Abstract
Current diagnostic and prognostic tests for prostate cancer require specialised laboratories and have low specificity for prostate cancer detection. As such, recent advancements in electrochemical devices for point of care (PoC) prostate cancer detection have seen significant interest. Liquid-biopsy detection of relevant circulating and exosomal nucleic acid markers presents the potential for minimally invasive testing. In combination, electrochemical devices and circulating DNA and RNA detection present an innovative approach for novel prostate cancer diagnostics, potentially directly within the clinic. Recent research in electrochemical impedance spectroscopy, voltammetry, chronoamperometry and potentiometric sensing using field-effect transistors will be discussed. Evaluation of the PoC relevance of these techniques and their fulfilment of the WHO’s REASSURED criteria for medical diagnostics is described. Further areas for exploration within electrochemical PoC testing and progression to clinical implementation for prostate cancer are assessed.
1. Introduction
Prostate cancer (PCa) presents a significant morbidity, where PCa mortality is the most common male-cancer-related death in 52 countries worldwide [1]. Prostate-specific antigen (PSA) testing is currently utilised to determine which men require further and more invasive testing. Population screening of PSA for PCa is not currently implemented, on account of the low specificity of the test to PCa [2,3]. Overtreatment and invasive diagnostic testing of men without PCa can result from high PSA levels in the blood, due to benign conditions [4]. Contrastingly, some PCa patients do not present with high circulating PSA concentrations, despite having the underlying disease.
Molecular diagnostic and prognostic testing for PCa has resulted in improved personalised medicine approaches for PCa. Nucleic acids, including DNA and RNA, can provide information on PCa presence, the chance of metastasis and the likelihood of clinical progression [5,6,7,8,9]. The Progensa prostate cancer antigen 3 (PCA3) mRNA assay has previously illustrated that nucleic acid molecular diagnostics can elevate the specificity of testing for PCa [10]. Specifically, the Progensa PCA3 urine test was utilised for PCa patients who had previously had a negative biopsy, to determine if they would require repeat biopsies [10]. However, a thorough economic evaluation of the Progensa PCA3 assay determined that the increase of quality-adjusted life years was not sufficient to accommodate the increased cost within the National Health Service in the United Kingdom [11]. The cell cycle risk (CCR) score (Myriad Genetics), Decipher score (Veracyte) and the genomic prostate score (GPS, Oncotype DX) are all marketed as prognostic mRNA panel tests [7,12,13]. While these tests represent the clinical utility of molecular diagnostics for PCa, each require specialised off-site laboratories and expensive equipment. Point of care (PoC) devices instead present the potential to bring personalised PCa diagnostics and prognostics directly within healthcare spaces. This could result in rapid multipanel testing of relevant biomarkers directly in the clinic, utilising non-specialised personnel.
Electrochemical biosensors for nucleic acid detection can result in exceptionally high sensitivities rivalling the current gold standard for nucleic acid amplification tests (NAATs), the quantitative polymerase chain reaction (qPCR). However, qPCR requires specialised laboratories and bulky thermal cycling equipment [14]. Relevant circulating and exosomal nucleic acid markers previously detected with qPCR for PCa diagnosis and prognosis will be described in this work. Detection of these relevant nucleic acid markers with PoC-compatible methodologies for PCa have also been reported and will also be explored in this review. Electrochemical biosensors can provide relatively simple, inexpensive and rapid detection of target analytes, through biomarker recognition, signal transduction and electronic readout (Figure 1) [15]. Electrochemical impedance spectroscopy (EIS), voltammetry, chronoamperometry and potentiometric sensing using field-effect transistors (FETs) will be discussed as electrochemical techniques for nucleic acid PoC PCa testing.
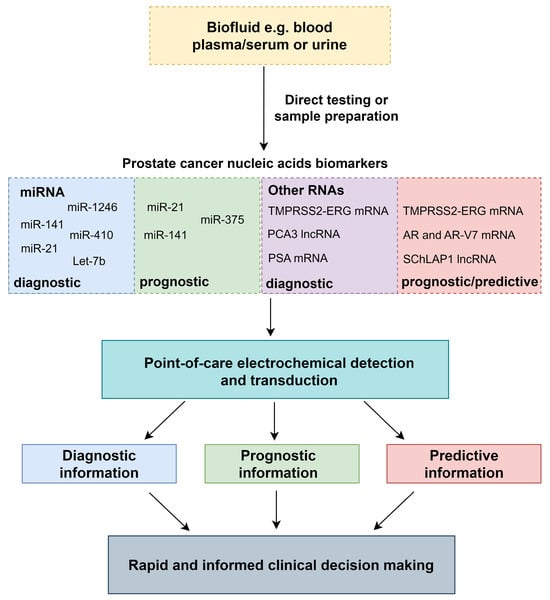
Figure 1.
The generic process of nucleic acid detection from biofluids to clinical decision making with PoC electrochemical devices.
The WHO has recommended that diagnostic PoC devices should adhere to the REASSURED criteria (Real-time connectivity, Ease of specimen collection, Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free or simple, and Deliverable to end users). Evaluation of current PoC tests for PCa will be discussed in relation to the REASSURED criteria, and further avenues for further research will be appraised.
2. Circulating and Exosomal Nucleic Acid Biomarkers for PCa Diagnosis and Prognosis
2.1. MicroRNAs
Detection of microRNAs—small (19–24 bp) single-stranded RNAs that predominantly regulate gene expression through the degradation of specific target mRNAs [16]—has seen significant interest in PCa for prospective diagnostics and prognostics. In blood and urine, microRNAs are inherently more stable than mRNAs [17,18]. As such, detection of microRNAs in both urine and blood could present easy, minimally invasive approaches to PoC nucleic acid detection. To date, several electrochemical PoC devices have targeted miR-21, miR-141, miR-410, miR-375, miR-1246 and Let7b for PCa diagnostics and prognostics [19,20,21,22,23].
In multiple cancers, including prostate, miR-21 and miR-141 are upregulated, and they are elevated in both the urine and blood of PCa patients relative to healthy men [24,25]. Correspondingly, they are both often utilised for PCa electrochemical diagnostic tests. Prognostically, miR-21 is associated with the epithelial-to-mesenchymal transition (EMT) in PCa, potentially linking miR-21 to more aggressive forms of the disease [26]. Meta-analysis of miR-21’s prognostic utility has indicated that the presence of miR-21 is strongly correlated with poor prognosis, a higher Gleason score and the prostate cancer stage [27]. When compared to healthy men, miR-141 is also upregulated in blood plasma and exosomes in PCa [24,28]. Metastatic PCa patients with elevated levels of miR-141 in serum also have more metastatic bone lesions, potentially highlighting the prognostic value of miR-141 detection [29].
It is also the case that miR-375 has been implicated in PCa and has previously been of interest for prognostic purposes. Higher levels of circulating miR-375 are present in more advanced disease, particularly within metastatic PCa, despite miR-375 having anti-invasive and anti-EMT properties [30,31,32,33]. Also, miR-375-related pathways are associated with taxane resistance in metastatic castration-resistant PCa mCRPC and differentiation to neuroendocrine PCa [31,34,35,36]. In combination with miR-141 detection, plasma miR-375 can also predict time to radiological progression [35]. As such, circulating miR-375 levels can provide useful prognostic and predictive information.
Furthermore, miR-410 has been successfully detected with PoC electrochemical devices for PCa [37], while miR-410-3p has previously been associated with the downregulation of the phosphatase and tensin homolog/protein kinase B/mammalian target of the rapamycin (PTEN/AKT/mTOR) pathway and is indicative of poorer PCa prognosis [38]. In PCa patients, miR-410-5p detection in blood serum was significantly higher when compared to healthy men [39]. As a result, miR-410 could provide relevant diagnostic information for PCa.
2.2. mRNAs and lncRNAs
While mRNAs and long non-coding RNAs (lncRNAs) are nucleic acid biomarkers prone to degradation, the development of the Progensa PCA3 assay has established that RNA detection can be relevant for early PCa diagnosis. Within this test, the relative quantity of PCA3 lncRNA is reported, relative to PSA mRNA concentration. Reported PCA3 score ratios above 25 can be utilised to improve the specificity of diagnostic testing when compared to PSA testing [10,40]. As such, several electrochemical devices aiming to improve upon current diagnostics utilise PCA3 lncRNA alone or in conjunction with PSA mRNA [41,42,43,44].
The gene fusion TMPRSS2-ERG has additionally been utilised as a predictive and prognostic marker for PCa. TMPRSS2-ERG is largely considered a PCa-specific biomarker and is indicative of an aggressive subset of the disease [45]. This fusion results in overexpression of the oncogene ERG driving progression towards metastasis [46,47]. In urine, TMPRSS2-ERG presence correlates with clinically significant PCa, tumour size and a high Gleason score at prostatectomy [48]. In the blood of mCRPC patients, several studies have recorded that patients with TMPRSS2-ERG mRNA presence respond poorly to taxane therapies, with reduced PSA progression-free survival and overall survival within this patient cohort [49,50]. TMPRSS2-ERG mRNA detection, therefore, can act as a biomarker for PCa prognosis or as a potential predictive marker for taxane resistance.
