Ratiometric Electrochemical Detection of Interleukin-6 Using Electropolymerized Methylene Blue and a Multi-Walled Carbon-Nanotube-Modified Screen-Printed Carbon Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of Chitosan-Carboxylic Multi-Wall Carbon Nanotubes
2.4. Preparation of Ratio Electrochemical Sensor
3. Results and Discussion
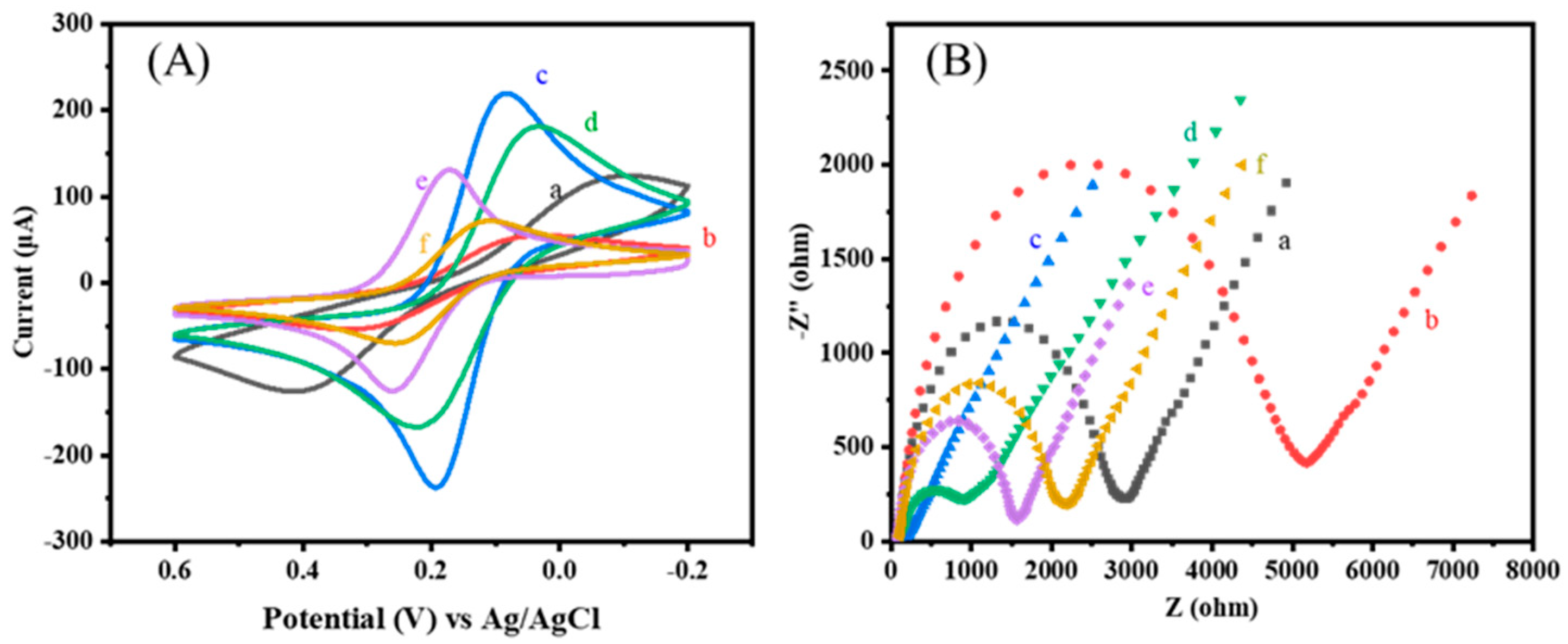
3.1. Characterizations
3.2. Construction Process and Optimization of Experimental Conditions for the Ratiometric Immunosensor
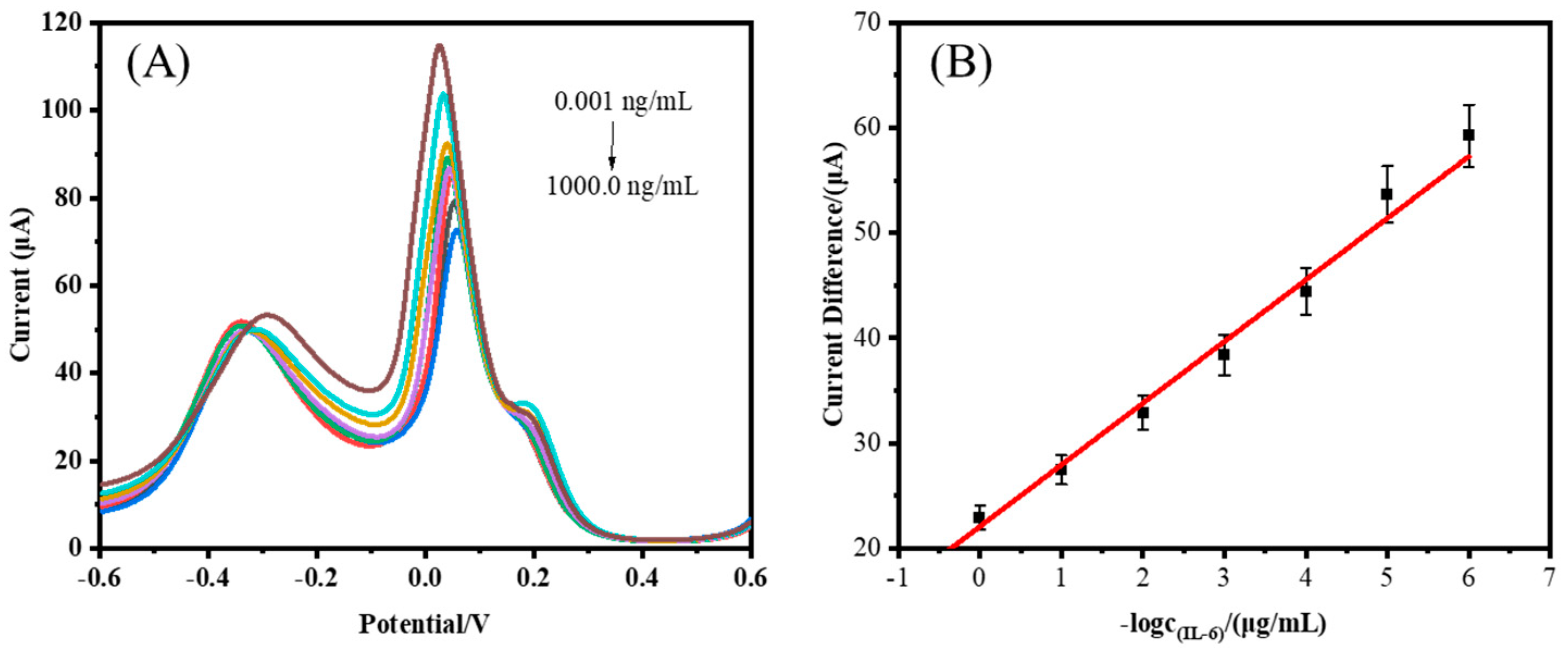
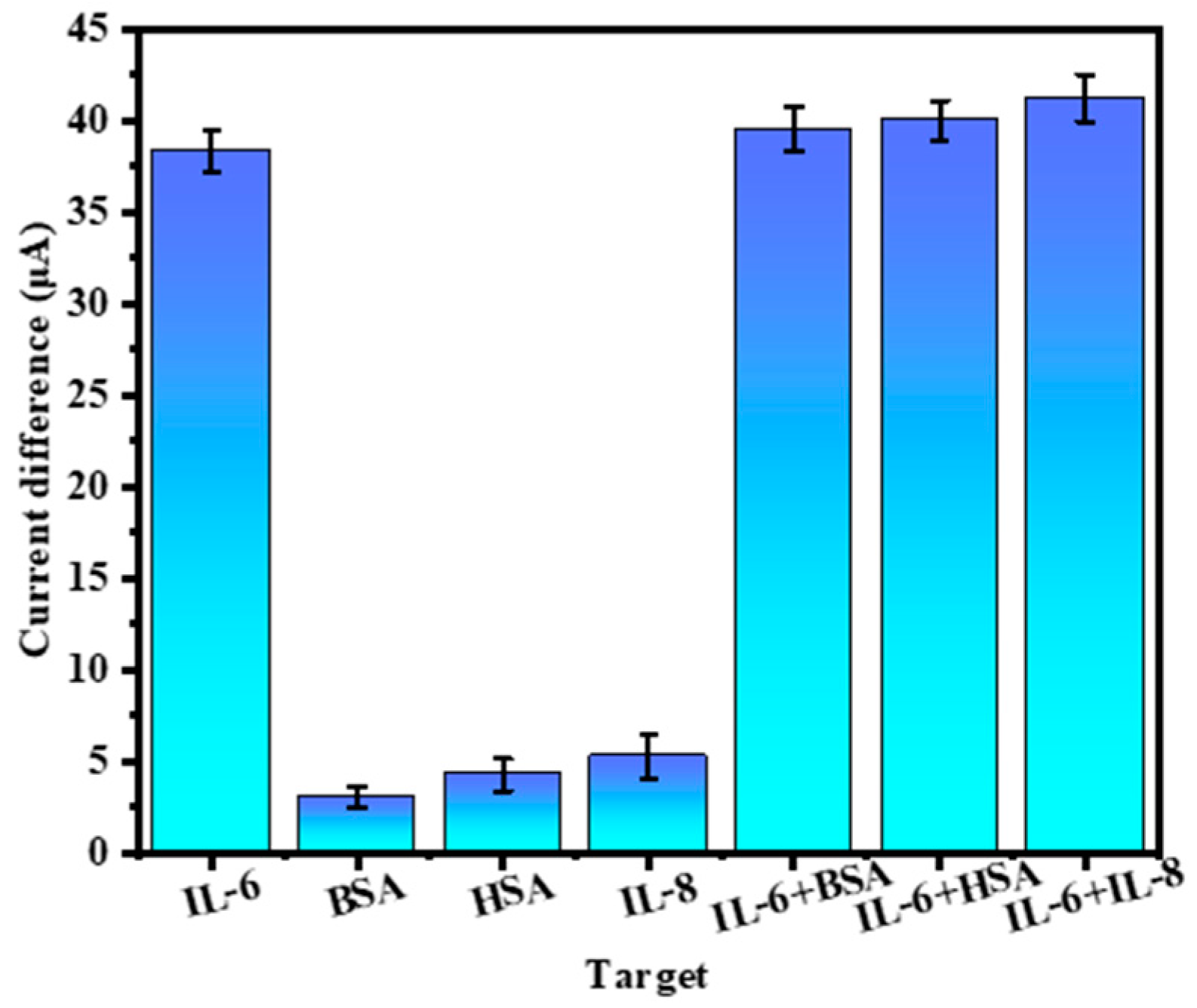
3.3. Performance Analysis of the Ratiometric Immunosensor
3.4. Application of IL-6 Sensor in Human Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, J.; Kim, D.; Park, J.; Kim, Y.-K.; Park Choo, H.-Y.; Woo, H.A. Novel Small Molecule Inhibitors Targeting the IL-6/STAT3 Pathway or IL-1β. Molecules 2022, 27, 2696. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Lamy de la Chapelle, M.; Marty, J.L. Recent Progresses in Optical Biosensors for Interleukin 6 Detection. Biosensors 2023, 13, 898. [Google Scholar] [CrossRef]
- Irimeș, M.-B.; Tertiș, M.; Oprean, R.; Cristea, C. Unrevealing the Connection between Real Sample Analysis and Analytical Method. The Case of Cytokines. Med. Res. Rev. 2024, 44, 23–65. [Google Scholar] [CrossRef]
- Scandurra, C.; Björkström, K.; Sarcina, L.; Imbriano, A.; Di Franco, C.; Österbacka, R.; Bollella, P.; Scamarcio, G.; Torsi, L.; Macchia, E. Single Molecule With a Large Transistor—Simot Cytokine IL-6 Detection Benchmarked against a Chemiluminescent Ultrasensitive Immunoassay Array. Adv. Mater. Technol. 2023, 8, 2201910. [Google Scholar] [CrossRef]
