Innovative Detection of Biomarkers Based on Chemiluminescent Nanoparticles and a Lensless Optical Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
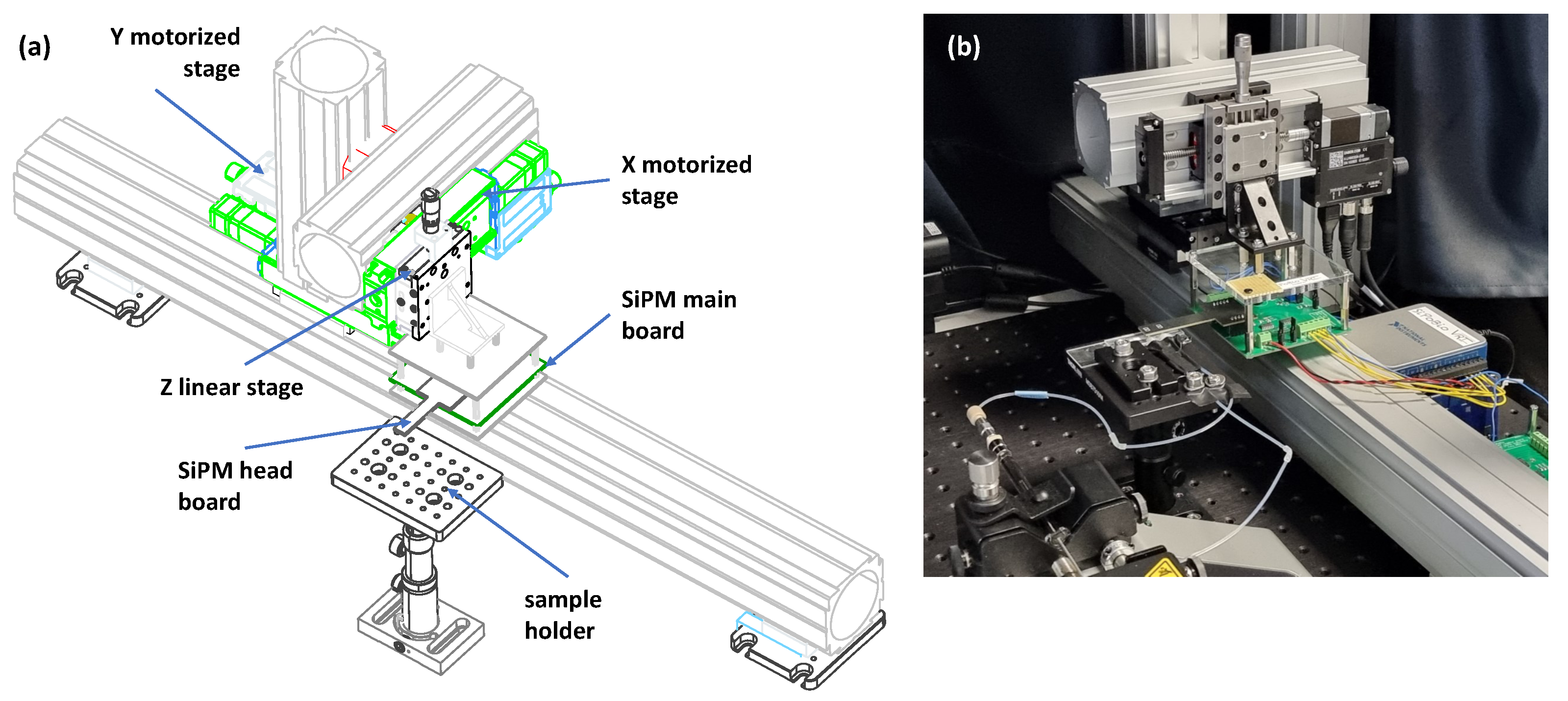
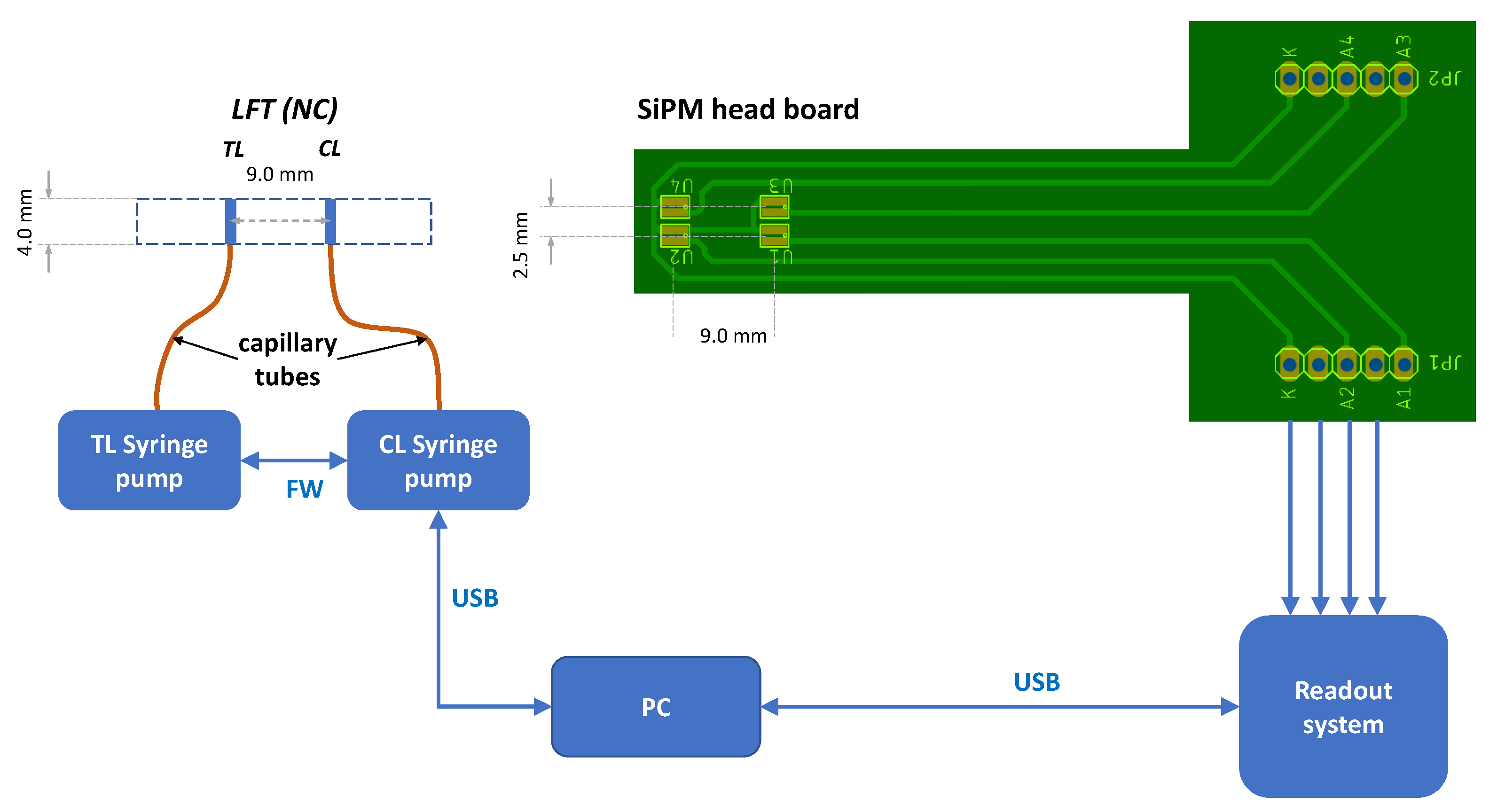
2.2. Setup of the Biosensor
2.3. Preparation of Gold Nanoparticles
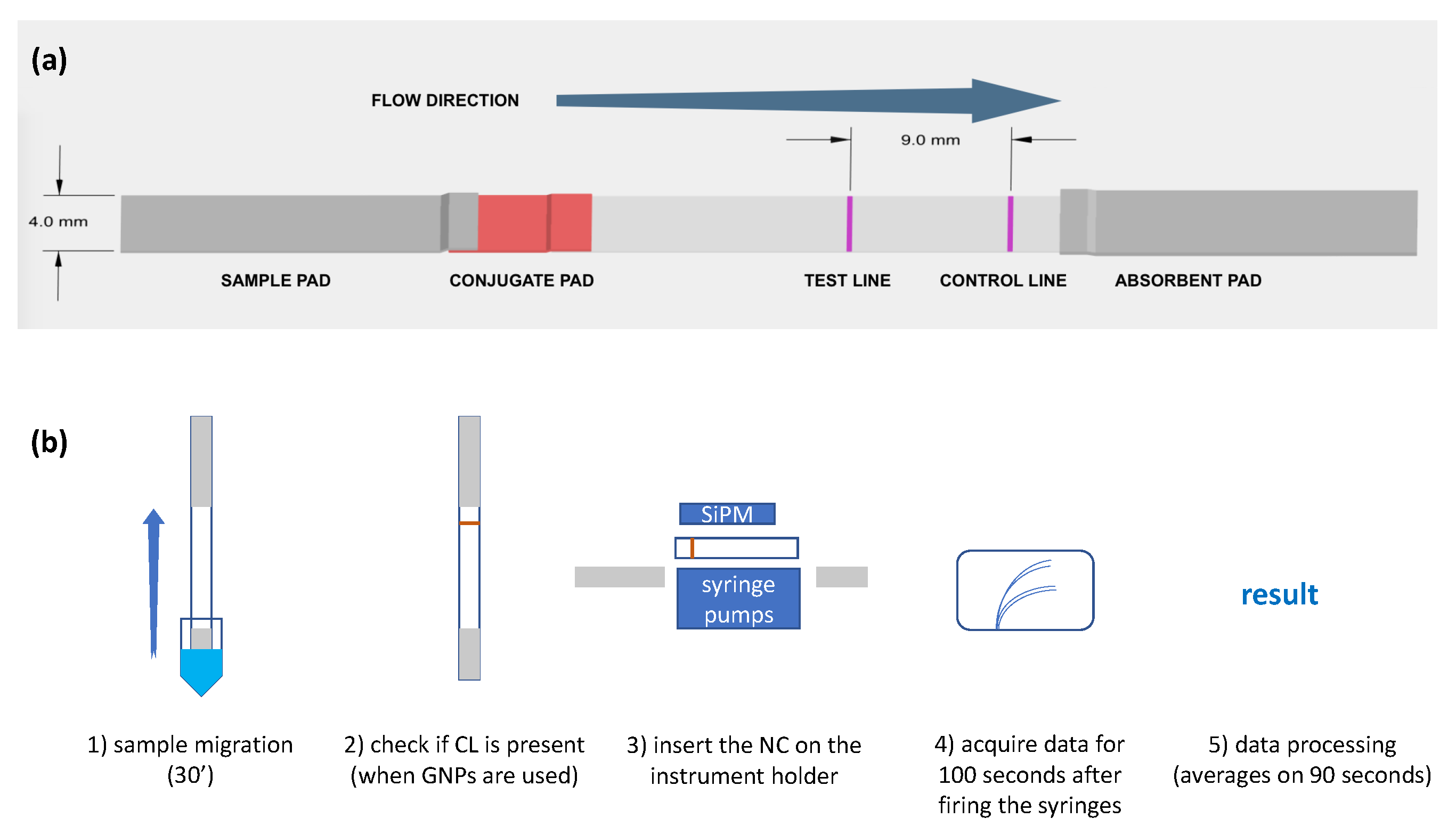
2.4. Fabrication of the LFT Strips
2.5. Detection Protocol of the CRP Biomarker
3. Results and Discussion
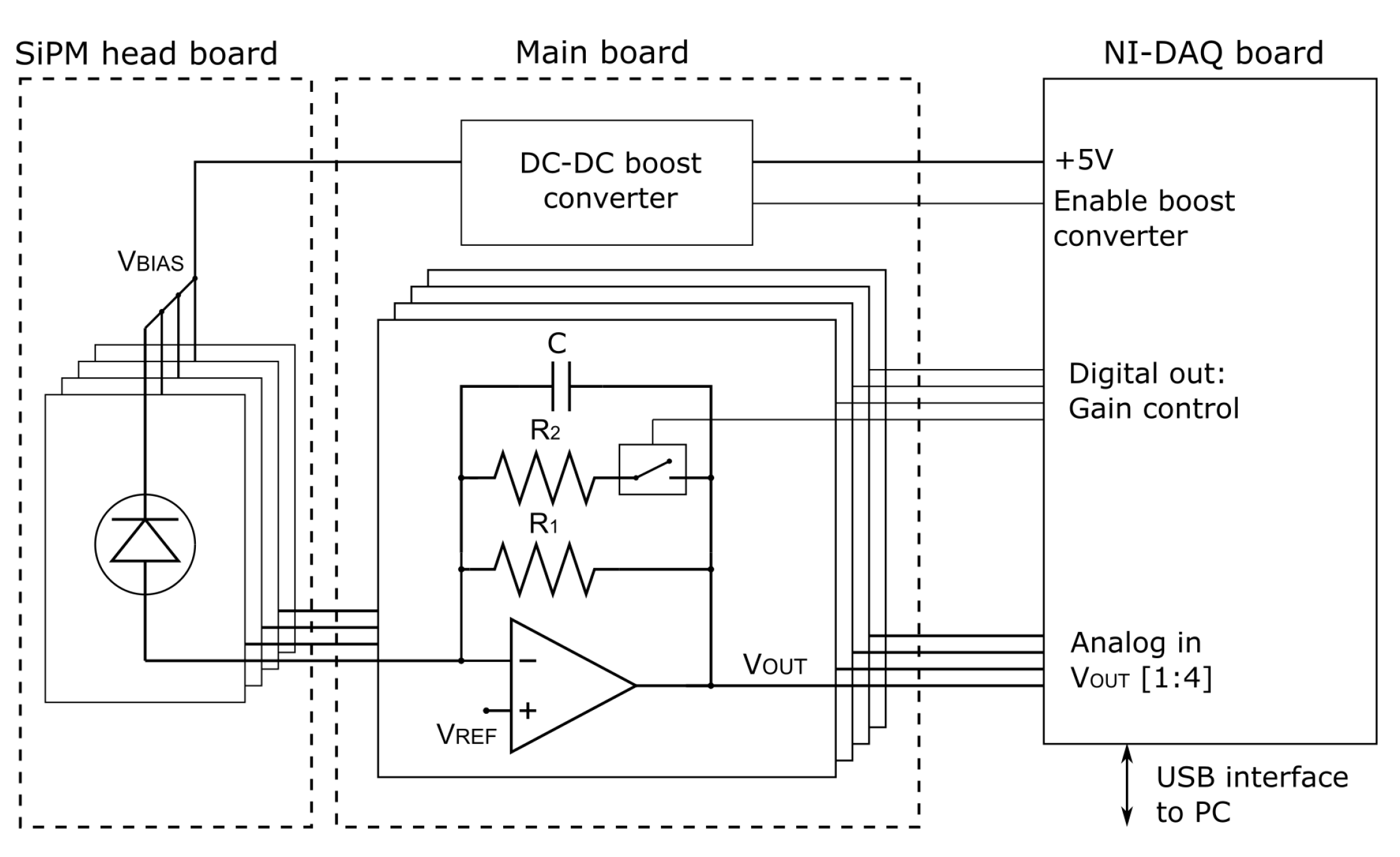
3.1. Description of the SiPM-Based Detector
3.2. Setup and Characterization of the LFT
3.3. Implementation of the LFT for Biomarkers Detection
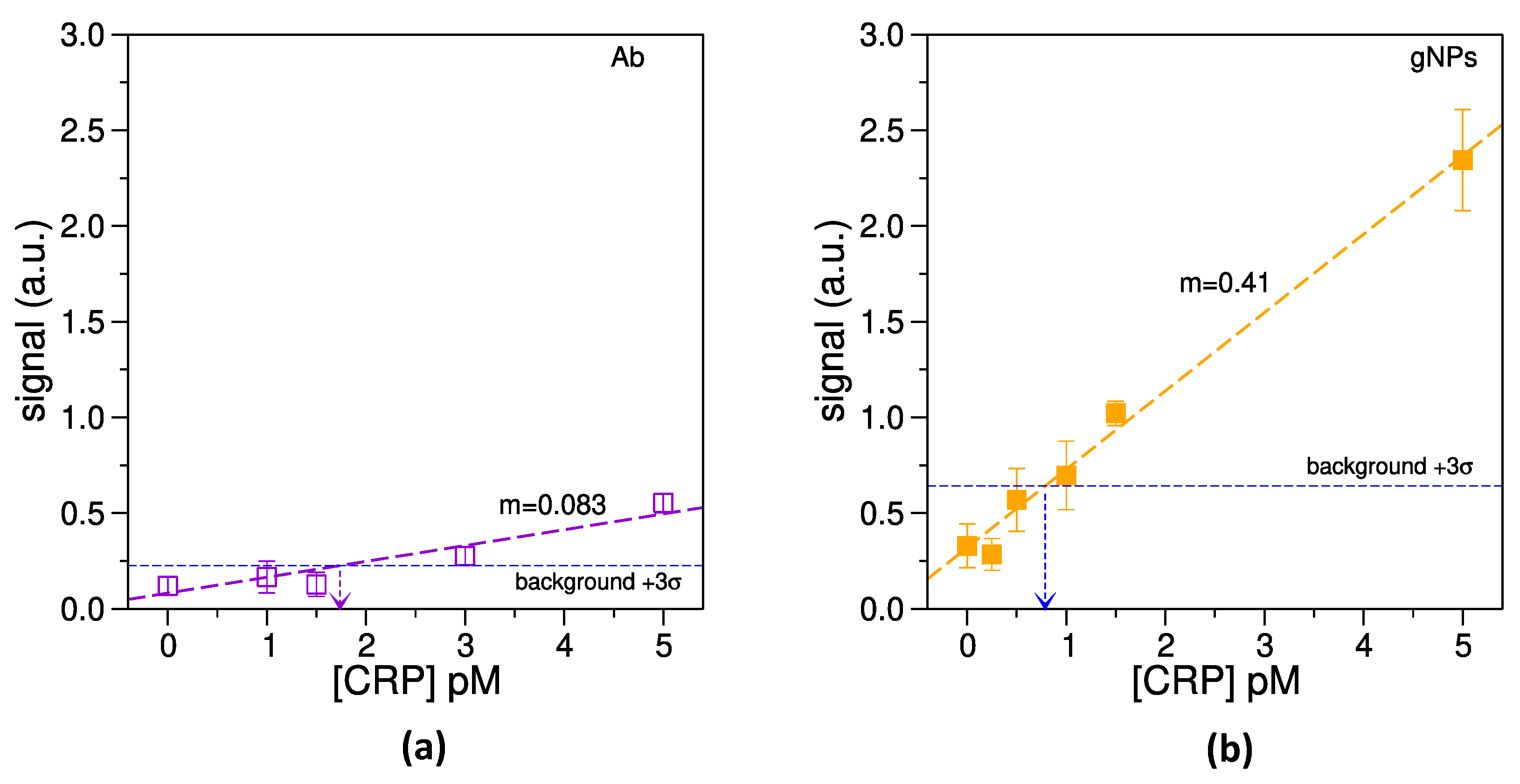
3.4. Estimation of the Limit of Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanzad, M.; Sarhangi, N.; Chimeh, S.E.; Ayati, N.; Afzali, M.; Khatami, F.; Nikfar, S.; Meybodi, H.R.A. Precision medicine journey through omics approach. J. Diabetes Metab. Disord. 2022, 21, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, X.; Jiang, C.; Zhao, H. Difficulties and challenges in the development of precision medicine. Clin. Genet. 