The androgen receptor (AR) signalling pathway, while maintaining a prostatic differentiated function in healthy men, can drive PCa growth and invasion [51]. Targeted therapies of the AR are utilised as first-line treatments for inoperable disease. Castration resistance in PCa results from resistance to these therapies and is indicative of late-stage and ultimately terminal disease [52]. Several mechanisms for castration resistance have been determined, including amplification of the AR or the presence of constitutively active AR variants (AR-Vs) [53,54]. Of note, AR-V7 is deficient in the ligand-binding domain region and, therefore, indicative of resistance to androgen-deprivation therapy [53,55]. Within the blood, AR mRNA amplification is associated with reduced overall survival (OS) and progression-free survival (PFS) in patients treated with androgen-deprivation therapies (ADT) [54]. On account of the low abundance of these mRNA biomarkers in the blood, ultrasensitive amplification strategies, including digital droplet PCR (ddPCR), have previously been utilised [56,57,58]. PoC devices, however, could provide valuable predictive and prognostic information with less complex detection methods.
2.3. Biofluid Considerations and Sample Preparation
Electrochemical PoC tests will either directly detect the nucleic acid markers from the desired biofluid or after sample preparation, once the biomarkers are extracted and purified. Blood, urine and exosomal nucleic acids can provide valuable information for PCa diagnostics and prognostics. Urine contains prostatic secretions and can be collected non-invasively, avoiding potentially painful procedures for use in diagnostic or prognostic tests [59]. The Progensa PCA3 assay test has previously used urine for PCa diagnostics [10]. For electrochemical devices that directly test from urine samples, robust analysis will be required, to account for the high variability of pH, metabolites and solid particulates between patients [59,60,61]. Ensuring that the sensitivity and signal transduction of these devices is maintained could reduce the likelihood of false negatives. For nucleic acid detection from urinary exosomes the gold standard is ultracentrifugation followed by purification. Alternative approaches will be required to avoid the use of the highly specialised, bulky laboratory equipment currently utilised for this technique [62].
Detection of nucleic acids in the blood can additionally be utilised for PCa diagnosis and prognosis [32,35,63]. Since intravasation of tumour cells from the primary tumour occurs during the progression to metastasis, the presence of nucleic acids within the blood can be utilised for prognostic purposes for PCa. Electrochemical detection of microRNAs has previously taken place directly from patient samples for PCa, despite their low abundance [20,64]. Other electrochemical techniques have additionally been shown to detect low levels of synthetic microRNAs spiked into serum or plasma [19,37,65]. Specialised kits for circulating RNA extraction from the blood can also be utilised that typically result in cell lysis, protein denaturation followed by RNA purification [66]. However, there can be significant variation in yield and purity between kits and extraction methodologies [67]; mRNAs are low-abundance and labile biomarkers in the blood. For example, AR-V7 mRNA has previously been recorded in the magnitude of 0–146 copies per mL of blood from mCRPC patients [57]. Therefore, direct detection of mRNA in blood plasma and serum is likely to require high volumes and highly sensitive detection methods to be viable. Fragmentation of the mRNA in the blood can additionally present challenges for detection [64]. However, on account of the limited time that mRNAs remain in the blood, they can provide valuable temporal information on the PCa tumour state. To take place successfully, mRNA detection in the blood is more likely to require sample preparation and purification. Incorporation of PoC sample preparation techniques for mRNA detection, therefore, would be essential to bringing PoC electrochemical devices directly into the clinic.
3. Point-of-Care Electrochemical Techniques
3.1. Electrochemical Impedance Spectroscopy
EIS is a widely used point-of-care electrochemical technique for the diagnosis of cancers and infectious diseases [19,68]. In faradaic EIS, a redox solution (often [Fe(CN)6]3−/4−) is utilised. With a signal-on EIS biosensor, the target analyte presence at the working electrode reduces the flux of the electron exchange of the redox reaction (Figure 2a) [69]. The perturbation of the solution is then measured when an alternating current or voltage is applied across a range of frequencies. The steady-state nature of EIS can allow for high-sensitivity devices that can often be label-free [70]. Like many electrochemical techniques, appropriate functionalisation of the working electrode in EIS can result in the detection of proteins, metabolites and nucleic acids [71,72,73,74,75].
Detection of long non-coding RNA (lncRNA) PCA3 through a low-cost impedimetric sensor was reported by Coatrini Soares et al. PCA3 overexpression is a PCa-specific biomarker, and, therefore, it is utilised for potential early PCa diagnosis [76]. Single-stranded DNA (ssDNA) complementary to a region of the PCA3 lncRNA was immobilised on multi-walled carbon nanotube (MWCNT)-coated interdigitated gold electrodes. Binding of the PCA3 lncRNA resulted in impedance of the redox reaction present in the electrolyte solution. The specificity of the device was successfully confirmed with RNA extracted from PCa and HeLa cell lines. Quantitative detection of synthetic PCA3 RNA was achieved down to 0.128 nM [73]. The subsequent alteration of the working electrode to a printed carbon electrode coated with chondroitin sulfate stabilised gold nanoparticles (AuNPs) improved the sensitivity of PCA3 lncRNA detection to 83 pM [77]. This work presents a potential low-cost, sensitive and specific biosensor for PCA3 lncRNA detection at the PoC. Further exploration of quantitative PCA3 detection directly within clinical urine samples would illustrate the potential of this biosensor for early diagnosis of PCa.
Aptamers as recognition elements have additionally seen success in the detection of PCA3 lncRNA for EIS. Takita et al. developed a biosensor with PoC potential, using a screen-printed carbon electrode (SPCE) and AuNPs that could easily immobilise the aptamer with thiol chemistries [43]. Previous validation of the aptamer established a high affinity and specificity for PCA3 lncRNA binding [78]. Detection of PCA3 lncRNA down to 1 fM was observed with this method, and 20 fM could be detected in artificial serum samples [43]. Specificity testing with cell lines would additionally aid the biosensor for clinical implementation.
Since microRNAs are small single-stranded RNA sequences, complementary ssDNA probes can often be utilised as simple bio-recognition elements. Yaman et al. successfully and sensitively detected miR-410 with EIS and an AuNP assembled peptides nanotube graphite sensor [37]. Binding of miR-410 to a complementary ssDNA probe impeded the flux of a redox probe, and this steric hindrance could be quantitatively determined as an increase in resistance. Detection of miR-410 presence could be easily distinguished from miR-192 and miR-200c microRNAs. Recovery of spiked-in microRNA into human serum within this work illustrated the use of the device directly with biological mediums [37].
Despite the valuable utility of EIS for PoC nucleic acid detection, PCa testing directly from clinical samples for microRNA and mRNA biomarker detection has not yet taken place. However, previous impedimetric-based sensors have been successful in detecting PCa metabolites in patient samples, or nucleic acid markers in other cancers [79,80]. This illustrates the potential for clinical sample testing with EIS PoC devices for PCa.
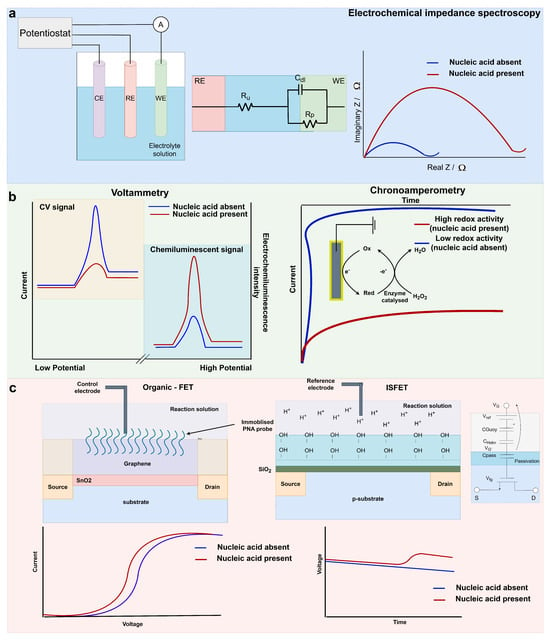
Figure 2.
(a) A simplistic three-electrode electrochemical system and the graphical output of an EIS on−switch system like that reported in [81]; (b) An example cyclic voltammetry signal (off−switch) and electrochemiluminescence (on−switch), as reported by [82] for miR−21 and miR−141 detection. An example chronoamperometry output and redox system for nucleic acid biomarker detection similar to that reported in [83]; (c) Organic graphene FET cross section and graphical interpretation of the sensor output reported by [21] and ISFET cross section ISFET macromodel, and graphical interpretation of a sensor output for nucleic acid biomarker detection in [84].
