonomidis, I.V.; Sharma, D.; Lloyd, B.; Wilm, B.J.; Wyss, M.; et al. MIDA: A Multimodal Imaging-Based Detailed Anatomical Model of the Human Head and Neck. PLoS ONE 2015, 10, e0124126. [Google Scholar] [CrossRef]
- Nahas, Z. Handbook of transcranial magnetic stimulation. J. Psychiatr. Neurosci. 2003, 28, 373–375. (In English) [Google Scholar]
- Tofts, P.S. The distribution of induced currents in magnetic stimulation of the nervous system. Phys. Med. Biol. 1990, 35, 1119. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Branston, N.M. The measurement of electric field, and the influence of surface charge, in magnetic stimulation. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials 1991, 81, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Cuffin, B.N. Developing a more focal magnetic stimulator. Part I: Some basic principles. J. Clin. Neurophysiol. 1991, 8, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family—Development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 2010, 55, N23. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-D.; Lisanby, S.H.; Peterchev, A.V. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: A finite element simulation study. J. Neural Eng. 2011, 8, 016007. [Google Scholar] [CrossRef]
- Grandori, F.; Ravazzani, P. Magnetic stimulation of the motor cortex-theoretical considerations. IEEE Trans. Biomed. Eng. 1991, 38, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Eaton, H. Electric field induced in a spherical volume conductor from arbitrary coils: Application to magnetic stimulation and MEG. Med. Biol. Eng. Comput. 1992, 30, 433–440. [Google Scholar] [CrossRef]
- Deng, Z.-D.; Lisanby, S.H.; Peterchev, A.V. Coil design considerations for deep transcranial magnetic stimulation. Clin. Neurophysiol. 2014, 125, 1202–1212. [Google Scholar] [CrossRef]
- Davey, K.; Epstein, C.M.; George, M.S.; Bohning, D.E. Modeling the effects of electrical conductivity of the head on the induced electric field in the brain during magnetic stimulation. Clin. Neurophysiol. 2003, 114, 2204–2209. [Google Scholar] [CrossRef]
- Hunold, A.; Machts, R.; Haueisen, J. Head phantoms for bioelectromagnetic applications: A material study. BioMedical Eng. OnLine 2020, 19, 87. [Google Scholar] [CrossRef]
- McClellan, R.O. Applications of swine in biomedical research. Lab. Anim. Care 1968, 18, 120–126. Available online: https://www.ncbi.nlm.nih.gov/pubmed/4230577 (accessed on 1 October 2023). [PubMed]
