Development of a Quantitative Digital Urinalysis Tool for Detection of Nitrite, Protein, Creatinine, and pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Reagents for Nitrite Detection
2.3. Preparation of Reagents for Protein Detection
2.4. Preparation of Reagents for Creatinine Detection
2.5. Preparation of Reagents for pH Detection
2.6. Colorimeter Measurement
2.7. Dipstick Measurement
3. Results and Discussion
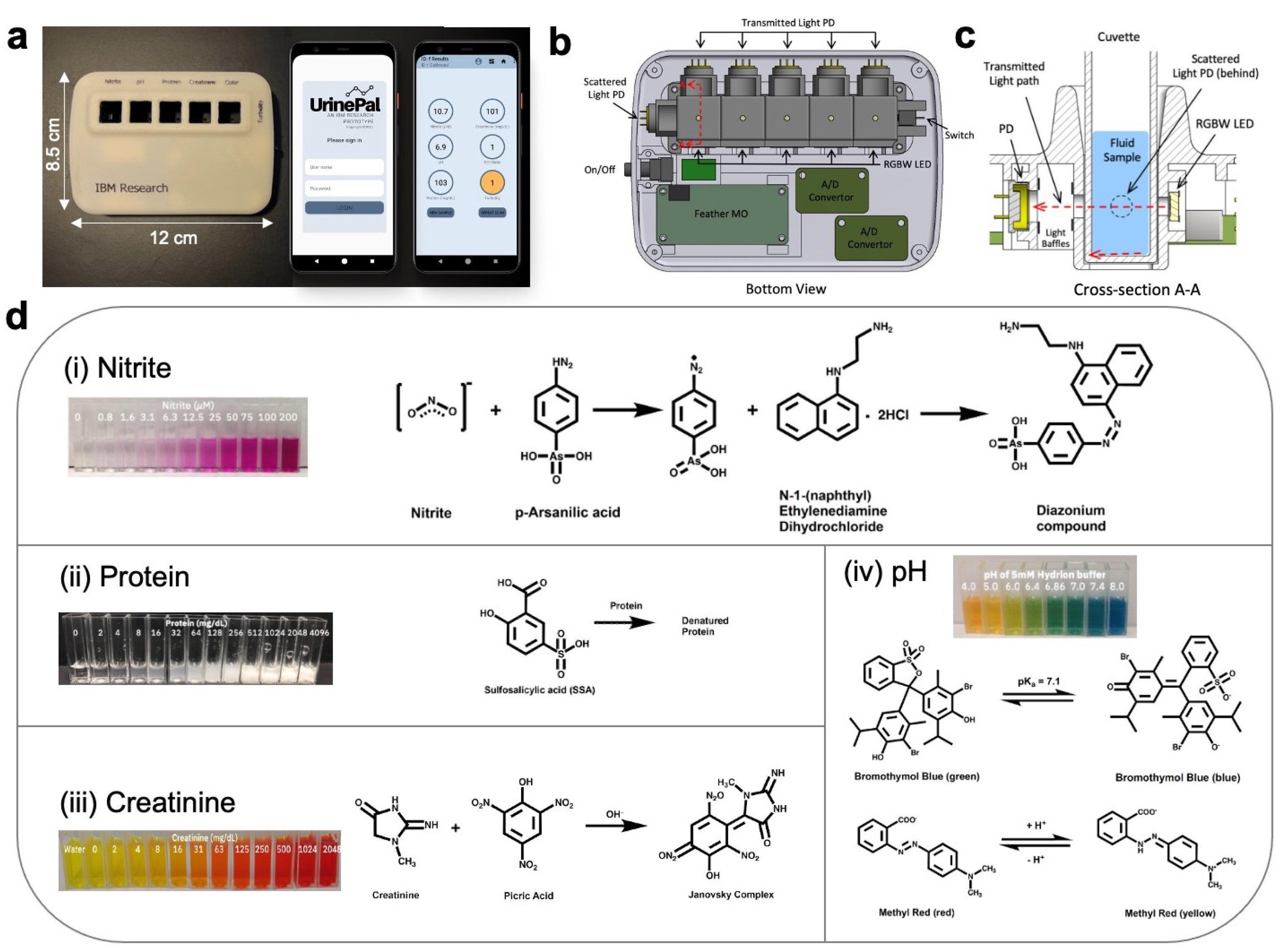
3.1. Colorimeter Device Design
3.2. Selection of Analyte Detection Reagents
3.3. Validation of Detection Assay Reagents
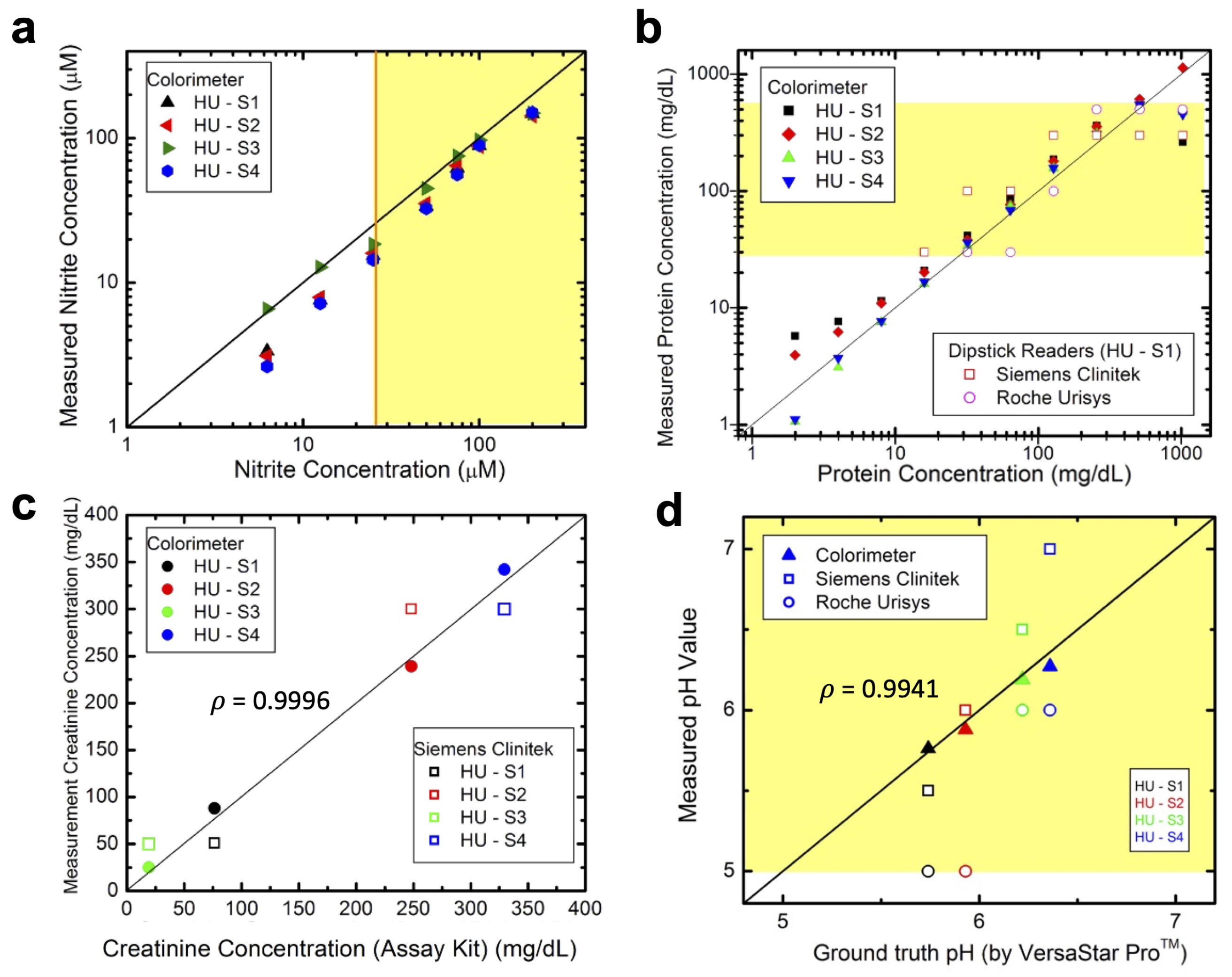
3.4. Analyte Detection in Artificial Urine
3.5. Analyte Detection in Human Urine
3.6. Color and Turbidity Measurements
3.7. Detection Reagent Stability
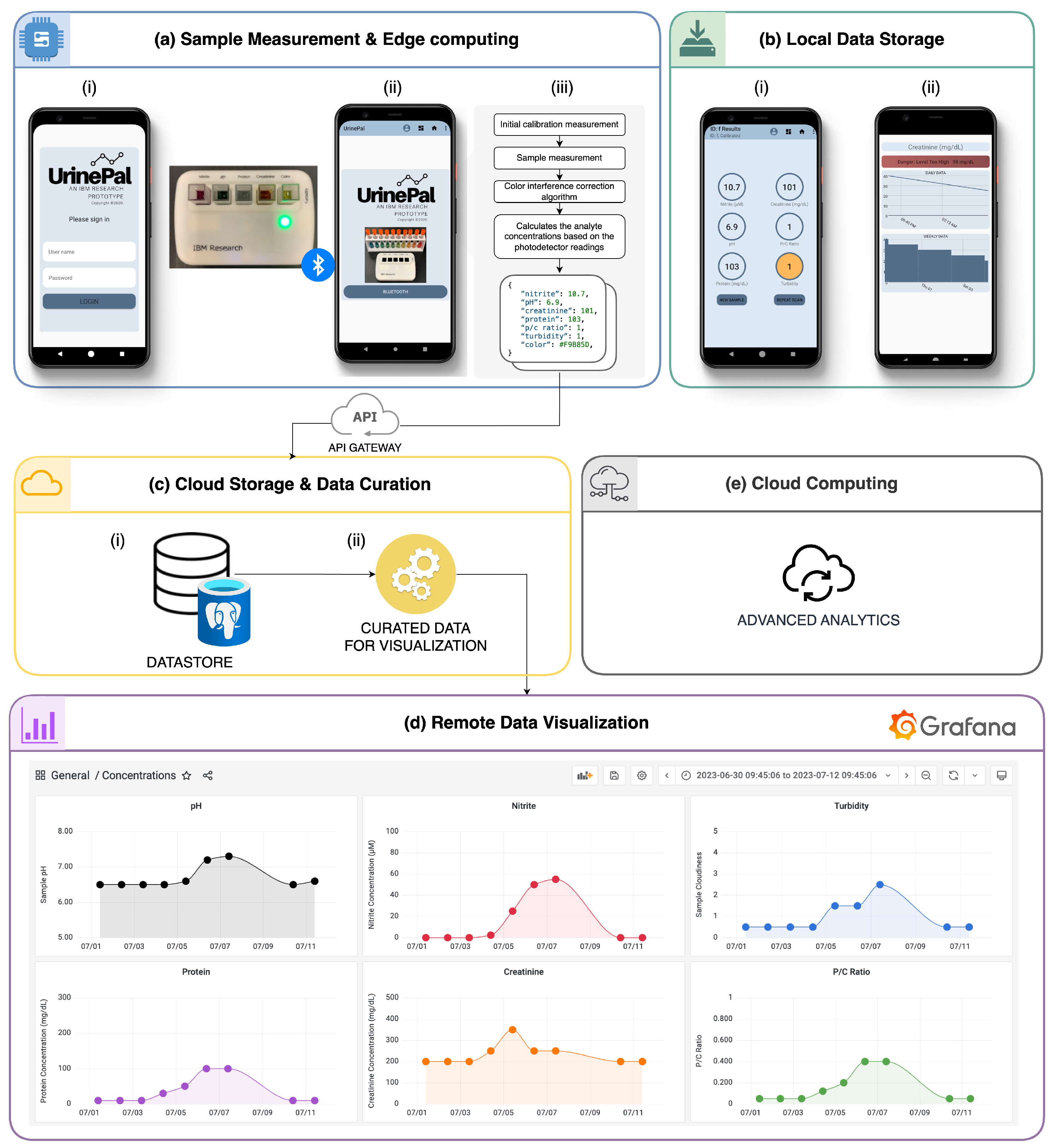
3.8. Cloud Connectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tucker, K.L.; Bowen, L.; Crawford, C.; Mallon, P.; Hinton, L.; Lee, M.; Oke, J.; Taylor, K.S.; Heneghan, C.; Bankhead, C.; et al. The feasibility and acceptability of self-testing for proteinuria during pregnancy: A mixed methods approach. Pregnancy Hypertens. 