3.2. Voltammetry and Chronoamperometry
3.2.1. Voltammetry
Voltammetry is utilised to observe changes in current when the potential is adjusted at a fixed rate. The half-cell potential of a target analyte can be observed with voltammetric biosensors. Common voltammetry techniques for use within biosensors include cyclic voltammetry (CV), differential-pulse voltammetry (DPV) and square-wave voltammetry (SWV). Cyclic voltammetry can be utilised to observe the oxidation and reduction potentials of species in solution through forward and reverse sweeps of potential with a potentiostat [85]. In the switch-off CV example shown in Figure 2b, the nucleic acid presence quenches the electron exchange of ferrocene at the working electrode, reducing the amplitude of the current during the CV scans. Usage of CV can result in the formation of low-cost and simplistic biosensors for nucleic acid detection [82,86]. In DPV, a linear ramp with small pulses is utilised. The current is recorded before each individual pulse, reducing the effect of the charging current [87,88]. In SWV, the staircase potential is superimposed with a square wave, and the current is sampled after each pulse [89]. As a result, both techniques have low capacitive currents and high sensitivities for analyte detection.
A CV and chemiluminescence biosensor developed by Feng et al. was utilised for multiplexed detection of miR-21 and miR-141 (Figure 2b) [82]. This biosensor comprised dual working electrodes coated in AuNPs. A ruthenium luminophore complex was attached to the working electrode surface. Hairpin DNA probes for miR-21 and miR-141 detection were attached to ferrocene and immobilised on the working electrode surface. The ferrocene acted as a quencher to the ruthenium complex. Specific microRNA binding to the DNA hairpin probe quantitatively unquenched the luminophore. Since the proximity of the ferrocene to the working electrode was abrogated, CV negative readings were also indicative of microRNA binding [82]. Detection of miR-21 and miR-141 occurred down to concentrations of 6.3 fM and 8.6 fM, respectively. Robust detection of spiked samples into human serum samples illustrated the potential of the biosensor for testing directly with clinical samples.
Similarly, DPV was also utilised as an electrochemical detection technique for miR-21 presence as a diagnostic test for PCa [65]. Cd2+ ions, which intercalated with the miR-21 phosphate backbone, provided the signal for the DPV detection. Immobilisation of the peptide nucleic acid recognition element was conducted with a dendritic gold (den-Au) nanostructure attached to SWCNTs on a doped tin oxide electrode [65]. Den-Au nanostructures can greatly increase the surface area of the biosensor for probe immobilisation [90]. A limit of detection of 0.01 fM was observed with this method, and a 10 fM miR-21 presence spiked into serum samples was observed with good recovery (97%). However, since miR-21 is also upregulated in bladder cancer, detection in urine is likely to not be specific to PCa [24,91]. For a PoC device, multiplexing of miR-21 with other PCa-specific nucleic acids or as a companion to PSA testing could alleviate this issue.
Furthermore, miR-21 and miR-141 simultaneous detection with SVW has been successful, with potential point-of-care deployment and a dual working electrode system [92]. In this instance, the addition of duplex-specific nuclease-assisted target-recycling signal amplification improved the sensitivity of the device. This allowed for the rebinding of the original target to the probe, to boost the signal from the analyte presence [92]. Extracted RNA from 22Rv1 PCa and MCF-7 breast cancer cell lines were utilised to confirm the specificity of the device.
Signal amplification with SWV has additionally allowed for miR-21 and miR-141 detection down to 0.1 fM [19]. Tian et al. reported a paper-based electrode system utilising molybdenum sulphide AuNPs. Enhancement of the electrochemical device with platinum/copper metal–organic frameworks allowed for the high sensitivity of the device. The DNA probe for miR-21 was bound to ferrocene, and the miR-141 probe was bound to methylene blue. Distinction of these two biomarkers was, therefore, possible through the different oxidation/reduction potentials of their respective electrochemical reporters. High recoveries of both microRNAs were additionally recorded down to 20 pM within spiked serum samples [19].
A more simplistic SWV technique for miR-375 detection saw improved sensitivity down to 11.7 aM and a limit of detection of 12.4 aM in diluted serum [23]. In this work, an ssDNA probe complementary to miR-375 was immobilised onto a gold electrode with a standard thiol chemistry approach. With this method, miR-375 was only observed in metastatic PCa cell lines and not in immortalised prostate epithelial cell lines [23]. The high sensitivity and simplistic development of this SWV detection platform could introduce a facile, amplification-free method for PoC microRNA detection.
3.2.2. Chronoamperometry
Chronoamperometry measures the current response over time during a single or double potential step. In the example shown in Figure 2b, the potential step induces a redox reaction involving 3,3′5,5′-tetramethylbenzidine (TMB) and horseradish peroxidase (HRP) when the target miRNA is present [83]. As a result, an observable shift in current over time is detected. Chronoamperometry has been utilised for PCa diagnostic purposes through the detection of PCA3 lncRNA and PSA mRNA [42]. Since the Progensa assay has previously established the clinical utility of detecting the ratio of PCA3 and PSA, emulation of this assay with PoC compatibility could result in a robust diagnostic test. Sanchez-Salcedo et al. utilised a sandwich assay for recognition of the two RNA biomarkers on individual working electrodes for multiplex detection. Binding of the target analytes resulted in the increased proximity of TMB to the working electrodes, to produce an electrochemical signal. The sensitivity of the device could detect PCA3 lncRNA and PSA mRNA down to 4.4 pM and 1.5 pM, respectively [42]. Importantly, detection of these biomarkers was additionally observed in PCa urine samples, where RNA was extracted and specifically enriched for PCA3 lncRNA and PSA mRNA. This work showed preliminary evidence of the clinical utility of the device. Further analysis with a larger cohort could determine the success of the device for early PCa diagnosis.
Similarly, chronoamperometry and reverse-transcription loop-mediated isothermal amplification (RT-LAMP) were utilised for PCA3 lncRNA and PSA mRNA detection [41]. LAMP is an isothermal amplification technique, often utilised for nucleic acid detection at PoC [93,94]. Here, LAMP was utilised to preamplify PCA3 lncRNA and PSA mRNA with a digoxigenin-bound dUTP. The LAMP amplicons were captured with strepavidin bound to an antidigoxigenin antibody and HRP. If the RNA target was present, accumulation of HRP occurred at the working electrode, and the reduction of benzoquinone to hydroquinone induced an electrochemical signal for chronoamperotery detection [41]. Extracted RNA from nine cell lines was utilised to robustly confirm the specificity of the electrochemical biosensor. Analysis with PCa and healthy urine samples established that PCA3 lncRNA and PCA3/PSA ratio were upregulated in PCa patients. All the steps required for this biosensor, except RNA extraction, can take place at the PoC. Evidence of quantitative RNA detection would further improve the value of PCA3/PSA ratios determined by this device.
An alternative chronoamperometry biosensor with an alternative isothermal reaction, recombinase polymerase amplification (RPA), was successful in detecting several nucleic acid targets, including TMPRSS2-ERG mRNA, PCA3 lncRNA, KLK2 mRNA (as an internal control) and SChLAP1 lncRNA. This work additionally presented a PoC methodology for sample preparation, utilising magnetic beads rendering extracted DNA and RNA sequences. RPA forward primers for each nucleic acid target were tethered to the working electrode, creating amplicons at the electrode surface in the presence of their respective targets. Peroxidase-mimicking nanozymes were subsequently added, which catalysed a redox reaction in the presence of the amplicons, rendering a detectable signal with chronoamperometry [95]. PCa cell lines (DuCaPs, LnCaPs and 22Rv1s) were utilised to confirm the specificity of the biosensor to the RNA targets. Simultaneous detection of the four nucleic acid targets in both the serum and the urine of PCa patients was achieved, and both biofluids were congruent for biomarker detection. Overexpression of these PCa biomarkers correlated with high-grade PCa [95]. This work illustrated a truly PoC method from sample to result, validated with a small cohort of PCa clinical samples within 30 min (Table 1).

Table 1.
Summary of point-of-care bioelectrical nucleic acid devices for prostate cancer diagnosis and prognosis.
Table 1.