- Swine in Biomedical Research. Lab. Anim. Sci. 1986, 36, 343–433. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3773443 (accessed on 1 October 2023).
- Book, S.A.; Bustad, L.K. The fetal and neonatal pig in biomedical research. J. Anim. Sci. 1974, 38, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Douglas, W.R. Of pigs and men and research: A review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972, 3, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S. Body farms. Forensic Sci. Med. Pathol. 2017, 13, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Hall, M.J.R.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Leg. Med. 2020, 134, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Gabriel, C. Cole-Cole parameters for the dielectric properties of porcine tissues as a function of age at microwave frequencies. Phys. Med. Biol. 2010, 55, N413. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.; Bialkowski, K.; Abbosh, A.; Mills, P.C.; Bradley, A.P. Closed-form equation to estimate the dielectric properties of biological tissues as a function of age. Bioelectromagnetics 2017, 38, 474–481. [Google Scholar] [CrossRef]
- Heller, L.; van Hulsteyn, D.B. Brain stimulation using electromagnetic sources: Theoretical aspects. Biophys. J. 1992, 63, 129–138. [Google Scholar] [CrossRef]
- Lomarev, M.P.; Kanchana, S.; Bara-Jimenez, W.; Iyer, M.; Wassermann, E.M.; Hallett, M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov. Disord. 2006, 21, 325–331. [Google Scholar] [CrossRef]
- Holczer, A.; Németh, V.L.; Vékony, T.; Vécsei, L.; Klivényi, P.; Must, A. Non-invasive Brain Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment—A State-of-the-Art Review on Methodological Characteristics and Stimulation Parameters. Front. Hum. Neurosci. 2020, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Pauri, F.; Pasqualetti, P.; Fini, R.; Forno, G.D.; Rossini, P.M. Motor cortex excitability in Alzheimer's disease: A transcranial magnetic stimulation study. Ann. Neurol. 2003, 53, 102–108. [Google Scholar] [CrossRef] [PubMed]


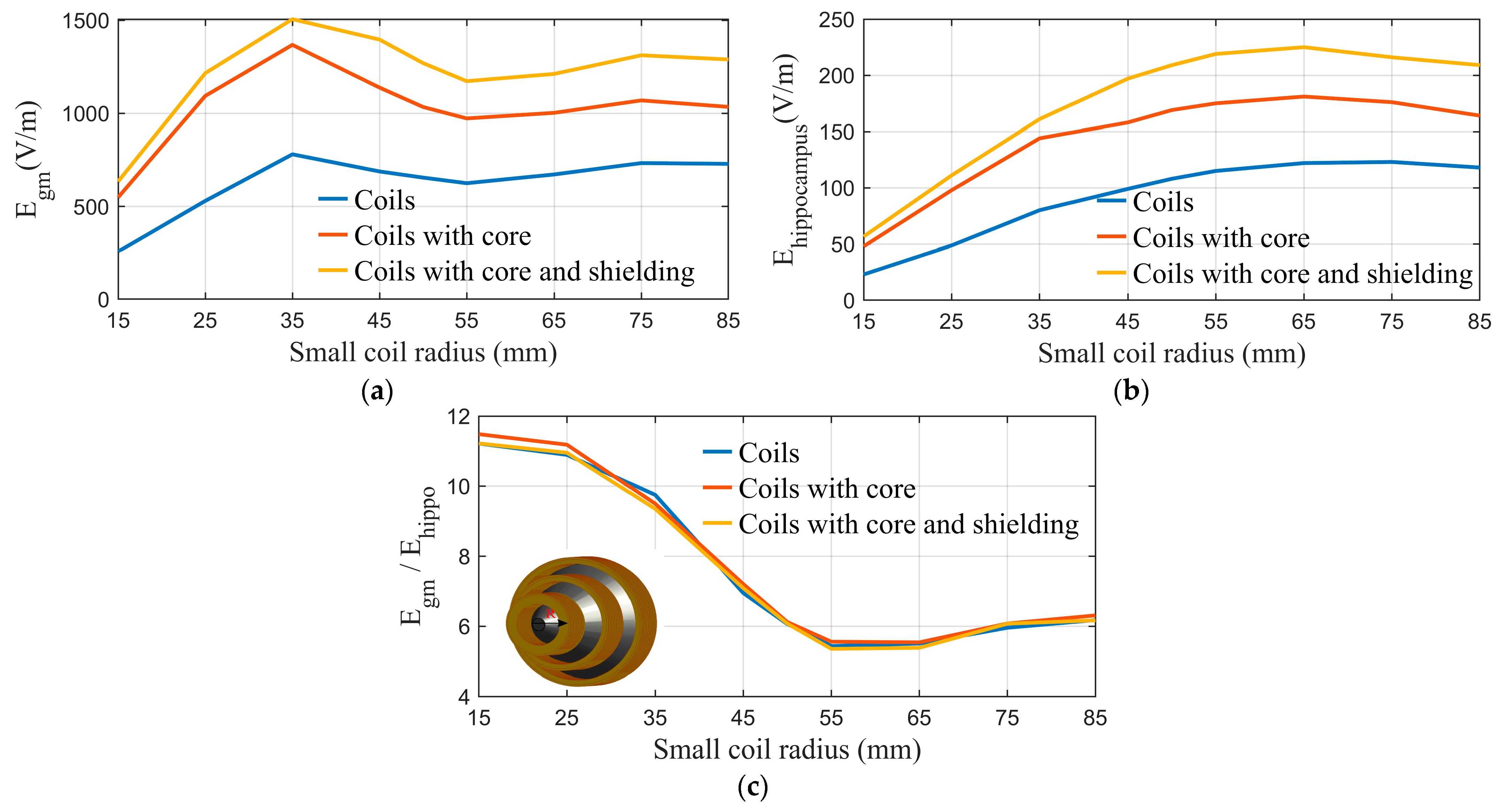
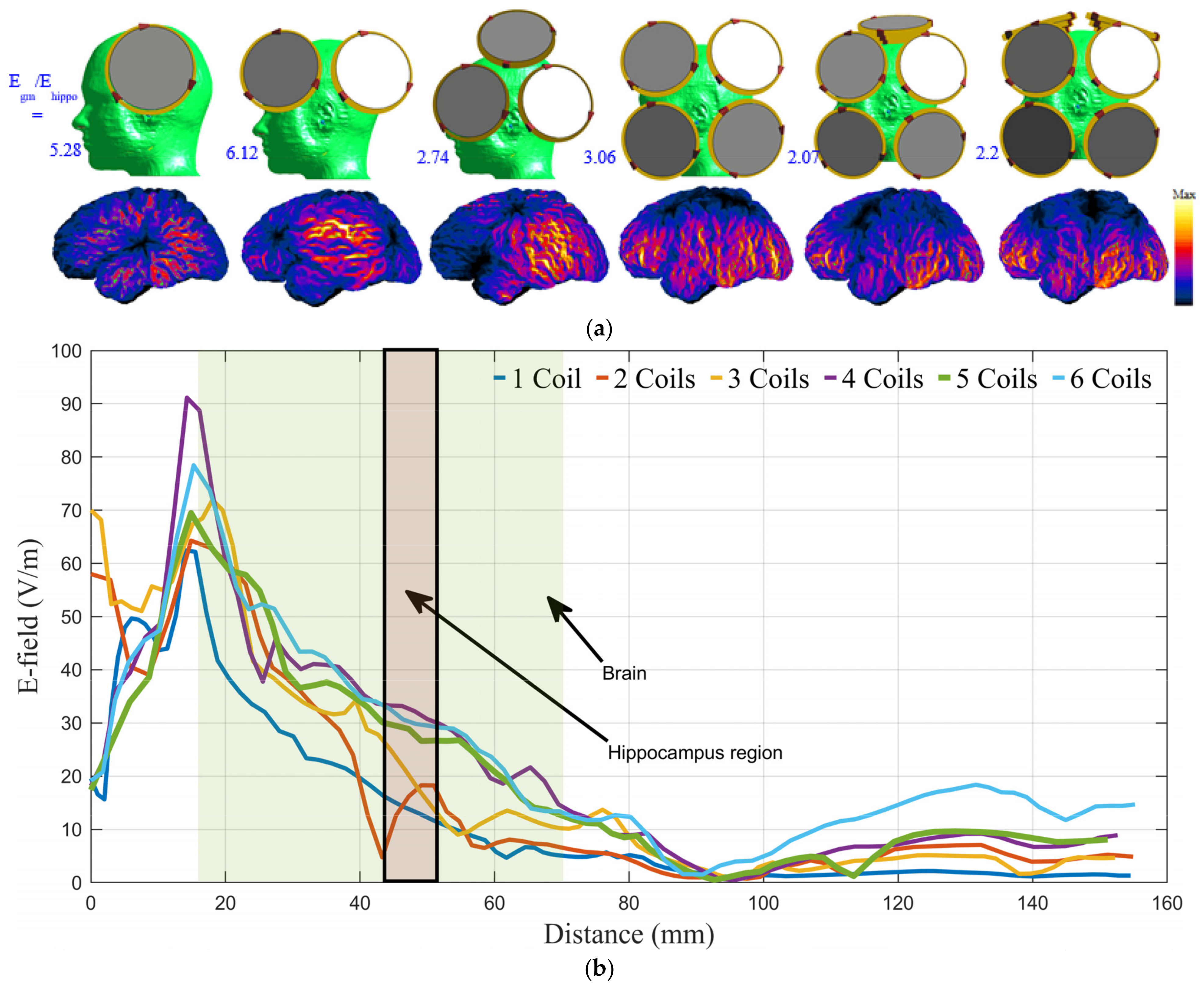

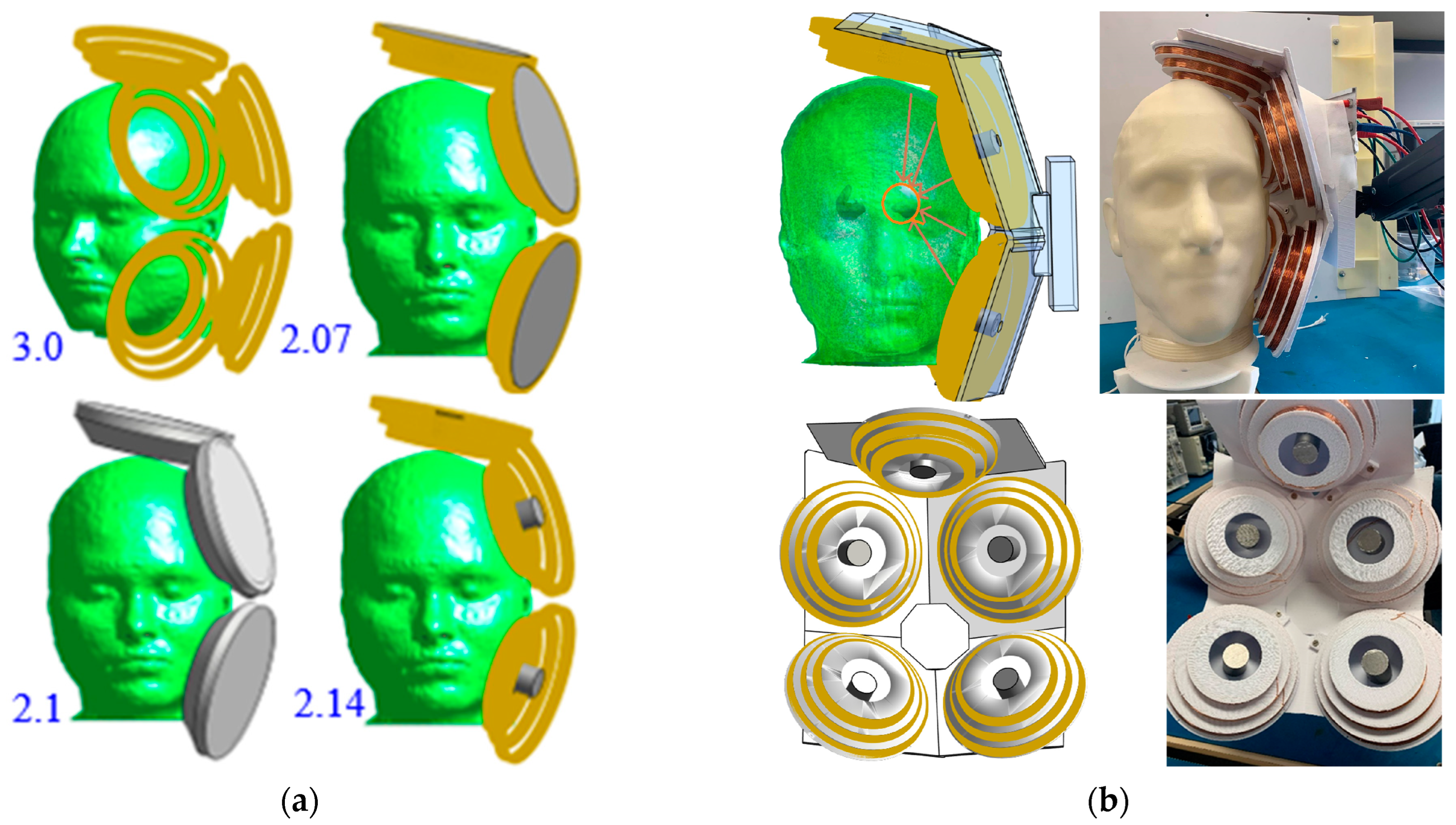
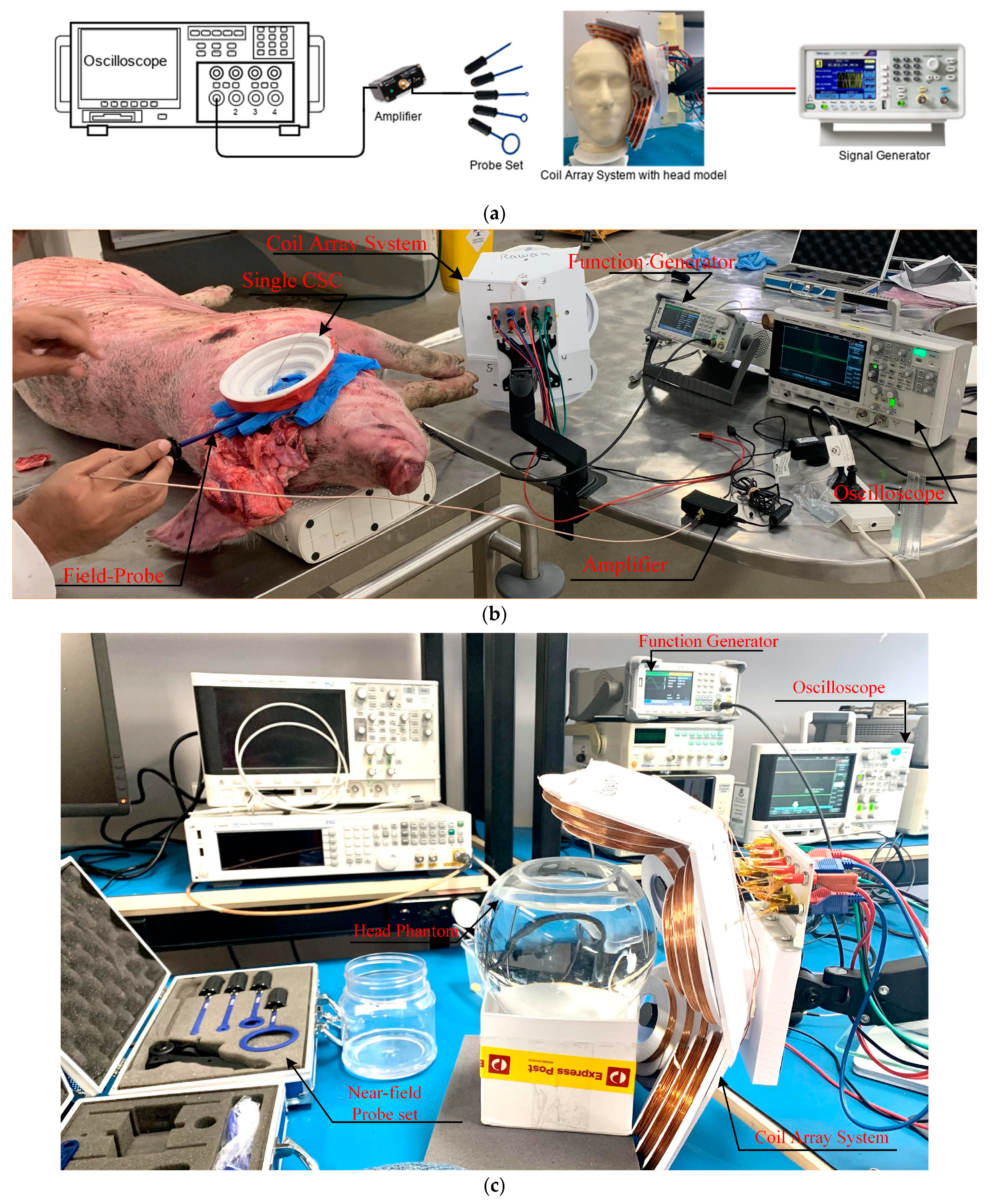


| Coil Geometry | First Coil | Second Coil | Third Coil |
|---|---|---|---|