- Wang, R.; Feng, Y.; Sun, M.; Jiang, Y.; Li, Z.; Cui, L.; Wei, L. Mvil6: Accurate Identification of IL-6-Induced Peptides Using Multi-View Feature Learning. Int. J. Biol. Macromol. 2023, 246, 125412. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.F.; Abaurrea, A.; Azcoaga, P.; Araujo, A.M.; Caffarel, M.M. New Perspectives in Cancer Immunotherapy: Targeting IL-6 Cytokine Family. J. Immunother. Cancer 2023, 11, e007530. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, A.K.; Negi, P.; Kar, A.; Mariappan, E.; Natarajan, J.; Namboori, P.K.K.; Selvaraj, J. Navigating IL-6: From Molecular Mechanisms to Therapeutic Breakthroughs. Cytokine Growth Factors Rev. 2024, 76, 48–76. [Google Scholar] [CrossRef]
- Li, T.; Yang, N.; Pan, X.; Zhang, X.; Xu, L. A Portable Microfluidic Photometric Detection Method Based on Enzyme Linked Immunoscatter Enhancement. Biosens. Bioelectron. 2024, 244, 115794. [Google Scholar] [CrossRef]
- Huang, J.; Chen, T.; Zhao, Y.; Li, D.; Huang, Q.; Cao, L.; Chen, J.; Chen, D.; Hu, L.; Liu, H. Colloidal Quantum Dots-Modified Electrochemical Sensor for High-Sensitive Extracellular Vesicle Detection. Chem. Eng. J. 2024, 487, 150616. [Google Scholar] [CrossRef]
- Tao, J.; Wang, Y.; Zhai, W.; Wang, M. A Core–Shell Aunrs@BUT-16 Nanocomposite for Enhancement Sers Detection and Efficient Removal of Deoxynivalenol. J. Adv. Res. 2024, 38237769. [Google Scholar] [CrossRef]
- Jiang, Y.; Sima, Y.; Liu, L.; Zhou, C.; Shi, S.; Wan, K.; Chen, A.; Tang, N.; He, Q.; Liu, J. Research Progress on Portable Electrochemical Sensors for Detection of Mycotoxins in Food and Environmental Samples. Chem. Eng. J. 2024, 485, 149860. [Google Scholar] [CrossRef]
- Zou, H.; Wu, W.; Zhou, J.; Deng, C. Silar Growth of ZnO Nss/Cds QDS on the Optical Fiber-Based Opto-Electrode With Guided In Situ Light and Its Application for the “Signal-on” Detection of Inflammatory Cytokine. Anal. Chem. 2024, 96, 5446–5454. [Google Scholar] [CrossRef]
- Gurusamy, L.; Karuppasamy, L.; Anandan, S.; Barton, S.C.; Chuang, Y.-H.; Liu, C.-H.; Wu, J.J. Review of Oxygen-Vacancies Nanomaterials for Non-Enzymatic Electrochemical Sensors Application. Coord. Chem. Rev. 2023, 484, 215102. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, J.; Pan, Y.; Wu, Q.; Ping, J. Wearable Electrochemical Sensors for Plant Small-Molecule Detection. Trends Plant Sci. 2024, 29, 219–231. [Google Scholar] [CrossRef]