Summary of point-of-care bioelectrical nucleic acid devices for prostate cancer diagnosis and prognosis.
| Bio-Electrical Detection Method | Bio-Recognition Element | Nucleic Acid Target | Limit of Detection | Quantitative Range | Endogenous Detection | References |
|---|---|---|---|---|---|---|
| EIS | ssDNA probe on chitosan and carbon nanotubes | PCA3 lncRNA | 0.128 nM | N/A | cell line | [73] |
| EIS | printed carbon electrode, chondroitin sulfate stabilised AuNPs and ssDNA probe | PCA3 lncRNA | 83 pM | N/A | N/A | [77] |
| EIS | SPCE, AuNPs and aptamer | PCA3 lncRNA | 1 fM | 0.1 pM to 10 nM | spiked artificial urine | [43] |
| EIS | AuNPs, peptide nanotubes and ssDNA probe | miR-410 | 3.9 fM | 10 fM to 300 pM | spiked serum | [37] |
| chronoamperometry | framework nucleic acid electrode and ssDNA probe | miR-21, miR-141 and Let-7a | 10 fM (miR-21) and 1 aM (miR-141) | 10 aM to 1 pM (miR-141) | cell line | [96] |
| chronoamperometry | RPA and peroxidase-mimicking nanozymes | TMPRSS2-ERG, PCA3, SChLAP1 and KLK2 nucleic acids | 50 copies | N/A | urine and serum samples | [95] |
| chronoamperometry | screen-printed carbon electrode and biotinylated ssDNA probe | exosomal miR-451 and miR-21 | 10 pM | 10 pM to 100 nM | extracted exosomal RNA from urine samples | [83] |
| chronoamperometry | gold nanoparticles and sandwich assay | PCA3 and PSA mRNA | 4.4 and 1.5 pM | 25 pM to 10 nM (PCA3), 25 pM to 1 nM (PSA) | extracted RNA from urine samples | [42] |
| chronoamperometry | RT-LAMP, magnetic beads and SPCE | PCA3 lncRNA and PSA mRNA | N/A | N/A | extracted RNA from urine samples | [41] |
| chemoluminescence and CV | AuNPs, Ru complexes and DNA probes | miR-21 and miR-141 | 6.3 and 8.6 fM | 0.02 pM to 150 pM (miR-21), 0.03 pM to 150 pM (miR-141) | N/A | [82] |
| DPV | SWCNT dendritic Au nanostructure and peptide nucleic acid probe | miR-21 | 0.01 fM | 0.01 fM to 1 M | spiked serum | [65] |
| SWV and EIS | MoS2/AuNPs/AgNW and signal amplification | miR-21 and miR-141 | 0.1 fM | 1 fM to 1 nM | spiked serum | [19] |
| SWV | redox labelled DNA hairpins on Au electrode and recycling signal amplification | miR-21 and miR-141 | 4.2 and 3.0 fM | 5 fM to 50 pM | cell lines | [92] |
| SWV | ssDNA probe and gold working electrode | miR-375 | 11.7 aM | 10 aM to 1 nM | cell lines and spiked serum | [23] |
| graphene FET | peptide nucleic acids immobilised on graphene oxide nanosheet | miR-21, miR-1246 and Let-7b | 10 fM | 10 fM to 10 nM | urine samples | [21] |
| solution-gated graphene FET | ssDNA probe immobolised on Au gate | miR-21 | 0.01 aM | 0.01 aM to 1 pM | blood serum patient samples | [20] |
| ISFET | target-specific RT-LAMP and pH-sensing passivation layer | AR-V7, TMPRSS2-ERG, YAP1 and AR-FL mRNA | 5–8 aM | 5–8 aM to 5–8 pM | cell lines, spiked serum and plasma | [84,97] |
MicroRNA detection with chronoamperometry has additionally resulted in highly sensitive PoC electrochemical devices. In research conducted by Wen et al. [96], miR-21 and miR-141 and Let-7a detection occurred with a framework nucleic acid (FNA) platform. Formation of a tetrahedral nucleic acid framework interface on the surface of a gold working electrode resulted in microRNA binding near the electrode surface. Localisation of HRP and TMB to the FNA network in the presence of microRNA was achieved with a tagged DNA reporter sequence. Synthetic miR-141 and miR-21 detection down to 1 aM and 10 fM, respectively, was achievable with this amplification-free method. Quantitative detection of miR-141 was additionally observed in PCa cell lines. This work could multiplex up to 16 individual sensors for microRNA detection [96]. Further expansion of microRNA probes for this FNA platform could result in simultaneous high-throughput detection of multiple nucleic acid markers for PCa diagnosis and prognosis.
For example, a screen-printed carbon electrode (SPCE)-based biosensor developed by Chiou et al. was capable of detecting exosomal miR-451 and miR-21 in PCa urine samples [83]. Screen-printing electrodes are a disposable, cheap alternative to traditional methods, ideal for PoC purposes [98]. NeutrAvidin binding to a biotinylated ssDNA probe was utilised to tether the probe to the screen-printed carbon electrode (SPCE)-based biosensor [83]. A further probe complementary to part of the microRNA sequence was tagged with fluorescein. Upon binding of the target microRNA, HRP attached to an anti-fluorescein antibody was localised to the SPCE for chronoamperometry measurement (Figure 2b). Selectivity of the device for these exosomal microRNAs was confirmed against miR-141 and miR-636. With this biosensor, a threshold of 220 nA for exosomal miR-451 could distinguish between PCa patients and a non-cancer group (BPH patients and healthy people). Exosomal miR-21 detection in PCa samples correlated with tumour size in PCa but was not elevated in PCa relative to the controls [83]. As such, detection of these two microRNAs could provide both diagnostic and prognostic utility directly in the clinic.
These works illustrate the versatility of chronoamperometry as a bioelectrical technique for detection of multiple nucleic acid biomarkers for PCa diagnostics and prognostics.
3.3. Potentiometric Sensing Using Field-Effect Transistors
FETs when used as sensing elements for diagnostics typically have portable instrumentation, low power usage and cost-effective manufacturing processes, making them an exemplary electrochemical technique for PoC purposes. In particular, some FETs can be utilised with unmodified complementary metal-oxide semiconductor (CMOS) technology, simplifying the manufacturing process of these devices. Organic FETS (OFETs) are typically functionalised with biorecognition elements for nucleic acid sensing. For example, miR-1246, miR-21 and let7b were simultaneously multipanel-detected with a reduced graphene oxide nanosheet and immobilised peptide nucleic acid microRNA probes [21]. Partitioning of the device for individual microRNA detection allowed for simultaneous detection of the three microRNAs. The formation of PNA-microRNA hybrids near the FET surface resulted in an increased electrostatic charge that could be detected as a shift in the gate voltage (Figure 2c). Crucially, urine samples could be added to this microfluidic device without the requirement for pre-processing for quantitative microRNA detection down to 10 fM within 20 min. Elevated microRNA levels were detected in six PCa patients compared to four healthy controls, and these were ratified with reverse transcription-qPCR [21]. This work provided a strong foundation for a non-invasive microRNA detection system for direct and rapid PoC testing. Evaluation of the clinical validity of microRNA detection with this device and a larger cohort of PCa patients would further strengthen its implementation in clinics.
Direct detection of miR-21 from PCa blood serum samples was also observed with a solution-gated graphene FET designed by Deng et al. [20]. Induced Dirac voltage shifts from the binding of miR-21 to a complementary ssDNA probe on the gold gate surface can sensitively detect a relevant biomarker presence. An exemplary quantitative sensitivity of 0.01 aM, approaching single-molecule detection, was observed with this device. A single change of one nucleotide in the miR-21 sequence resulted in lower Dirac voltage shifts, confirming the specificity of the biosensor [20]. Direct testing in clinical blood serum samples of miR-21 could successfully distinguish between PCa patients and patients with benign prostatic hyperplasia (BPH). Since PSA testing is additionally elevated in other prostatic diseases, including BPH, circulating miR-21 PoC detection could provide additional diagnostic information for PCa patients.
Ion-sensitive field-effect transistors (ISFETs) are an FET that can detect the concentration of ions in solution through a passivation (sensing) layer. The passivation layer can detect changes in pH in the solution above through the hydroxylation of the layer detecting pertubations in ion concentration in the solution above [99]. ISFETs can be utilised directly with unmodified complementary metal-oxide semiconductor (CMOS) technology, increasing the ease of manufacture and implementation of ISFET PoC devices [100].
Previous utilisation of ISFETs with replacement of the traditional Si3N4 passivation layer with rare-earth oxide films has resulted in detection of AR-V7 cDNA for prediction of PCa drug resistance [74]. However, the need for qPCR pre-amplification of AR-V7 cDNA before detection of biomarker presence with an ssDNA probe currently limits this technique for a PoC setting. Adjustment to isothermal amplification techniques for pre-amplification could allow for direct PoC detection utilising this ISFET biosensor.
The authors have previously published RT-LAMP reactions coupled directly with ISFET biosensors and have previously been successful at detecting endogenous expression of PCa nucleic acid biomarkers with a handheld device named Lacewing [84,97,101]. Amplification reactions produce a proton for each nucleotide addition to a DNA strand [102]. When the target nucleic acid is present, the pH change from the amplification reaction can be detected with the ISFET biosensor (Figure 2c). This biosensor does not necessitate functionalisation of the ISFET surface, reducing the associated cost and complexity of the device. With this method, AR-V7, AR-FL, TMPRSS2-ERG and YAP1 mRNA were sensitively detected in real-time within 30 min with the Lacewing device. Down to 5 aM mRNA of each target (8 aM for AR-FL) was achievable with this technique, and specificity was confirmed with RNA extracted from PCa cell lines [84,97]. Synthetic detection in both serum and plasma additionally took place. Testing of this electrochemical device with clinical samples could result in valuable prognostic and predictive data for rapid clinical decision making.
4. REASSURED Criteria and Future Directions for PCa PoC Devices
The electrochemical devices explored in this review present simple, sensitive and specific methods with PoC compatibility for PCa diagnostics and prognostics (Table 1). However, establishment of an electrochemical device or procedure that fulfils the REASSURED criteria has not yet been developed for PCa.