| Outer Diameter (mm) | 62 | 64 | 30 |
| Inner Diameter (mm) | 56 | 40 | 20 |
| Height (mm) | 10 | 10 | 10 |
| Number of Turns | 30 | 30 | 60 |
| Wire Diameter (mm) | 1.2 | 1.2 | 1.2 |
| Number of Layers | 10 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Yosef, R.; Sultan, K.; Mobashsher, A.T.; Zare, F.; Mills, P.C.; Abbosh, A. Shielded Cone Coil Array for Non-Invasive Deep Brain Magnetic Stimulation. Biosensors 2024, 14, 32. https://doi.org/10.3390/bios14010032
Abu Yosef R, Sultan K, Mobashsher AT, Zare F, Mills PC, Abbosh A. Shielded Cone Coil Array for Non-Invasive Deep Brain Magnetic Stimulation. Biosensors. 2024; 14(1):32. https://doi.org/10.3390/bios14010032
Chicago/Turabian StyleAbu Yosef, Rawan, Kamel Sultan, Ahmed Toaha Mobashsher, Firuz Zare, Paul C. Mills, and Amin Abbosh. 2024. "Shielded Cone Coil Array for Non-Invasive Deep Brain Magnetic Stimulation" Biosensors 14, no. 1: 32. https://doi.org/10.3390/bios14010032
APA StyleAbu Yosef, R., Sultan, K., Mobashsher, A. T., Zare, F., Mills, P. C., & Abbosh, A. (2024). Shielded Cone Coil Array for Non-Invasive Deep Brain Magnetic Stimulation. Biosensors, 14(1), 32. https://doi.org/10.3390/bios14010032