- Ting, W.-T.; Wang, M.-J.; Howlader, M.M.R. Interleukin-6 Electrochemical Sensor Using Poly(O-Phenylenediamine)-Based Molecularly Imprinted Polymer. Sens. Actuators B-Chem. 2024, 404, 135282. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Dou, Y.; Liu, X.; Song, S.; Jiang, H.; Fan, C. Electrochemical Biosensor for Point-of-Care Testing of Low-Abundance Biomarkers of Neurological Diseases. Anal. Chem. 2024, 96, 10332–10340. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiang, J.; Han, J.; Man, M.; Chen, L.; Li, B. An Electrochemical Molecularly Imprinted Microfluidic Paper-Based Chip for Detection of Inflammatory Biomarkers IL-6 and Pct. Analyst 2023, 148, 5896–5904. [Google Scholar] [CrossRef]
- Vessella, T.; Zhang, H.; Zhou, Z.; Cui, F.; Zhou, H.S. In-Situ Synthesized V(2)Ct(X) Mxene-Based Immune Tag for the Electrochemical Detection of Interleukin 6 (IL-6) from Breast Cancer Cells. Biosens. Bioelectron. 2023, 237, 115512. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Zeng, W.; Zhou, S.; Zhang, W.; Ma, J. A New Ratiometric Molecularly Imprinted Electrochemical Sensor for the Detection of Sunset Yellow Based on Gold Nanoparticles. Food Chem. 2023, 413, 135600. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Tian, Y.; Geng, Y.; Wang, J.; Ma, M. A Ratiometric Electrochemical Sensing Strategy Based on the Self-Assembly of Co Nc/Cnt and Methylene Blue for Effective Detection of the Food Additive Tert-Butylhydroquinone. Talanta 2024, 266, 125024. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, Y.; Yang, Y.; Liu, H.; Chen, Z.; Guo, L.; Li, L. A Novel Ratiometric Imprinted Electrochemical Sensing Bisphenol a Using Mip With Alkenyl Ferrocene as a Functional Monomer and Cof-Derived Porous Carbon as a Sensitive Element. Electrochim. Acta 2024, 475, 143672. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, K.; Ma, H.; Xiong, Q.; Li, Y.; Sun, M.; Wang, X.; Wang, Y.; Wu, C.; Su, G.; et al. Nanoarchitectonics of on–off Ratiometric Signal Amplified Electrochemical Sensor for Chlorpromazine With Molecularly Imprinted Polymer Based on Ni-Mof/Fe-Mof-5 Hybrid Au Nanoparticles. Sep. Purif. Technol. 2023, 327, 124858. [Google Scholar] [CrossRef]
- Chen, Y.; Su, X.; Wu, Z.; Deng, X.; Zhang, Y.; Zhao, Z.; Wei, Z.; Sun, S. Sensitive Sensing of Gla and Isl Based on Highly Conductivity Nitrogen-Doped Carbon Synergistic Dual-Template Molecularly Imprinted Ratiometric Electrochemical Sensor. Biosens. Bioelectron. 2024, 259, 116384. [Google Scholar] [CrossRef] [PubMed]
- Krasley, A.T.; Li, E.; Galeana, J.M.; Bulumulla, C.; Beyene, A.G.; Demirer, G.S. Carbon Nanomaterial Fluorescent Probes and Their Biological Applications. Chem. Rev. 2024, 124, 3085–3185. [Google Scholar] [CrossRef]
- Chu, H.; Hu, X.; Lee, C.Y.; Zhang, A.; Ye, Y.; Wang, Y.; Chen, Y.; Yan, X.; Wang, X.; Wei, J.; et al. A Wearable Electrochemical Fabric for Cytokine Monitoring. Biosens. Bioelectron. 2023, 232, 115301. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Sun, J.; Liu, M.; Huang, J.; Dong, J.; Guo, Z.; Guo, Y.; Sun, X. Molecularly Imprinted Polymers-Aptamer Electrochemical Sensor Based on Dual Recognition Strategy for High Sensitivity Detection of Chloramphenicol. Food Chem. 2024, 437, 137933. [Google Scholar] [CrossRef]
- El Fazdoune, M.; Bahend, K.; Oubella, M.; Ben Jadi, S.; El Guerraf, A.; Bazzaoui, E.A.; García-García, F.J.; Martinis, J.I.; Bazzaoui, M. Poly(Methylene Blue) Modified Pla-Cb Conductive 3D Printer Filament as a Promising Platform for Electrochemical Sensing of Uric Acid. J. Polym. Environ. 2023, 32, 2105–2119. [Google Scholar] [CrossRef]