Many of the electrochemical techniques utilised for PCa diagnostics and prognostics have been employed for microRNA, mRNA and lncRNA detection platforms. EIS devices alone have not yet been utilised for clinical sample testing. SWV, DPV, chronoamperometry and solution gated-graphene FET are all capable of detecting attomolar concentrations of microRNAs. These exemplary sensitivities could allow for direct testing in blood or urine samples from PCa patients. Attomolar sensitivities for mRNA detection have also been observed with ISFETs and chronoamperometry electrochemical devices [84,95]. The majority of electrochemical devices have also been checked for specificity, either through detection in cell lines or through synthetic nucleic acids. Several electrochemical devices for microRNA detection are capable of discerning between the desired target and a sequence with one base pair mismatch [20,23]. Given the amount of redundancy in microRNA sequences, this testing is valuable, to reduce the likelihood of false positives [103]. In order for the electrochemical PoC tests to be clinically viable they should have high sensitivities and be robustly ratified for specificity testing, ideally with synthetic and endogenous RNA.
Several studies within this review have detected multiple endogenous nucleic acids from clinical samples from blood, urine or their extracts. Many of these studies report an ascribed benefit to detecting relevant PCa microRNAs or mRNAs biomarkers, but typically within small cohorts [42,95]. Previous validation of non-PoC nucleic acid tests for PCa has required robust clinical studies before deployment. This necessitates multicentre validation clinical studies to explore the robustness of the PoC tests and extensive investigation of improved patient outcomes when provided with clinical information from the developed assays [5,13,104,105]. In addition, while all the devices in this review have PoC potential, testing within a clinical setting has not taken place. Translation of these devices directly into hospitals will be crucial to establishing the devices’ utility and position within the current clinical framework. Fulfilment of the user-friendly stipulation in the REASSURED criteria would ideally require devices to be tested with non-specialised personnel.
Multiplex testing has additionally been successful with electrochemical PoC testing for PCa diagnostics. Quantitation of up to four nucleic acid markers simultaneously has been achieved for PCa Poc testing [95]. Other electrochemical devices have additionally shown capacity for expansion of multiplex capability. For example, Wen et al. utilised a 16X sensor chip where creation of further ssDNA probes could expand upon the three microRNA targets utilised in the study [96]. To combat the inherent heterogeneity of PCa, FDA-approved molecular testing typically utilises large panels of nucleic acid markers. The GPS, Decipher Score and CCR detect the expression of 17, 22 and 46 genes, respectively [5,7,106]. Augmentation of developed PoC PCa devices to accommodate large arrays of nucleic acid markers for simultaneous detection will likely improve the diagnostic or prognostic capabilities of the device.
A singular microRNA-detecting PoC device for PCa diagnosis and prognosis allows for direct testing from urine samples with minimal processing [20]. The ease of processing of this device allows for easy implementation of the devices into clinics [20]. Despite this, many other papers have illustrated the utility of microRNA detection directly from serum, which can be easily extracted from whole blood by a clinical laboratory. Alternatively, several papers have reported PoC-compatible sample preparation techniques that allow for appropriate conversion of blood or urine into relevant media for analyte detection in PCa [41,95]. Especially, in instances where copy numbers of the nucleic acid target in the biofluid are low—for example, circulating mRNA in the blood—some degree of PoC sample preparation is likely to be required for relevant PoC detection strategies.
Within the work explored within this review, Koo et al., Deng et al. and Kim et al. currently present the most advanced PoC tests towards clinical implementation [20,21,95]. Koo et al. have developed a multiplex chronoamperometry device capable of PoC sample preparation and subsequent nucleic acid detection from both urine and blood. Corroboration of biomarker presence within multiple biofluids could result in a more robust diagnostic and risk-prediction device. Additionally, the utilisation of 30 PCa patient samples and five healthy donors presents a relatively large patient cohort compared to many of the other PoC electrochemical devices. The devices developed by Kim et al. and Deng et al. are both capable of microRNA testing directly from patient urine and blood, respectively. The work from Deng et al. presented the highest sensitivity for nucleic acid detection, with miR-21 observed down to 0.01 aM. Expansion of this device to detect multiple microRNAs simultaneously would greatly increase its value for PCa diagnostics. Kim et al., while exhibiting a lower sensitivity of 10 fM, were capable of multiplex detection of three microRNAs directly from urine samples. As such, this device is more likely to provide valuable clinical information for a larger range of PCa patients. Expansion of the patient cohort from the initial proof of concept study, however, will be required, to robustly confirm the validity of the device in aiding PCa diagnosis.
While ctDNA can provide both valuable diagnostic and prognostic information for PCa, current PoC electrochemical techniques have not detected this type of biomarker. However, ctDNA has been utilised for molecular diagnostics for PCa. Prediction of resistance to poly(ADP-ribose) polymerase (PARP) inhibitors with BRCA1/BRCA2 mutational status has resulted in FDA-approved tests for PCa from Myriad Genetics and FoundationOne [107]. A non-PoC electrochemical biosensor has also detected levels of DNA methylation in circulation, appropriately distinguishing between PCa patients and healthy men [108]. In other cancer types, PoC detection of ctDNA cell line and synthetic DNA with ISFETs and LAMP has previously been established [109,110]. Therefore, ctDNA presents potential as a relevant biomarker for detection in liquid biopsies, and future PoC tests could utilise this nucleic acid subtype for predictive purposes in PCa.
Current PoC electrochemical device platforms present exceptional potential for the future of PCa diagnostic and prognostic testing. Personalised medicine approaches for PCa through electrochemical testing could result in rapid, cheap and easy-to-use biosensors capable of providing clinically relevant information at the PoC.
Author Contributions
J.B.: writing—original draft preparation, conceptualization, visualisation; M.K.: writing—review and editing, conceptualisation, visualisation; C.L.B.: writing—review and editing, conceptualisation, visualisation; P.G.: writing–review and editing, conceptualisation, visualisation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a Cancer Research UK Imperial Convergence Science Centre PhD studentship (C24523/A27435).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank members of the Bevan and Georgiou laboratories for insightful discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PoC | point of care |
| PCa | prostate cancer |
| PSA | prostate-specific antigen |
| ADT | androgen-deprivation therapies |
| AR | androgen receptor |
| PCA3 | prostate cancer antigen 3 |
| ssDNA | single-stranded DNA |
| EIS | electrochemical impedance spectroscopy |
| AuNP | gold nanoparticle |
| SPCE | screen-printed carbon electrode |
| MWCNT | multi-walled carbon nanotubes |
| CV | cyclic voltammetry |
| SWV | square-wave voltammetry |
| DPV | differential-pulse voltammetry |
| FET | field-effect transistors |
| ISFET | ion-sensitive FET |
| PTEN | phosphatase and tensin homolog |
| AKT | protein kinase B |