2018, 12, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Thompson, D.; Lieberman, E.S. Use of a random urinary protein-to-creatinine ratio for the diagnosis of significant proteinuria during pregnancy. Am. J. Obstet. Gynecol. 2001, 185, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Vuljanić, D.; Dojder, A.; Špoljaric, V.; Saračević, A.; Dukić, L.; Leniček-Krleža, J.; Vlašic-Tanasković, J.; Maradin, I.; Grzunov, A.; Vogrinc, Z.; et al. Analytical verification of 12 most commonly used urine dipsticks in Croatia: Comparability, repeatability and accuracy. Biochem. Med. 2019, 29, 010708. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kapoor, S.; Gupta, R.C. Comparison of urinary protein: Creatinine index and dipsticks for detection of microproteinuria in diabetes mellitus patients. J. Clin. Diagn. Res. 2013, 7, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.E.; Miller, D.L.; Shi, W.; Wenzler, D.; Elkhoury, F.F.; Patel, N.D.; Sur, R.L. Optimization of urinary dipstick pH: Are multiple dipstick pH readings reliably comparable to commercial 24-hour urinary pH? Investig. Clin. Urol. 2017, 58, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Kwon, M.; Ryu, S.; Woo, H.; Park, H. Performance Evaluation of three URiSCAN devices for routine urinalysis. J. Clin. Lab. Anal. 2016, 30, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Devillé, W.L.J.M.; Yzermans, J.C.; van Dujin, N.P.; Bezemer, P.D.; van der Windt, D.A.W.M.; Bouter, L.M. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004, 4, 1–4. [Google Scholar] [CrossRef]
- Abbott Architect System Specifications. Available online: https://cdn.pepperapps.io/diagnostics-cms/public/5fc549120bc133350e910860?signature=eyJhbGciOiJkaXIiLCJlbmMiOiJBMTI4Q0JDLUhTMjU2In0..sOpYOs97DOUIXCrbYACHAg.eFme2LJj_5YS0-zGx34yIPSGuz8urpdWwZuxojzSsmvkPPB1xmxwT6rwTeMQBsteqhKdWNGcBhJtDyXGYd9Ly0iDhvG_qA5jGYUfvwZFlSvFEqw0-Va2SE_OlaUru_2DdCpuroEBgH7oXj5pd88hKM0eiLzSQjlW3PaH-_mUFLK930qmcQcQyymQqQFtHNlJ.oIoycVemSs3FlC9NbCAkIg (accessed on 21 January 2024).
- Healthcare at the Speed of Life. Available online: https://healthy.io/ (accessed on 28 December 2023).
- Minuteful Kidney Test. Available online: https://lp.healthy.io/wp-content/uploads/2022/11/Minuteful-KT-UML-UK-GEN_4_20220810.pdf (accessed on 21 January 2024).
- BD Acquires Smartphone-Powered COVID Test Partner Scanwell Health. Available online: https://www.fiercebiotech.com/medtech/bd-acquires-smartphone-powered-covid-test-partner-scanwell-health (accessed on 28 December 2023).
- Smith, G.T.; Dwork, N.; Khan, S.A.; Millet, M.; Magar, K.; Javanmardb, M.; Bowden, A.K.E. Robust dipstick urinalysis using a low-cost, micro-volume slipping manifold and mobile phone platform. Lab Chip 2016, 16, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.F.; Nagi, R.; Sadeghi, K.; Phillips, S.; Ozcan, A. Albumin testing in urine using a smart-phone. Lab Chip 2013, 13, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Siu, V.S.; Lu, M.; Hsieh, K.Y.; Raines, K.; Asaad, Y.A.; Patel, K.; Afzali-Ardakani, A.; Wen, B.; Budd, R. Toward a quantitative colorimeter for point-of-care nitrite detection. ACS Omega 2022, 7, 11126–11134. [Google Scholar] [CrossRef] [PubMed]
- WS2812B: Intelligent Control LED Integrated Light Source. Available online: https://cdn-shop.adafruit.com/product-files/3094/WS2812B.pdf (accessed on 28 December 2023).