- Negahdary, M.; Buoro, R.M.; Bacil, R.P.; Santos, B.G.; Angnes, L. Design of an Electrochemical Aptasensor in the Presence of an Array of Gold Nanostructure and a Go-Mwcnts Nanocomposite: Application in Diagnosis of Alzheimer’s Disease. Microchim. Acta 2023, 190, 409. [Google Scholar] [CrossRef]
- Li, Y.-J.; Yang, L.-L.; Ni, L.; Xiong, J.-M.; He, J.-Y.; Zhou, L.-D.; Luo, L.; Zhang, Q.-H.; Yuan, C.-S. Constructing Electrochemical Sensor Using Molecular-Imprinted Polysaccharide for Rapid Identification and Determination of L-Tryptophan in Diet. Food Chem. 2023, 425, 136486. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Khajehsharifi, H.; Hashemnia, S.; Solati, Z.; Azimpanah, R.; Shahrokhian, S. Evaluation of Molecular Imprinted Polymerized Methylene Blue/Aptamer as a Novel Hybrid Receptor for Cardiac Troponin I (Ctni) Detection at Glassy Carbon Electrodes Modified with New Biosynthesized Znonps. Sens. Actuators B-Chem. 2020, 320, 128316. [Google Scholar] [CrossRef]
- Chu, Z.; Guo, J.; Guo, J. Up-Conversion Luminescence System for Quantitative Detection of IL-6. IEEE Trans. NanoBiosci. 2023, 22, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Mahani, M.; Faghihi-Fard, M.; Divsar, F.; Torkzadeh-Mahani, M.; Khakbaz, F. Ultrasensitive Fret-Based Aptasensor for Interleukin-6 as a Biomarker for Covid-19 Progression Using Nitrogen-Doped Carbon Quantum Dots and Gold Nanoparticles. Microchim. Acta 2022, 189, 472. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, X.; Fan, M.; Ruan, S.; Peng, S.; You, R.; Chen, J.; Lu, Y. Sers-Based Self-Calibrating Aptamer Sensor for Selective Detection of IL-6. Sens. Actuators B-Chem. 2023, 374, 132828. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Corrigan, D.K. Comparing Nanobody and Aptamer-Based Capacitive Sensing for Detection of Interleukin-6 (IL-6) at Physiologically Relevant Levels. Anal. Bioanal. Chem. 2023, 415, 7035–7045. [Google Scholar] [CrossRef]




| Detection Method | Concentration Range/(ng/mL) | LOD/(ng/mL) | Reference |