| mTOR | mammalian target of rapamycin |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Kwiatkowski, M.; Lujan, M.; Maattanen, L.; Lilja, H.; Denis, L.J.; et al. The European Randomized Study of Screening for Prostate Cancer— Prostate Cancer Mortality at 13 Years of Follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Donovan, J.L.; Turner, E.L.; Metcalfe, C.; Young, G.J.; Walsh, E.I.; Lane, J.A.; Noble, S.; Oliver, S.E.; Evans, S.; et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: The CAP randomized clinical trial. JAMA-J. Am. Med. Assoc. 2018, 319, 883–895. [Google Scholar] [CrossRef]
- McNally, C.J.; Ruddock, M.W.; Moore, T.; McKenna, D.J. Biomarkers that differentiate benign prostatic hyperplasia from prostate cancer: A literature review. Cancer Manag. Res. 2020, 12, 5225–5241. [Google Scholar] [CrossRef]
- Canter, D.J.; Branch, C.; Shelnutt, J.; Foreman, A.J.; Lehman, A.M.; Sama, V.; Edwards, D.K.; Abran, J. The 17-Gene Genomic Prostate Score Assay Is Prognostic for Biochemical Failure in Men with Localized Prostate Cancer after Radiation Therapy at a Community Cancer Center. Adv. Radiat. Oncol. 2023, 8, 101193. [Google Scholar] [CrossRef]
- Fine, N.D.; LaPolla, F.; Epstein, M.; Loeb, S.; Dani, H. Genomic classifiers for treatment selection in newly diagnosed prostate cancer. BJU Int. 2019, 124, 578–586. [Google Scholar] [CrossRef]
- Dal Pra, A.; Ghadjar, P.; Hayoz, S.; Liu, V.Y.; Spratt, D.E.; Thompson, D.J.; Davicioni, E.; Huang, H.C.; Zhao, X.; Liu, Y.; et al. Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy—An ancillary study of the SAKK 09/10 randomized clinical trial. Ann. Oncol. 2022, 33, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Oh-hohenhorst, S.J.; Lange, T. Role of Metastasis-Related microRNAs in Prostate Cancer. Cancers 2021, 13, 4492. [Google Scholar] [CrossRef]
- Rana, S.; Valbuena, G.N.; Curry, E.; Bevan, C.L.; Keun, H.C. MicroRNAs as biomarkers for prostate cancer prognosis: A systematic review and a systematic reanalysis of public data. Br. J. Cancer 2022, 126, 502–513. [Google Scholar] [CrossRef]
- Gittelman, M.C.; Hertzman, B.; Bailen, J.; Williams, T.; Koziol, I.; Henderson, R.J.; Efros, M.; Bidair, M.; Ward, J.F. PCA3 molecular urine test as a predictor of repeat prostate biopsy outcome in men with previous negative biopsies: A prospective multicenter clinical study. J. Urol. 2013, 190, 64–69. [Google Scholar] [CrossRef]
- Nicholson, A.; Mahon, J.; Boland, A.; Beale, S.; Dwan, K.; Fleeman, N.; Hockenhull, J.; Dundar, Y. The clinical effectiveness and cost-effectiveness of the PROGENSA® prostate cancer antigen 3 assay and the prostate health index in the diagnosis of prostate cancer: A systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 1–191. [Google Scholar] [CrossRef] [PubMed]
- Tward, J.D.; Schlomm, T.; Bardot, S.; Canter, D.J.; Scroggins, T.; Freedland, S.J.; Lenz, L.; Flake, D.D.; Cohen, T.; Brawer, M.K.; et al. Personalizing Localized Prostate Cancer: Validation of a Combined Clinical Cell-cycle Risk (CCR) Score Threshold for Prognosticating Benefit From Multimodality Therapy. Clin. Genitourin. Cancer 2021, 19, 296–304. [Google Scholar] [CrossRef]
- Brooks, M.A.; Thomas, L.; Magi-Galluzzi, C.; Li, J.; Crager, M.R.; Lu, R.; Abran, J.; Aboushwareb, T.; Klein, E.A. GPS Assay Association with Long-Term Cancer Outcomes: Twenty-Year Risk of Distant Metastasis and Prostate Cancer–Specific Mortality. JCO Precis. Oncol. 2021, 5, 442–449. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; Von Stetten, F. Loop-mediated isothermal amplification (LAMP)-review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, N.; De Guire, V. MiRNAs: Their discovery, biogenesis and mechanism of action. Clin. Biochem. 2013, 46, 842–845. [Google Scholar] [CrossRef]
- Mall, C.; Rocke, D.M.; Durbin-Johnson, B.; Weiss, R.H. Stability of miRNA in human urine supports its biomarker potential. Biomarkers Med. 2013, 7, 623–631. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper based analytical devices for simultaneously electrochemical detection of microRNAs. Anal. Chim. Acta 2019, 1058, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Ren, Z.; Zhang, H.; Li, Z.; Xue, C.; Wang, J.; Zhang, D.; Yang, H.; Wang, X.; Li, J. Unamplified and Real-Time Label-Free miRNA-21 Detection Using Solution-Gated Graphene Transistors in Prostate Cancer Diagnosis. Adv. Sci. 2023, 10, e2205886. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Cho, Y.S.; Kim, Y.; Tae, J.H.; No, T.I.; Shim, J.S.; Jeong, Y.; Kang, S.H.; Lee, K.H. Electrical Cartridge Sensor Enables Reliable and Direct Identification of MicroRNAs in Urine of Patients. ACS Sens. 2021, 6, 833–841. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Li, B.; Liu, J.; Xu, J.J.; Chen, H.Y. Dual-Mode SERS and Electrochemical Detection of miRNA Based on Popcorn-like Gold Nanofilms and Toehold-Mediated Strand Displacement Amplification Reaction. Anal. Chem. 2021, 93, 6120–6127. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Kim, Y.J.; Jeong, J.Y.; Kim, Y.J. Label-free electrochemical quantification of microRNA-375 in prostate cancer cells. J. Electroanal. Chem. 2019, 846, 113127. [Google Scholar] [CrossRef]
- Ghorbanmehr, N.; Gharbi, S.; Korsching, E.; Tavallaei, M.; Einollahi, B.; Mowla, S.J. miR-21-5p, miR-141-3p, and miR-205-5p levels in urine—promising biomarkers for the identification of prostate and bladder cancer. Prostate 2019, 79, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Porzycki, P.; Ciszkowicz, E.; Semik, M.; Tyrka, M. Combination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognition. Int. Urol. Nephrol. 2018, 50, 1619–1626. [Google Scholar] [CrossRef]
- Arisan, E.D.; Rencuzogullari, O.; Freitas, I.L.; Radzali, S.; Keskin, B.; Kothari, A.; Warford, A.; Uysal-Onganer, P. Upregulated wnt-11 and mir-21 expression trigger epithelial mesenchymal transition in aggressive prostate cancer cells. Biology 2020, 9, 52. [Google Scholar] [CrossRef]
- Stafford, M.Y.; Willoughby, C.E.; Walsh, C.P.; McKenna, D.J. Prognostic value of miR-21 for prostate cancer: A systematic review and meta-analysis. Biosci. Rep. 2022, 42, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, Y.; Wang, K.J.; Deng, Z.; Zhang, W.; Shen, H.F. Plasma exosomal miR-125a-5p and miR-141-5p as non-invasive biomarkers for prostate cancer. Neoplasma 2021, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Qin, X.J.; Cao, D.L.; Zhu, Y.; Yao, X.D.; Zhang, S.L.; Dai, B.; Ye, D.W. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J. Androl. 2013, 15, 231–235. [Google Scholar] [CrossRef]
- Wei, J.; Lu, Y.; Wang, R.; Xu, X.; Liu, Q.; He, S.; Pan, H.; Liu, X.; Yuan, B.; Ding, Y.; et al. MicroRNA-375: Potential cancer suppressor and therapeutic drug. Biosci. Rep. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- Selth, L.A.; Das, R.; Townley, S.L.; Coutinho, I.; Hanson, A.R.; Centenera, M.M.; Stylianou, N.; Sweeney, K.; Soekmadji, C.; Jovanovic, L.; et al. A ZEB1-miR-375-YAP1 pathway regulates epithelial plasticity in prostate cancer. Oncogene 2017, 36, 24–34. [Google Scholar] [CrossRef]
- Cai, S.; Pataillot-Meakin, T.; Shibakawa, A.; Ren, R.; Bevan, C.L.; Ladame, S.; Ivanov, A.P.; Edel, J.B. Single-molecule amplification-free multiplexed detection of circulating microRNA cancer biomarkers from serum. Nat. Commun. 2021, 12, 3515. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, G.A.; Shibakawa, A.; Patel, H.; Sita-Lumsden, A.; Zivi, A.; Rama, N.; Bevan, C.L.; Ladame, S. Amplification-free detection of circulating microRNA biomarkers from body fluids based on fluorogenic oligonucleotide-templated reaction between engineered peptide nucleic acid probes: Application to prostate cancer diagnosis. Anal. Chem. 2016, 88, 8091–8098. [Google Scholar] [CrossRef]
- Wang, Y.; Lieberman, R.; Pan, J.; Zhang, Q.; Du, M.; Zhang, P.; Nevalainen, M.; Kohli, M.; Shenoy, N.K.; Meng, H.; et al. miR-375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1. Mol. Cancer 2016, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.H.; Osther, P.J.; Assenholt, J.; Madsen, J.S.; Hansen, T.F. Circulating miR-141 and miR-375 are associated with treatment outcome in metastatic castration resistant prostate cancer. Sci. Rep. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, D.; Liston, M.; Patel, N.; Akoto, T.; Lui, B.; Yang, T.L.; To, D.M.; Majid, S.; Dahiya, R.; Tabatabai, Z.L.; et al. MicroRNA determinants of neuroendocrine differentiation in metastatic castration-resistant prostate cancer. Oncogene 2020, 39, 7209–7223. [Google Scholar] [CrossRef] [PubMed]