- Pugia, M.J.; Lott, J.A.; Profitt, J.A.; Cast, T.K. High-sensitivity dye binding assay for albumin in urine. J. Clin. Lab Anal. 1999, 13, 180–187. [Google Scholar] [CrossRef]
- Franke, G.; Salvati, M.; Sommer, R.G. Composition and Device for Urinary Protein Assay and Method of Using the Same. US Patent 5,326,707, 5 July 1994. [Google Scholar]
- Cahill, S.E.; Pugia, M.J.; Schaeper, R.J. Method for the Detection of Protein in Urine. US Patent 5,593,895, 14 January 1997. [Google Scholar]
- Watanabe, N.; Kamei, S.; Ohkubo, A.; Yamanaka, M.; Ohsawa, S.; Makino, K.; Tokuda, K. Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clin. Chem. 1986, 32, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Dube, J.; Girouard, J.; Leclerc, P.; Douville, P. Problems with the estimation of urine protein by automated assay. Clin. Biochem. 2005, 38, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yalamti, P.; Karra, M.L.; Bhongir, A.V. Comparison of urinary total protein by four different methods. Indian J. Clin. Biochem. 2016, 31, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Peake, M.; Whiting, M. Measurement of serum creatinine—Current status and future goals. Clin. Biochem. Rev. 2006, 27, 173–184. [Google Scholar] [PubMed]
- Franklin, L.U.; Saches, M.E. pH-Indicating Material and Cat Litter Containing Same. US Patent 5,267,532, 7 December 1993. [Google Scholar]
- Smith, J.V. Automated Analyzer Testing of Urine for Presence of a pH Abnormality. US Patent 6,468,805, 22 October 2022. [Google Scholar]
- Collins, G.F. pH Indicator Unit. US Patent 3,146,070, 25 August 1964. [Google Scholar]
- Wen, B.; Siu, V.S.; Buleje, I.; Hsieh, K.Y.; Itoh, T.; Zimmerli, L.; Hinds, N.; Eyigöz, E.; Dang, B.; von Cavallar, S.; et al. Health Guardian Platform: A technology stack to accelerate discovery in digital health research. In Proceedings of the IEEE International Conference for Digital Health (ICDH), Barcelona, Spain, 11–15 July 2022; pp. 40–46. [Google Scholar] [CrossRef]
- Mijuskovic, A.; Chiumento, A.; Bemthuis, R.; Aldea, A.; Havinga, P. Resource management techniques for cloud/fog and edge computing: An evaluation framework and classification. Sensors 2021, 21, 1832. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Knottenbelt, W.J.; Wolter, K. An efficient application partitioning algorithm in mobile environments. IEEE Trans. Parallel Distrib. Syst. 2016, 30, 1464–1480. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siu, V.S.; Lu, M.; Hsieh, K.Y.; Wen, B.; Buleje, I.; Hinds, N.; Patel, K.; Dang, B.; Budd, R. Development of a Quantitative Digital Urinalysis Tool for Detection of Nitrite, Protein, Creatinine, and pH. Biosensors 2024, 14, 70. https://doi.org/10.3390/bios14020070
Siu VS, Lu M, Hsieh KY, Wen B, Buleje I, Hinds N, Patel K, Dang B, Budd R. Development of a Quantitative Digital Urinalysis Tool for Detection of Nitrite, Protein, Creatinine, and pH. Biosensors. 2024; 14(2):70. https://doi.org/10.3390/bios14020070
Chicago/Turabian StyleSiu, Vince S., Minhua Lu, Kuan Yu Hsieh, Bo Wen, Italo Buleje, Nigel Hinds, Krishna Patel, Bing Dang, and Russell Budd. 2024. "Development of a Quantitative Digital Urinalysis Tool for Detection of Nitrite, Protein, Creatinine, and pH" Biosensors 14, no. 2: 70. https://doi.org/10.3390/bios14020070
APA StyleSiu, V. S., Lu, M., Hsieh, K. Y., Wen, B., Buleje, I., Hinds, N., Patel, K., Dang, B., & Budd, R. (2024). Development of a Quantitative Digital Urinalysis Tool for Detection of Nitrite, Protein, Creatinine, and pH. Biosensors, 14(2), 70. https://doi.org/10.3390/bios14020070