2019, 95, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Lianidou, E.; Pantel, K. Liquid biopsies. Genes Chromosom. Cancer 2019, 58, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M. Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics 2021, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Murthy, V.; Takahashi, H.; Huyser, M.; Okano, M.; Tokumaru, Y.; Rashid, O.M.; Matsuyama, R.; Endo, I.; Takabe, K. Urine as a Source of Liquid Biopsy for Cancer. Cancers 2021, 13, 2652. [Google Scholar] [CrossRef] [PubMed]
- Aro, K.; Wei, F.; Wong, D.T.; Tu, M. Saliva Liquid Biopsy for Point-of-Care Applications. Front. Public Health 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.; Gao, S.; Wang, B.; Kong, H.; Yang, G.; Shu, W.; Xu, P.; Wei, G. Two-Dimensional Material-Based Electrochemical Sensors/Biosensors for Food Safety and Biomolecular Detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Altug, H.; Oh, S.H.; Maier, S.A.; Homola, J. Advances and applications of nanophotonic biosensors. Nat. Nanotechnol. 2022, 17, 5–16. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Nardo, F.D.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef]
- Han, G.; Kim, M. Highly Sensitive Chemiluminescence-Based Lateral Flow Immunoassay for Cardiac Troponin I Detection in Human Serum. Sensors 2020, 20, 2593. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Jin, E.; Lee, M.H. Portable Chemiluminescence-Based Lateral Flow Assay Platform for the Detection of Cortisol in Human Serum. Biosensors 2021, 11, 191. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Raphael Wong, H.T. (Ed.) Lateral Flow Immunoassay; Humana: Totowa, NJ, USA, 2009. [Google Scholar]
- Roda, A.; Mirasoli, M.; Michelini, E.; Fusco, M.D.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 342848. [Google Scholar] [CrossRef] [PubMed]
- Volanakis, J. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023, 61, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.A.; Blum, L.J. Applications of the luminol chemiluminescent reaction in analytical chemistry. Anal. Bioanal. Chem. 2006, 385, 546–554. [Google Scholar] [CrossRef]