- Yaman, Y.T.; Vural, O.A.; Bolat, G.; Abaci, S. One-pot synthesized gold nanoparticle-peptide nanotube modified disposable sensor for impedimetric recognition of miRNA 410. Sens. Actuators Chem. 2020, 320, 128343. [Google Scholar] [CrossRef]
- Zhang, T.; Austin, R.G.; Park, S.E.; Runyambo, D.; Boominathan, R.; Rao, C.; Bronson, E.; Anand, M.; Healy, P.; George, D.J.; et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer (mPC). J. Clin. Oncol. 2018, 36, 191. [Google Scholar] [CrossRef]
- Wang, J.; Ye, H.; Zhang, D.; Hu, Y.; Yu, X.; Wang, L.; Zuo, C.; Yu, Y.; Xu, G.; Liu, S. MicroRNA-410-5p as a potential serum biomarker for the diagnosis of prostate cancer. Cancer Cell Int. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Merriel, S.W.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022, 20, 54. [Google Scholar] [CrossRef]
- Moranova, L.; Stanik, M.; Hrstka, R.; Campuzano, S.; Bartosik, M. Electrochemical LAMP-based assay for detection of RNA biomarkers in prostate cancer. Talanta 2022, 238, 123064. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-los Santos-Álvarez, N.; Lobo-Castañón, M.J. Dual electrochemical genosensor for early diagnosis of prostate cancer through lncRNAs detection. Biosens. Bioelectron. 2021, 192, 113520. [Google Scholar] [CrossRef] [PubMed]
- Takita, S.; Nabok, A.; Mussa, M.; Kitchen, M.; Lishchuk, A.; Smith, D. Ultrasensitive prostate cancer marker PCA3 detection with impedimetric biosensor based on specific label-free aptamers. Biosens. Bioelectron. X 2024, 18, 100462. [Google Scholar] [CrossRef]
- Soares, R.R.; Neumann, F.; Caneira, C.R.; Madaboosi, N.; Ciftci, S.; Hernández-Neuta, I.; Pinto, I.F.; Santos, D.R.; Chu, V.; Russom, A.; et al. Silica bead-based microfluidic device with integrated photodiodes for the rapid capture and detection of rolling circle amplification products in the femtomolar range. BIosens. Bioelectron. 2019, 128, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hägglöf, C.; Hammarsten, P.; Strömvall, K.; Egevad, L.; Josefsson, A.; Stattin, P.; Granfors, T.; Bergh, A. TMPRSS2-ERG expression predicts prostate cancer survival and associates with stromal biomarkers. PLoS ONE 2014, 9, e86824. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.a.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.w.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Leshem, O.; Madar, S.; Kogan-Sakin, I.; Kamer, I.; Goldstein, I.; Brosh, R.; Cohen, Y.; Jacob-Hirsch, J.; Ehrlich, M.; Ben-Sasson, S.; et al. TMPRSS2/ERG promotes epithelial to mesenchymal transition through the ZEB1/ZEB2 axis in a prostate cancer model. PLoS ONE 2011, 6, e21650. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Aubin, S.M.; Siddiqui, J.; Lonigro, R.J.; Sefton-Miller, L.; Miick, S.; Williamsen, S.; Hodge, P.; Meinke, J.; Blase, A.; et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci. Transl. Med. 2011, 3, 94ra72. [Google Scholar] [CrossRef]
- Reig, Ò.; Marín-Aguilera, M.; Carrera, G.; Jiménez, N.; Paré, L.; García-Recio, S.; Gaba, L.; Pereira, M.V.; Fernández, P.; Prat, A.; et al. TMPRSS2-ERG in Blood and Docetaxel Resistance in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 709–713. [Google Scholar] [CrossRef]
- Marín-Aguilera, M.; Reig, Ò.; Milà-Guasch, M.; Font, A.; Domènech, M.; Rodríguez-Vida, A.; Carles, J.; Suárez, C.; del Alba, A.G.; Jiménez, N.; et al. The influence of treatment sequence in the prognostic value of TMPRSS2-ERG as biomarker of taxane resistance in castration-resistant prostate cancer. Int. J. Cancer 2019, 145, 1970–1981. [Google Scholar] [CrossRef]
- Estébanez-Perpiñá, E.; Bevan, C.L.; McEwan, I.J. Eighty years of targeting androgen receptor activity in prostate cancer: The fight goes on. Cancers 2021, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Ferraldeschi, R.; Welti, J.; Luo, J.; Attard, G.; De Bono, J.S. Targeting the androgen receptor pathway in castration-resistant prostate cancer: Progresses and prospects. Oncogene 2014, 34, 1745–1757. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, J.; Luber, B.; Wang, H.; Lu, C.; Chen, Y.; Zhu, Y.; Taylor, M.N.; Carducci, M.A.; Eisenberger, M.A.; Luo, J.; et al. Clinical significance of AR mRNA quantification from circulating tumor cells (CTCs) in men with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone (Abi) or enzalutamide (Enza). J. Clin. Oncol. 2017, 35, 132. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first & second-line Abiraterone & Enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Stitz, R.; Stoiber, F.; Silye, R.; Vlachos, G.; Andaloro, S.; Rebhan, E.; Dunzinger, M. Clinical Implementation of a Noninvasive, Multi-Analyte Droplet Digital PCR Test to Screen for Androgen Receptor Alterations. J. Mol. Diagn. 2024, 26, 467–478. [Google Scholar] [CrossRef]
- Nimir, M.; Ma, Y.; Jeffreys, S.A.; Opperman, T.; Young, F.; Khan, T.; Ding, P.; Chua, W.; Balakrishnar, B.; Cooper, A.; et al. Detection of AR-v7 in liquid biopsies of castrate resistant prostate cancer patients: A comparison of AR-v7 analysis in circulating tumor cells, circulating tumor RNA and exosomes. Cells 2019, 8, 688. [Google Scholar] [CrossRef]
- Del Re, M.; Conteduca, V.; Crucitta, S.; Gurioli, G.; Casadei, C.; Restante, G.; Schepisi, G.; Lolli, C.; Cucchiara, F.; Danesi, R.; et al. Androgen receptor gain in circulating free DNA and splicing variant 7 in exosomes predict clinical outcome in CRPC patients treated with abiraterone and enzalutamide. Prostate Cancer Prostatic Dis. 2021, 24, 524–531. [Google Scholar] [CrossRef]
- Eskra, J.N.; Rabizadeh, D.; Pavlovich, C.P.; Catalona, W.J.; Luo, J. Approaches to urinary detection of prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 362–381. [Google Scholar] [CrossRef]
- Wang, T.; Tang, L.; Lin, R.; He, D.; Wu, Y.; Zhang, Y.; Yang, P.; He, J. Individual variability in human urinary metabolites identifies age-related, body mass index-related, and sex-related biomarkers. Mol. Genet. Genom. Med. 2021, 9, e1738. [Google Scholar] [CrossRef]
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K.T. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Garimella, S.; Clay-Gilmour, A.; Vojtech, L.; Armstrong, B.; Bessonny, M.; Stamatikos, A. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. J. Pers. Med. 2022, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yuan, X.; Wang, J.; Wang, Y.; Li, S. The diagnostic value of miRNA-141 in prostate cancer: A systematic review and meta-analysis. Blood Genom. 2020, 4, 53–59. [Google Scholar] [CrossRef]
- Qin, Y.; Yao, J.; Wu, D.C.; Nottingham, R.M.; Mohr, S.; Hunicke-Smith, S.; Lambowitz, A.M. High-throughput sequencing of human plasma RNA by using thermostable group II intron reverse transcriptases. Rna 2016, 22, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Sabahi, A.; Salahandish, R.; Ghaffarinejad, A.; Omidinia, E. Electrochemical nano-genosensor for highly sensitive detection of miR-21 biomarker based on SWCNT-grafted dendritic Au nanostructure for early detection of prostate cancer. Talanta 2020, 209, 120595. [Google Scholar] [CrossRef]
- Paul, R.; Ostermann, E.; Wei, Q. Advances in point-of-care nucleic acid extraction technologies for rapid diagnosis of human and plant diseases. Biosens. Bioelectron. 2020, 169, 112592. [Google Scholar] [CrossRef]
- Sriram, H.; Khanka, T.; Kedia, S.; Tyagi, P.; Ghogale, S.; Deshpande, N.; Chatterjee, G.; Rajpal, S.; Patkar, N.V.; Subramanian, P.G.; et al. Improved protocol for plasma microrna extraction and comparison of commercial kits. Biochem. Medica 2021, 31, 030705. [Google Scholar] [CrossRef]
- Lasserre, P.; Balansethupathy, B.; Vezza, V.J.; Butterworth, A.; Macdonald, A.; Blair, E.O.; McAteer, L.; Hannah, S.; Ward, A.C.; Hoskisson, P.A.; et al. SARS-CoV-2 Aptasensors Based on Electrochemical Impedance Spectroscopy and Low-Cost Gold Electrode Substrates. Anal. Chem. 2022, 94, 2126–2133. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Garg, S.; Sachdeva, A.; Peeters, M.; McClements, J. Point-of-Care Prostate Specific Antigen Testing: Examining Translational Progress toward Clinical Implementation. ACS Sens. 2023, 8, 3643–3658. [Google Scholar] [CrossRef]
- Mwanza, D.; Adeniyi, O.; Tesfalidet, S.; Nyokong, T.; Mashazi, P. Capacitive label-free ultrasensitive detection of PSA on a covalently attached monoclonal anti-PSA antibody gold surface. J. Electroanal. Chem. 2022, 927, 116983. [Google Scholar] [CrossRef]