|---|---|---|---|
| Homemade instruments | 1–200 | 1 | [31] |
| FRET | 1.5–5.9 × 10−3 | 8.2 × 10−4 | [32] |
| SERS | 0.1–1000 | 0.05 | [33] |
| EIS | 0.01–10 | 5 × 10−2 | [34] |
| DPV | 0.01–5 | 3.5 × 10−3 | [18] |
| DPV | 0.001–1000 | 5.4 × 10−4 | This work |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| t-test/p | 0.95 | 0.99 | 0.99 | 0.98 | 0.99 |
| Serum Samples | Added/(pg/mL) | Found/(pg/mL) | Recovery/% | RSD/% |
|---|---|---|---|---|
| 1 | 5.0 | 5.152 ± 0.17 | 103.0 | 3.29 |
| 2 | 10.0 | 10.254 ± 0.26 | 102.5 | 2.53 |
| 3 | 20.0 | 18.984 ± 0.68 | 94.9 | 3.58 |
| 4 | 50.0 | 51.024 ± 0.98 | 102.0 | 1.92 |
| 5 | 100.0 | 98.976 ± 2.88 | 99.0 | 2.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, F.; Wang, C.; Li, H.; Xu, Y.; Sun, S. Ratiometric Electrochemical Detection of Interleukin-6 Using Electropolymerized Methylene Blue and a Multi-Walled Carbon-Nanotube-Modified Screen-Printed Carbon Electrode. Biosensors 2024, 14, 457. https://doi.org/10.3390/bios14100457
Liu Z, Liu F, Wang C, Li H, Xu Y, Sun S. Ratiometric Electrochemical Detection of Interleukin-6 Using Electropolymerized Methylene Blue and a Multi-Walled Carbon-Nanotube-Modified Screen-Printed Carbon Electrode. Biosensors. 2024; 14(10):457. https://doi.org/10.3390/bios14100457
Chicago/Turabian StyleLiu, Zhuo, Fengyu Liu, Chaofan Wang, Hongjuan Li, Yongqian Xu, and Shiguo Sun. 2024. "Ratiometric Electrochemical Detection of Interleukin-6 Using Electropolymerized Methylene Blue and a Multi-Walled Carbon-Nanotube-Modified Screen-Printed Carbon Electrode" Biosensors 14, no. 10: 457. https://doi.org/10.3390/bios14100457
APA StyleLiu, Z., Liu, F., Wang, C., Li, H., Xu, Y., & Sun, S. (2024). Ratiometric Electrochemical Detection of Interleukin-6 Using Electropolymerized Methylene Blue and a Multi-Walled Carbon-Nanotube-Modified Screen-Printed Carbon Electrode. Biosensors, 14(10), 457. https://doi.org/10.3390/bios14100457