- Gundacker, S.; Heering, A. The silicon photomultiplier: Fundamentals and applications of a modern solid-state photon detector. Phys. Med. Biol. 2020, 65, 17TR01. [Google Scholar] [CrossRef] [PubMed]
- Pasquardini, L.; Pancheri, L.; Potrich, C.; Ferri, A.; Piemonte, C.; Lunelli, L.; Napione, L.; Comunanza, V.; Alvaro, M.; Vanzetti, L.; et al. SPAD aptasensor for the detection of circulating protein biomarkers. Biosens. Bioelectron. 2015, 68, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Aggarwal, K.; Vinitha T., U.; Nguyen, T.; Han, J.; Ahn, C.H. A new microchannel capillary flow assay (MCFA) platform with lyophilized chemiluminescence reagents for a smartphone-based POCT detecting malaria. Microsyst. Nanoeng. 2020, 6, 5. [Google Scholar] [CrossRef]
- Ruffinatti, F.A.; Lomazzi, S.; Nardo, L.; Santoro, R.; Martemiyanov, A.; Dionisi, M.; Tapella, L.; Genazzani, A.A.; Lim, D.; Distasi, C.; et al. Assessment of a Silicon-Photomultiplier-Based Platform for the Measurement of Intracellular Calcium Dynamics with Targeted Aequorin. ACS Sens. 2020, 5, 2388–2397. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Montali, L.; Lopreside, A.; Fragapane, F.; Iacoangeli, F.; Roda, A.; Bocci, V.; D’Elia, M.; Michelini, E. Ultrasensitive On-Field Luminescence Detection Using a Low-Cost Silicon Photomultiplier Device. Anal. Chem. 2021, 93, 7388–7393. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, W.; Chu, K.; Wei, X.; Smith, Z.J. Highly Sensitive, Portable Detection System for Multiplex Chemiluminescence Analysis. Anal. Chem. 2023, 95, 14762–14769. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Akerström, B.; Björck, L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J. Biol. Chem. 1986, 261, 10240–10247. [Google Scholar] [CrossRef]
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1787–1791. [Google Scholar] [CrossRef]
- Kim, W.; Cho, H.Y.; Jeong, B.; Byun, S.; Huh, J.; Kim, Y.J. Synergistic Use of Gold Nanoparticles (AuNPs) and “Capillary Enzyme-Linked Immunosorbent Assay (ELISA)” for High Sensitivity and Fast Assays. Sensors 2018, 18, 55. [Google Scholar] [CrossRef]
- Oh, Y.K.; Joung, H.A.; Han, H.S.; Suk, H.J.; Kim, M.G. A three-line lateral flow assay strip for the measurement of C-reactive protein covering a broad physiological concentration range in human sera. Biosens. Bioelectron. 2014, 61, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Guo, J.; Wang, J.; Zhang, Y. Electrochemical detection of C-reactive protein using Copper nanoparticles and hybridization chain reaction amplifying signal. Anal. Biochem. 2017, 539, 1–7. [Google Scholar] [CrossRef] [PubMed]
- CRP Human Instant ELISA™ Kit. Available online: https://www.thermofisher.com/elisa/product/CRP-Human-Instant-ELISA-Kit/BMS288INST (accessed on 5 April 2024).
- Human CRP ELISA Kit (C-Reactive protein), Fluorescent. Available online: https://www.abcam.com/en-it/products/elisa-kits/human-crp-elisa-kit-c-reactive-protein-fluorescent-ab278042#tab=support (accessed on 5 April 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potrich, C.; Palmara, G.; Frascella, F.; Pancheri, L.; Lunelli, L. Innovative Detection of Biomarkers Based on Chemiluminescent Nanoparticles and a Lensless Optical Sensor. Biosensors 2024, 14, 184. https://doi.org/10.3390/bios14040184
Potrich C, Palmara G, Frascella F, Pancheri L, Lunelli L. Innovative Detection of Biomarkers Based on Chemiluminescent Nanoparticles and a Lensless Optical Sensor. Biosensors. 2024; 14(4):184. https://doi.org/10.3390/bios14040184
Chicago/Turabian StylePotrich, Cristina, Gianluca Palmara, Francesca Frascella, Lucio Pancheri, and Lorenzo Lunelli. 2024. "Innovative Detection of Biomarkers Based on Chemiluminescent Nanoparticles and a Lensless Optical Sensor" Biosensors 14, no. 4: 184. https://doi.org/10.3390/bios14040184
APA StylePotrich, C., Palmara, G., Frascella, F., Pancheri, L., & Lunelli, L. (2024). Innovative Detection of Biomarkers Based on Chemiluminescent Nanoparticles and a Lensless Optical Sensor. Biosensors, 14(4), 184. https://doi.org/10.3390/bios14040184