- Soares, J.C.; Soares, A.C.; Rodrigues, V.C.; Melendez, M.E.; Santos, A.C.; Faria, E.F.; Reis, R.M.; Carvalho, A.L.; Oliveira, O.N. Detection of the Prostate Cancer Biomarker PCA3 with Electrochemical and Impedance-Based Biosensors. ACS Appl. Mater. Interfaces 2019, 11, 46645–46650. [Google Scholar] [CrossRef]
- Weng, W.H.; Jhou, C.H.; Xie, H.X.; Pan, T.M. Label-Free Detection of AR-V7 mRNA in Prostate Cancer Using Yb2Ti2O7–Based Electrolyte-Insulator-Semiconductor Biosensors. J. Electrochem. Soc. 2016, 163, B710–B717. [Google Scholar] [CrossRef]
- Kinnamon, D.; Ghanta, R.; Lin, K.C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef] [PubMed]
- Mugoni, V.; Ciani, Y.; Nardella, C.; Demichelis, F. Circulating RNAs in prostate cancer patients. Cancer Lett. 2022, 524, 57–69. [Google Scholar] [CrossRef]
- Rodrigues, V.C.; Soares, J.C.; Soares, A.C.; Braz, D.C.; Melendez, M.E.; Ribas, L.C.; Scabini, L.F.; Bruno, O.M.; Carvalho, A.L.; Reis, R.M.; et al. Electrochemical and optical detection and machine learning applied to images of genosensors for diagnosis of prostate cancer with the biomarker PCA3. Talanta 2021, 222, 121444. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, K.; Neves, A.F.; Rocha, R.M.; Faria, P.R.; Alves, P.T.; Souza, A.G.; Fujimura, P.T.; Santos, F.A.; Araújo, T.G.; Ward, L.S.; et al. Prostate-specific RNA aptamer: Promising nucleic acid antibody-like cancer detection. Sci. Rep. 2015, 5, 12090. [Google Scholar] [CrossRef]
- Abdelbaset, R.; Shawky, S.M.; Abdullah, M.A.; Morsy, O.E.; Yahia, Y.A.; Ghallab, Y.H.; Matboli, M.; Ismail, Y. A new label free spiral sensor using impedance spectroscopy to characterize hepatocellular carcinoma in tissue and serum samples. Sci. Rep. 2024, 14, 13155. [Google Scholar] [CrossRef]
- Farokhi, S.; Roushani, M. Flower-like core-shell nanostructures based on natural asphalt coated with Ni-LDH nanosheets as an electrochemical platform for prostate cancer biomarker sensing. Microchim. Acta 2023, 190, 198. [Google Scholar] [CrossRef]
- Takita, S.; Nabok, A.; Lishchuk, A.; Mussa, M.H.; Smith, D. Detection of Prostate Cancer Biomarker PCA3 with Electrochemical Apta-Sensor. Eng. Proc. 2022, 16, 8. [Google Scholar] [CrossRef]
- Feng, X.; Gan, N.; Zhang, H.; Li, T.; Cao, Y.; Hu, F.; Jiang, Q. Ratiometric biosensor array for multiplexed detection of microRNAs based on electrochemiluminescence coupled with cyclic voltammetry. Biosens. Bioelectron. 2016, 75, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.E.; Yu, K.J.; Pang, S.N.; Yang, Y.L.; Pang, S.T.; Weng, W.H. A Highly Sensitive Urinary Exosomal miRNAs Biosensor Applied to Evaluation of Prostate Cancer Progression. Bioengineering 2022, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, J.; Kalofonou, M.; Pataillot-Meakin, T.; Powell, S.M.; Fernandes, R.C.; Moser, N.; L. Bevan, C.; Georgiou, P. Detection of YAP1 and AR-V7 mRNA for Prostate Cancer Prognosis Using an ISFET Lab-On-Chip Platform. ACS Sens. 2022, 7, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Chooto, P. Cyclic Voltammetry and Its Applications; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Simões, F.R.; Xavier, M.G. 6-Electrochemical Sensors. In Nanoscience and its Applications; Micro and Nano Technologies; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N.B.T.N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 155–178. [Google Scholar] [CrossRef]
- Venton, B.J.; DiScenza, D.J. Chapter 3—Voltammetry. In Electrochemistry for Bioanalysis; Patel, B.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–50. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-wave voltammetry. ChemTexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Li, F.; Han, X.; Liu, S. Development of an electrochemical DNA biosensor with a high sensitivity of fM by dendritic gold nanostructure modified electrode. Biosens. Bioelectron. 2011, 26, 2619–2625. [Google Scholar] [CrossRef]
- Yang, F.K.; Tian, C.; Zhou, L.X.; Guan, T.Y.; Chen, G.L.; Zheng, Y.Y.; Cao, Z.G. The value of urinary exosomal microRNA-21 in the early diagnosis and prognosis of bladder cancer. Kaohsiung J. Med. Sci. 2024, 40, 660–670. [Google Scholar] [CrossRef]
- Yang, C.; Dou, B.; Shi, K.; Chai, Y.; Xiang, Y.; Yuan, R. Multiplexed and amplified electronic sensor for the detection of microRNAs from cancer cells. Anal. Chem. 2014, 86, 11913–11918. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.M.; Dey, S.; Trau, M. A Sample-to-Targeted Gene Analysis Biochip for Nanofluidic Manipulation of Solid-Phase Circulating Tumor Nucleic Acid Amplification in Liquid Biopsies. ACS Sens. 2018, 3, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, L.; Li, J.; Lin, M.; Liu, G.; Liang, W.; Xu, L.; Li, Y.; Zuo, X.; Ren, S.; et al. DNA Framework-Mediated Electrochemical Biosensing Platform for Amplification-Free MicroRNA Analysis. Anal. Chem. 2020, 92, 4498–4503. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, J.; Kalofonou, M.; Franklin, S.; Powell, S.M.; Pataillot-Meakin, T.; Moser, N.; Bevan, C.L.; Georgiou, P. Handheld ISFET Lab-on-Chip Detection of TMPRSS2-ERG and AR mRNA for Prostate Cancer Prognostics. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Moser, N.; Keeble, L.; Rodriguez-Manzano, J.; Georgiou, P. ISFET arrays for lab-on-chip technology: A review. In Proceedings of the 2019 26th IEEE International Conference on Electronics, Circuits and Systems, ICECS 2019, Genoa, Italy, 27–29 November 2019; pp. 57–60. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. ISFET characteristics in CMOS and their application to weak inversion operation. Sens. Actuators B Chem. 2009, 143, 211–217. [Google Scholar] [CrossRef]
- Rodriguez-Manzano, J.; Malpartida-Cardenas, K.; Moser, N.; Pennisi, I.; Cavuto, M.; Miglietta, L.; Moniri, A.; Penn, R.; Satta, G.; Randell, P.; et al. Handheld point-of-care system for rapid detection of SARS-CoV-2 extracted RNA in under 20 min. ACS Cent. Sci. 2021, 7, 307–317. [Google Scholar] [CrossRef]
- Chen, C.Y. DNA polymerases drive DNA sequencing-by-synthesis technologies: Both past and present. Front. Microbiol. 2014, 5, 305. [Google Scholar] [CrossRef]
- Kehl, T.; Backes, C.; Kern, F.; Fehlmann, T.; Ludwig, N.; Meese, E.; Lenhof, H.P.; Keller, A. About miRNAs, miRNA seeds, target genes and target pathways. Oncotarget 2017, 8, 107167–107175. [Google Scholar] [CrossRef]
- Lokhandwala, P.M.; Riel, S.L.; Haley, L.; Lu, C.; Chen, Y.; Silberstein, J.; Zhu, Y.; Zheng, G.; Lin, M.T.; Gocke, C.D.; et al. Analytical Validation of Androgen Receptor Splice Variant 7 Detection in a Clinical Laboratory Improvement Amendments (CLIA) Laboratory Setting. J. Mol. Diagn. 2017, 19, 115–125. [Google Scholar] [CrossRef]
- Sokoll, L.J.; Ellis, W.; Lange, P.; Noteboom, J.; Elliott, D.J.; Deras, I.L.; Blase, A.; Koo, S.; Sarno, M.; Rittenhouse, H.; et al. A multicenter evaluation of the PCA3 molecular urine test: Pre-analytical effects, analytical performance, and diagnostic accuracy. Clin. Chim. Acta 2008, 389, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Canter, D.J.; Freedland, S.; Rajamani, S.; Latsis, M.; Variano, M.; Halat, S.; Tward, J.; Cohen, T.; Stone, S.; Schlomm, T.; et al. Analysis of the prognostic utility of the cell cycle progression (CCP) score generated from needle biopsy in men treated with definitive therapy. Prostate Cancer Prostatic Dis. 2020, 23, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Su, J.; Zhao, Z.; Shao, Y.; Dou, Y.; Li, F.; Deng, W.; Shi, J.; Li, Q.; Zuo, X.; et al. DNA Framework-Supported Electrochemical Analysis of DNA Methylation for Prostate Cancers. Nano Lett. 2020, 20, 7028–7035. [Google Scholar] [CrossRef]
- Mantikas, K.T.; Moser, N.; Gulli, C.; Cunningham, D.; Georgiou, P.; Simillis, C.; Kalofonou, M. Detection of the Colorectal Cancer TP53 p.R248W Mutation on a Lab-on-Chip ISFET Platform. In Proceedings of the 2023 IEEE BioSensors Conference, BioSensors 2023—Proceedings, London, UK, 30 July–1 August 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Alexandrou, G.; Moser, N.; Mantikas, K.T.; Rodriguez-Manzano, J.; Ali, S.; Coombes, R.C.; Shaw, J.; Georgiou, P.; Toumazou, C.; Kalofonou, M. Detection of Multiple Breast Cancer ESR1 Mutations on an ISFET Based Lab-on-Chip Platform. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 380–389. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).